Hepatitis C Elimination: Opportunities and Challenges in 2023
Abstract
1. Introduction
2. World Health Organization (WHO) Initiative
3. Access and Barriers
4. Actions Required
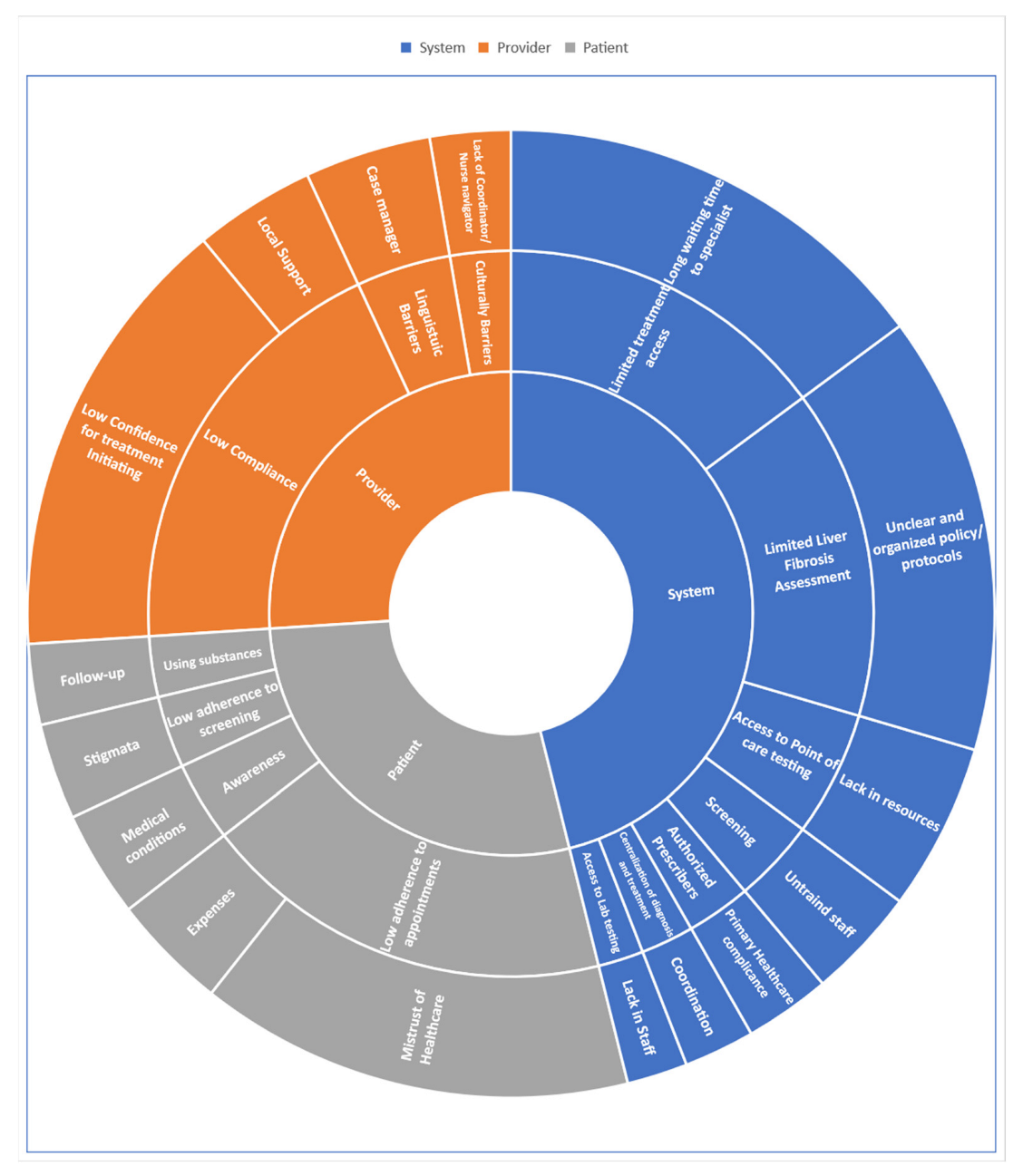
5. System-Level Barriers
6. Provider-Level Barriers
7. Patient-Level Barriers
8. Telemedicine
9. Models for HCV Healthcare
10. Cascade of Care
11. Cost Effectiveness of HCV Screening and Elimination
12. Discussion
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 15 February 2023).
- Abu-Freha, N.; Jacob, B.M.; Elhoashla, A.; Afawi, Z.; Abu-Hammad, T.; Elsana, F.; Paz, S.; Etzion, O. Chronic hepatitis C: Diagnosis and treatment made easy. Eur. J. Gen. Pract. 2022, 28, 102–108. [Google Scholar] [CrossRef] [PubMed]
- EASL. EASL recommendations on treatment of hepatitis C final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Ghany, M.G.; Morgan, T.R. AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C guidance 2019 update: American association for the study of liver diseases–infectious diseases society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 2020, 71, 686–721. [Google Scholar] [CrossRef] [PubMed]
- Shiha, G.; Soliman, R.; Hassan, A.A.; Mikhail, N.N.H. Changes in hepatic fibrosis and incidence of HCC following direct-acting antiviral treatment of F3 chronic hepatitis c patients: A prospective observational study. Hepatoma Res. 2022, 8, 29. [Google Scholar] [CrossRef]
- Zakareya, T.; Elhelbawy, M.; Elzohry, H.; Eltabbakh, M.; Deif, M.; Abbasy, M. Long-Term Impact of Hepatitis C Virus Eradication on Liver Stiffness in Egyptian Patients. Can. J. Gastroenterol. Hepatol. 2021, 20, 4961919. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 2017, 153, 996–1005.e1. [Google Scholar] [CrossRef] [PubMed]
- Li, D.K.; Ren, Y.; Fierer, D.S.; Rutledge, S.; Shaikh, O.S.; Lo Re, V., III; Simon, T.; Abou Samra, A.-B.; Chung, R.T.; Butt, A.A. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An Archives study. Hepatology 2018, 67, 2244–2253. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Green, P.K.; Berry, K. HCV eradication induced by direct acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. 2017, 68, 25–32. [Google Scholar] [CrossRef]
- Shiha, G.; Mousa, N.; Soliman, R.; Nnh Mikhail, N.; Adel Elbasiony, M.; Khattab, M. Incidence of HCC in chronic hepatitis C patients with advanced hepatic fibrosis who achieved SVR following DAAs: A prospective study. J. Viral Hepat. 2020, 27, 671–679. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf;jsessionid=60A93ADD1A191FF6A0FA823314D24C43?sequence=1 (accessed on 28 February 2023). (WHO, 2016).
- Yeo, Y.H.; Gao, X.; Wang, J.; Li, Q.; Su, X.; Geng, Y.; Huang, R.; Wu, C.; Ji, F.; Sundaram, V.; et al. The impact of COVID-19 on the cascade of care of HCV in the US and China. Ann. Hepatol. 2022, 27, 100685. [Google Scholar] [CrossRef]
- Blach, S.; Kondili, L.A.; Aghemo, A.; Cai, Z.; Dugan, E.; Estes, C.; Gamkrelidze, I.; Ma, S.; Pawlotsky, J.M.; Razavi-Shearer, D.; et al. Impact of COVID-19 on global HCV elimination efforts. J. Hepatol. 2021, 74, 31–36. [Google Scholar] [CrossRef]
- Hoenigl, M.; Abramovitz, D.; Flores Ortega, R.E.; Martin, N.K.; Reau, N. Sustained Impact of the Coronavirus Disease 2019 Pandemic on Hepatitis C Virus Treatment Initiations in the United States. Clin. Infect. Dis. 2022, 75, e955–e961. [Google Scholar] [CrossRef] [PubMed]
- Kondili, L.A.; Buti, M.; Riveiro-Barciela, M.; Maticic, M.; Negro, F.; Berg, T.; Craxì, A. Impact of the COVID-19 pandemic on hepatitis B and C elimination: An EASL survey. JHEP Rep. 2022, 4, 100531. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/bitstream/handle/10665/273174/9789241550345-eng.pdf (accessed on 5 March 2023).
- Ferraro, M.; Painter, M. Hepatitis C—Screening, diagnosis, management & treatment. Osteopath. Fam. Phys. 2019, 11, 12–19. [Google Scholar]
- Guss, D.; Sherigar, J.; Rosen, P.; Mohanty, S.R. Diagnosis and Management of Hepatitis C Infection in Primary Care Settings. J. Gen. Intern. Med. 2018, 33, 551–557. [Google Scholar] [CrossRef]
- WHO Recommendation. Available online: https://apps.who.int/iris/bitstream/handle/10665/254621/9789241549981-eng.pdf?sequence=1 (accessed on 21 March 2023).
- Liu, L.; Zhang, M.; Hang, L.; Kong, F.; Yan, H.; Zhang, Y.; Feng, X.; Gao, Y.; Wang, C.; Ma, H. Evaluation of a new point-of-care oral anti-HCV test for screening of hepatitis C virus infection. Virol. J. 2020, 17, 14. [Google Scholar] [CrossRef]
- Lee, S.R.; Yearwood, G.D.; Guillon, G.B.; Kurtz, L.A.; Fischl, M.; Friel, T.; Berne, C.T.; Kardos, K.W. Evaluation of a rapid, point-of-care test device for the diagnosis of hepatitis C infection. J. Clin. Virol. 2010, 48, 15–17. [Google Scholar] [CrossRef]
- Cha, Y.J.; Park, Q.; Kang, E.-S.; Yoo, B.C.; Park, K.U.; Kim, J.-W.; Hwang, Y.-S.; Kim, M.H. Performance evaluation of the OraQuick hepatitis C virus rapid antibody test. Ann. Lab. Med. 2013, 33, 184–189. [Google Scholar] [CrossRef]
- Pallarés, C.; Carvalho-Gomes, A.; Hontangas, V.; Conde, I.; Di Maira, T.; Aguilera, V.; Benlloch, S.; Berenguer, M.; López-Labrador, F.X. Performance of the OraQuick hepatitis C virus antibody test in oral fluid and fingerstick blood before and after treatment-induced viral clearance. J. Clin. Virol. 2018, 102, 77–83. [Google Scholar] [CrossRef]
- Litwin, A.H.; Drolet, M.; Nwankwo, C.; Torrens, M.; Kastelic, A.; Walcher, S.; Somaini, L.; Mulvihill, E.; Ertl, J.; Grebely, J. Perceived barriers related to testing, management and treatment of HCV infection among physicians prescribing opioid agonist therapy: The C-SCOPE Study. J. Viral Hepatol. 2019, 26, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.C.; Figueiredo-Mendes, C.; Villela-Nogueira, C.A.; Marcellin, P. Staging Fibrosis in Chronic Viral Hepatitis. Viruses 2022, 14, 660. [Google Scholar] [CrossRef]
- Isakov, V.; Nikityuk, D. Elimination of HCV in Russia: Barriers and Perspective. Viruses 2022, 11, 14–790. [Google Scholar] [CrossRef] [PubMed]
- Shilton, S.; Markby, J.; Sem, X.; Siva, S.; Zain, R.; Menetrey, C.; Yuswan, F.; Nasir, N.; Andrieux-Meyer, I.; -Easterbrook, P.; et al. Feasibility, effectiveness, and lessons learned from the introduction of decentralised HCV testing and treatment at primary healthcare clinics in three regions in Malaysia. J. Hepatol. 2021, 75, S660. [Google Scholar]
- Mandel, E.; Underwood, K.; Capraru, C.; Shah, H.; Janssen, H.; Feld, J.; Biondi, M. Working towards elimination by understanding province to province variability in the HCV cascade of care in Canada. Hepatology 2021, 74, 417A. [Google Scholar]
- Patel, A.; Wang, S.; Gallipani, A.; Brogden, R.; Xu, B.; Adedeji, M.; Yango, J. Partnership for elimination: Ambulatory care clinical pharmacists dismantling barriers to hepatitis C cure. Hepatology 2021, 74, 570A. [Google Scholar]
- Grebely, J.; Tyndall, M.W. Management of HCV and HIV infection among people who inject drugs. Curr. Opin. HIV AIDS 2011, 6, 501–507. [Google Scholar] [CrossRef]
- Myles, A.; Mugford, G.J.; Zhao, J.; Krahn, M.; Wang, P.P. Physicians’ attitudes and practice toward treating injection drug users with hepatitis C: Results from a national specialist survey in Canada. Can. J. Gastroenterol. 2011, 25, 135. [Google Scholar] [CrossRef]
- Litwin, A.H.; Kunins, H.V.; Berg, K.M.; Federman, A.D.; Heavner, K.K.; Gourevitch, M.N.; Arnsten, J.H. Hepatitis C management by addiction medicine physicians: Results from a national survey. J. Subst. Abus. Treat. 2007, 33, 99–105. [Google Scholar] [CrossRef]
- Malespin, M.; Harris, C.; Kanar, O.; Jackman, K.; Smotherman, C.; Johnston, A.; Ferm, J.; de Melo, S.W., Jr.; Scolapio, J.S.; Nelson, D.R.; et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann. Hepatol. 2019, 18, 304–309. [Google Scholar] [CrossRef]
- Omboni, S.; Padwal, R.S.; Alessa, T.; Benczúr, B.; Green, B.B.; Hubbard, I.; Kario, K.; Khan, N.A.; Konradi, A.; Logan, A.G.; et al. The worldwide impact of telemedicine during COVID-19: Current evidence and recommendations for the future. Connect Health 2022, 1, 7–35. [Google Scholar] [CrossRef]
- Solari-Twadell, P.A.; Flinter, M.; Rambur, B.; Renda, S.; Witwer, S.; Vanhook, P.; Poghosyan, L. The impact of the COVID-19 pandemic on the future of telehealth in primary care. Nurs. Outlook 2022, 70, 315–322. [Google Scholar] [CrossRef]
- Reicher, S.; Sela, T.; Toren, O. Using Telemedicine During the COVID-19 Pandemic: Attitudes of Adult Health Care Consumers in Israel. Front. Public Health 2021, 9, 653553. [Google Scholar] [CrossRef] [PubMed]
- Talal, A.H.; Sofikitou, E.M.; Wang, K.; Dickerson, S.; Jaanimägi, U.; Markatou, M. High Satisfaction with Patient-Centered Telemedicine for Hepatitis C Virus Delivered to Substance Users: A Mixed-Methods Study. Telemed. e-Health 2023, 29, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.; Tam, L.; Xu, A.; Osborn, K.; Sheffrin, M.; Gould, C.; Schillinger, E.; Martin, M.; Mesias, M. Barriers to Telemedicine Video Visits for Older Adults in Independent Living Facilities: Mixed Methods Cross-sectional Needs Assessment. JMIR Aging 2022, 5, e34326. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Cobo, C.; Mateo, M.; Blasco, A.J.; Cabezas, J.; Llerena, S.; Fortea, J.I.; Lázaro, P.; Crespo, J. Telemedicine efficiently improves access to hepatitis C management to achieve HCV elimination in the penitentiary setting. Int. J. Drug Policy 2021, 88, 103031. [Google Scholar] [CrossRef]
- Gajarawala, S.N.; Pelkowski, J.N. Telehealth Benefits and Barriers. J. Nurse Pract. 2021, 17, 218–221. [Google Scholar] [CrossRef]
- Bobb, R.; Malayala, S.V.; Ajayi, E.; Wimbush, A.; Bobb, A. Hepatitis C Treatment in Persons Who Inject Drugs in a Medication Assisted Treatment Program: A Retrospective Review of an Integrated Model. J. Prim. Care Community Health 2023, 14, 21501319231164884. [Google Scholar] [CrossRef]
- Nisingizwe, M.P.; Makuza, J.D.; Janjua, N.Z.; Bansback, N.; Hedt-Gauthier, B.; Serumondo, J.; Remera, E.; Law, M.R. The Cascade of Care for Hepatitis C Treatment in Rwanda: A Retrospective Cohort Study of the 2017–2019 Mass Screening and Treatment Campaign. Viruses 2023, 15, 661. [Google Scholar] [CrossRef] [PubMed]
- Mendlowitz, A.B.; Bremner, K.E.; Krahn, M.; Walker, J.D.; Wong, W.W.L.; Sander, B.; Jones, L.; Isaranuwatchai, W.; Feld, J.J. Characterizing the cascade of care for hepatitis C virus infection among Status First Nations peoples in Ontario: A retrospective cohort study. CMAJ 2023, 195, E499–E512. [Google Scholar] [CrossRef]
- Yoo, S.H.; Kim, M.; Kim, S.; Lee, J.I.; Lee, K.S.; Lee, H.W.; Lim, J.H. The care cascade for hepatitis C virus and prognosis of chronic hepatitis C patients treated with antiviral agents in a tertiary hospital. BMC Gastroenterol. 2023, 23, 116. [Google Scholar] [CrossRef] [PubMed]
- Opstaele, L.; Bielen, R.; Bourgeois, S.; Moreno, C.; Nevens, F.; Robaeys, G.; Robaeys, G.; van Vlierberghe, H. Who to screen for hepatitis C? A cost-effectiveness study in Belgium of comprehensive hepatitis C screening in four target groups. Acta Gastro Enterol. Belgica 2019, 82, 379–387. [Google Scholar]
- Krauth, C.; Rossol, S.; Ortsäter, G.; Kautz, A.; Krüger, K.; Herder, B.; Stahmeyer, J.T. Elimination of hepatitis C virus in Germany: Modelling the cost-effectiveness of HCV screening strategies. BMC Infect. Dis. 2019, 19, 1019. [Google Scholar] [CrossRef] [PubMed]
- Gountas, I.; Sypsa, V.; Papatheodoridis, G.; Souliotis, K.; Athanasakis, K.; Razavi, H.; Hatzakis, A. Economic evaluation of the hepatitis C elimination strategy in Greece in the era of affordable direct-acting antivirals. World J. Gastroenterol. 2019, 25, 1327–1340. [Google Scholar] [CrossRef]
- Lim, A.G.; Scott, N.; Walker, J.G.; Hamid, S.; Hellard, M.; Vickerman, P. Health and economic benefits of achieving hepatitis C virus elimination in Pakistan: A modelling study and economic analysis. PLoS Med. 2021, 18, e1003818. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.; Kuschel, C.; Pedrana, A.; Schroeder, S.; Howell, J.; Thompson, A.; Wilson, D.P.; Hellard, M. A model of the economic benefits of global hepatitis C elimination: An investment case. Lancet Gastroenterol. Hepatol. 2020, 5, 940–947. [Google Scholar] [CrossRef]
- Ministry of Health and Population; El-Zanaty and Associates. Egypt Health Issues Survey 2015; Ministry of Health and Population: Cairo, Egypt, 2015; Available online: http://dhsprogram.com/pubs/pdf/FR313/FR313.pdf (accessed on 19 April 2023).
- Omran, D.; Alboraie, M.; Zayed, R.A.; Wifi, M.N.; Naguib, M.; Eltabbakh, M.; Abdellah, M.; Sherief, A.F.; Maklad, S.; Eldemellawy, H.H.; et al. Towards hepatitis C virus elimination: Egyptian experience, achievements and limitations. World J. Gastroenterol. 2018, 24, 4330–4340. [Google Scholar] [CrossRef]
- El-Akel, W.; El-Sayed, M.H.; El Kassas, M.; El-Serafy, M.; Khairy, M.; Elsaeed, K.; Kabil, K.; Hassany, M.; Shawky, A.; Yosry, A.; et al. National treatment programme of hepatitis C in Egypt: Hepatitis C virus model of care. J. Viral Hepatol. 2017, 24, 262–267. [Google Scholar] [CrossRef]
- Waked, I.; Esmat, G.; Elsharkawy, A.; El-Serafy, M.; Abdel-Razek, W.; Ghalab, R.; Elshishiney, G.; Salah, A.; Abdel Megid, S.; Kabil, K.; et al. Screening and Treatment Program to Eliminate Hepatitis C in Egypt. N. Engl. J. Med. 2020, 382, 1166–1174. [Google Scholar] [CrossRef]
- Tatar, M.; Keeshin, S.W.; Mailliard, M.; Wilson, F.A. Cost-effectiveness of Universal and Targeted Hepatitis C Virus Screening in the United States. JAMA Netw. Open 2020, 3, e2015756. [Google Scholar] [CrossRef]
- Chhatwal, J.; Kanwal, F.; Roberts, M.S.; Dunn, M.A. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann. Intern. Med. 2015, 162, 397–406. [Google Scholar] [CrossRef]
- Scott, D.N.; Palmer, M.A.; Tidhar, M.T.; Stoove, P.M.; Sacks-Davis, D.R.S.; Doyle, A.J.S.; Pedrana, D.A.J.; Thompson, P.A.; Wilson, P.D.P.; Hellard, P.M. Assessment of the cost-effectiveness of Australia’s risk-sharing agreement for direct-acting antiviral treatments for hepatitis C: A modelling study. Lancet Reg. Health West. Pac. 2021, 18, 100316. [Google Scholar] [CrossRef] [PubMed]
- Jack, K.; Thomson, B.; Patterson, A. PTU-102 “you can’t treat ‘em ‘till you get the security right”: Prison officers views about hepatitis C testing and treatment in prisons. Gut 2015, 64, A106. [Google Scholar] [CrossRef]
- Thornton, J. Hepatitis Fund aims to accelerate viral hepatitis elimination. Lancet 2023, 401, 1414–1415. [Google Scholar] [CrossRef] [PubMed]
| System-Level | Provider Level | Patients Level | |
|---|---|---|---|
| Screening | Screening high-risk patients (PWID, prisoners, and others) | Proactive action: patient invitation for screening | Stopping substance abuse |
| Modeling high-risk patient screening according to medical files | Being aware with cultural and linguistic barriers | Keeping appointments | |
| Universal screening in countries with high HCV prevalence | Adherence to screening | ||
| Linkage to care | Improving HCV antibody and viral load testing accessibility and availability | Preparing patients for treatment (laboratory test including antibody and HCV viral load) | Compliance with preparation before treatment, and laboratory testing |
| Availability of the point-of-care/self-testing according to the local needs | Case and resource management at the local level | Insurance coverage | |
| Availability of testing for non-invasive liver disease severity assessment (FIB-4, APRI, Fibrotest, FibroScan) | Using doctor-to-doctor virtual consultation if available | ||
| Decentralization of the patient’s investigation/ patient’s preparation for treatment | |||
| Treatment | Universal access to treatment | Be convinced treatment is possible, easy, and simple | Paying medical expenses |
| Shortening wait time for specialist consultation | Appropriate education for HCV treatment initiation, in regions where providers are authorized to do so | Adherence to treatment | |
| Using telemedicine throughout all phases | |||
| Decentralization of treatment approval and prescriptions | |||
| Adding prescribers (internist, family doctors, or pharmacists) according to the local needs | |||
| Clear healthcare policy/national program/protocols | |||
| Special consideration for people who inject drugs, refugees, and prisoners | |||
| Other elements | Education and training of the providers | Increasing knowledge, education Staff familiarity with the process of screening, investigation and treatment | Continuing follow-up |
| Increasing awareness among the population regarding the importance of the screening and treatment | Using telemedicine when possible | ||
| Other manpower Nurse navigator |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, G.; Ezra, L.; Abu-Freha, N. Hepatitis C Elimination: Opportunities and Challenges in 2023. Viruses 2023, 15, 1413. https://doi.org/10.3390/v15071413
Taha G, Ezra L, Abu-Freha N. Hepatitis C Elimination: Opportunities and Challenges in 2023. Viruses. 2023; 15(7):1413. https://doi.org/10.3390/v15071413
Chicago/Turabian StyleTaha, Gadeer, Levy Ezra, and Naim Abu-Freha. 2023. "Hepatitis C Elimination: Opportunities and Challenges in 2023" Viruses 15, no. 7: 1413. https://doi.org/10.3390/v15071413
APA StyleTaha, G., Ezra, L., & Abu-Freha, N. (2023). Hepatitis C Elimination: Opportunities and Challenges in 2023. Viruses, 15(7), 1413. https://doi.org/10.3390/v15071413






