Viral Loads in Skin Samples of Patients with Monkeypox Virus Infection: A Systematic Review and Meta-Analysis
Abstract
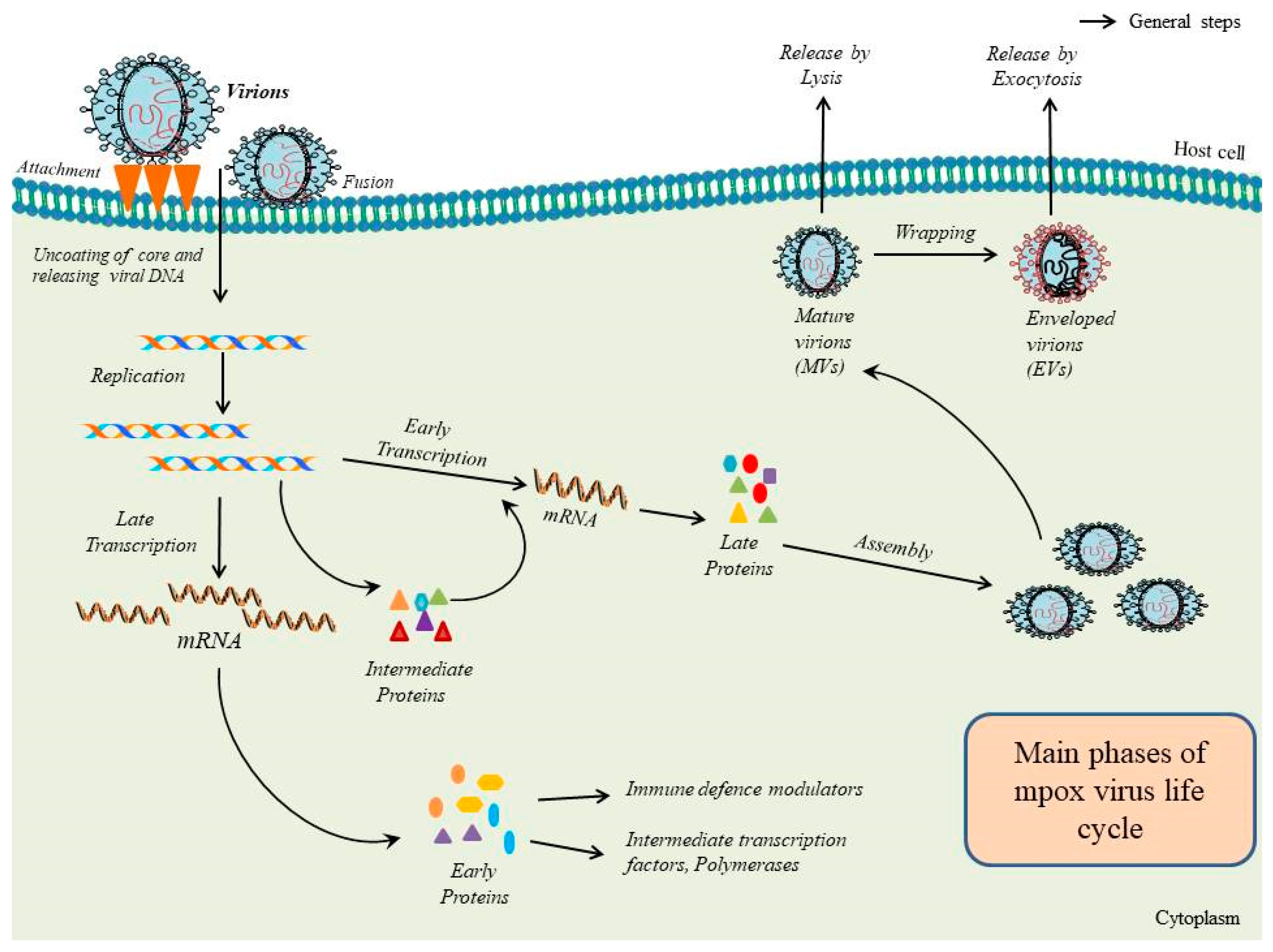
1. Introduction
2. Methods
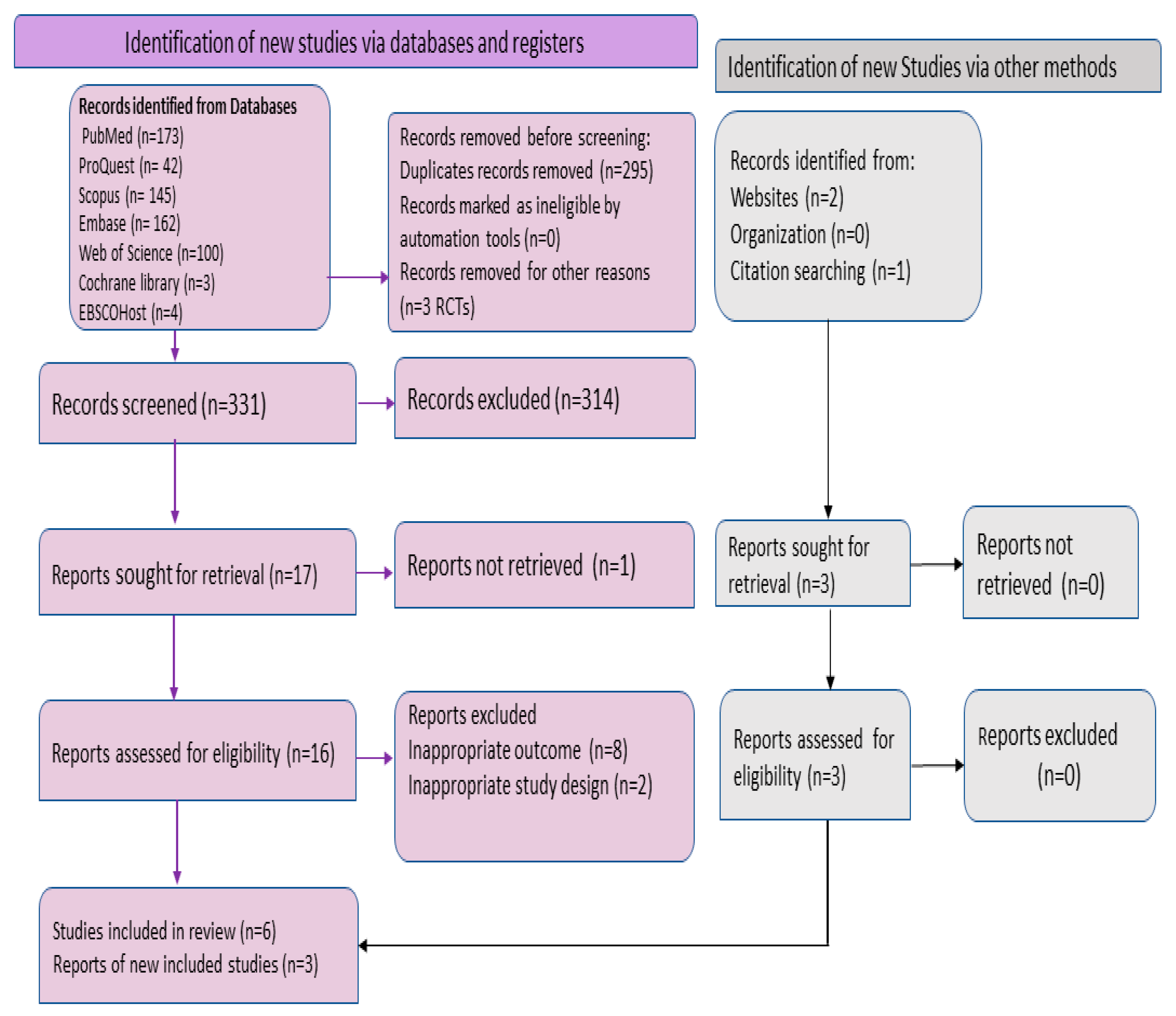
2.1. Search Strategy and Selection Criteria
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Management
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Selection Criteria and Baseline Characteristics of Included Studies
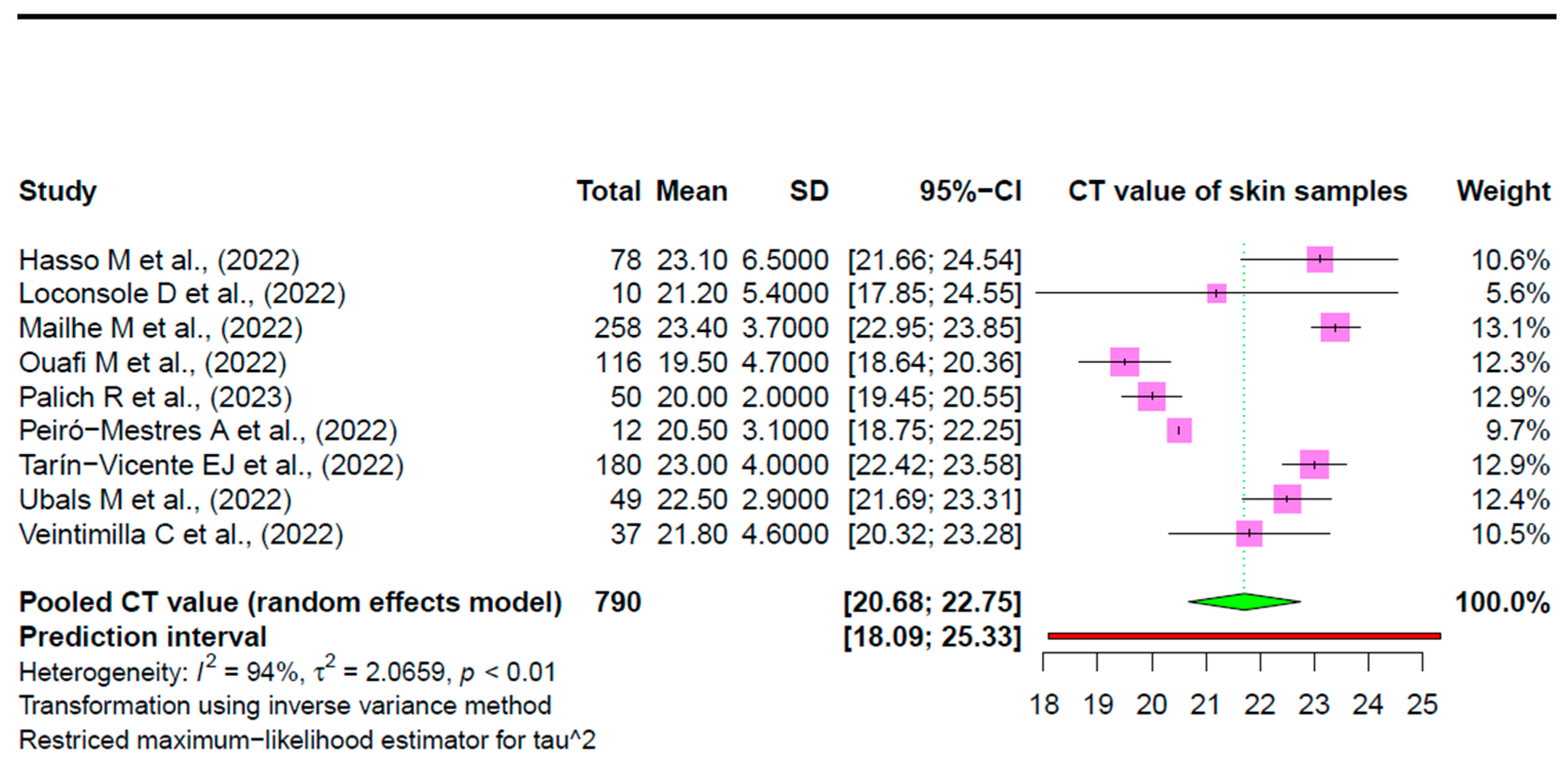
| Authors (YOP) | Study Design | Ct Mean (±SD) | Number of Mpox-Confirmed Cases from Whom Skin Samples Were Taken | Prevalence of Mpox Viral DNA in Skin Samples (%) | Age (Years) Median | Gender | Region |
|---|---|---|---|---|---|---|---|
| Hasso M et al., (2022) [41] | RC | 23.1 (6.5) | 78 | 43.60% | 38 * | All males | Ontario, Canada |
| Loconsole D et al., (2022) [39] | PO | 21.2 (5.4) | 10 | 100% | 36.7 | 8 males (6 MSM) and 2 females | Southern Italy |
| Mailhe M et al., (2022) [38] # | PO | 23.4 (3.7) | 258 | 98% | 35 | Majority of males, including MSM, except 1 female and 1 transgender female | France |
| Ouafi M et al., (2022) [35] # | COS | 19.5 (4.7) | 116 | 100% | 37 | All males (including mostly MSM), except 1 female | Northern France |
| Palich R et al., (2023) [17] # | CS | 20 (2) | 50 | 88% | 34 | All males (49 MSM and 1 MSW) | Paris, France |
| Peiró-Mestres A et al., (2022) [18] | PO | 20.5 (3.1) | 12 | 100% | 38.5 | All males (MSM) | Barcelona, Spain |
| Tarín-Vicente EJ et al., (2022) [40] | PO | 23 (4) | 180 | 99% | 37 | Majority males, including gay, bisexual, and MSM, except for a few heterosexual males or females | Madrid and Barcelona, Spain |
| Ubals M et al., (2022) [36] # | PO | 22.5 (2.9) | 49 | 100% | 33.5 | All males | Spain |
| Veintimilla C et al., (2022) [37] | PO | 21.8 (4.6) | 37 | 97% | 31 | All males (MSM) | Madrid, Spain |
3.2. Pooled Prevalence
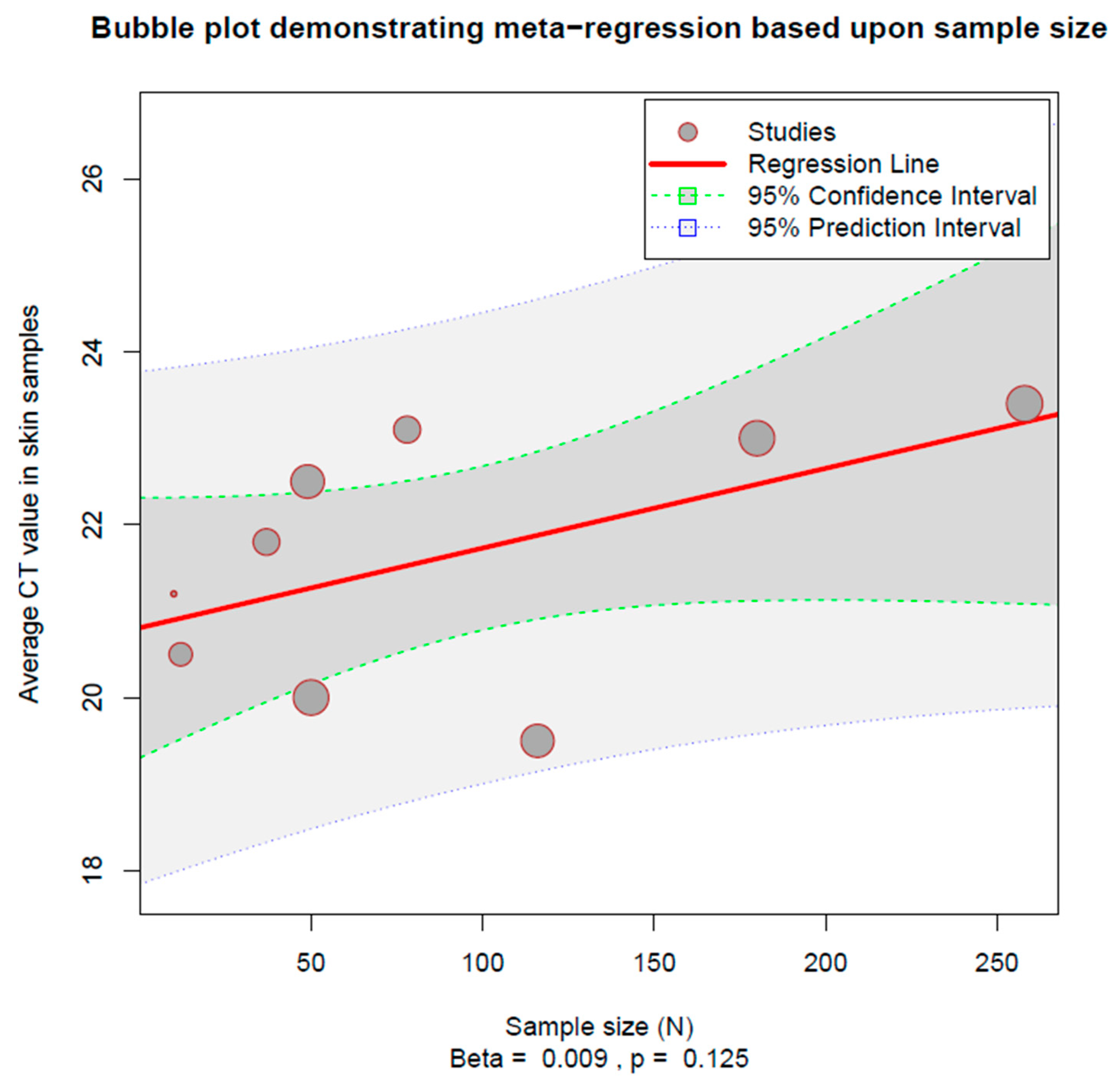
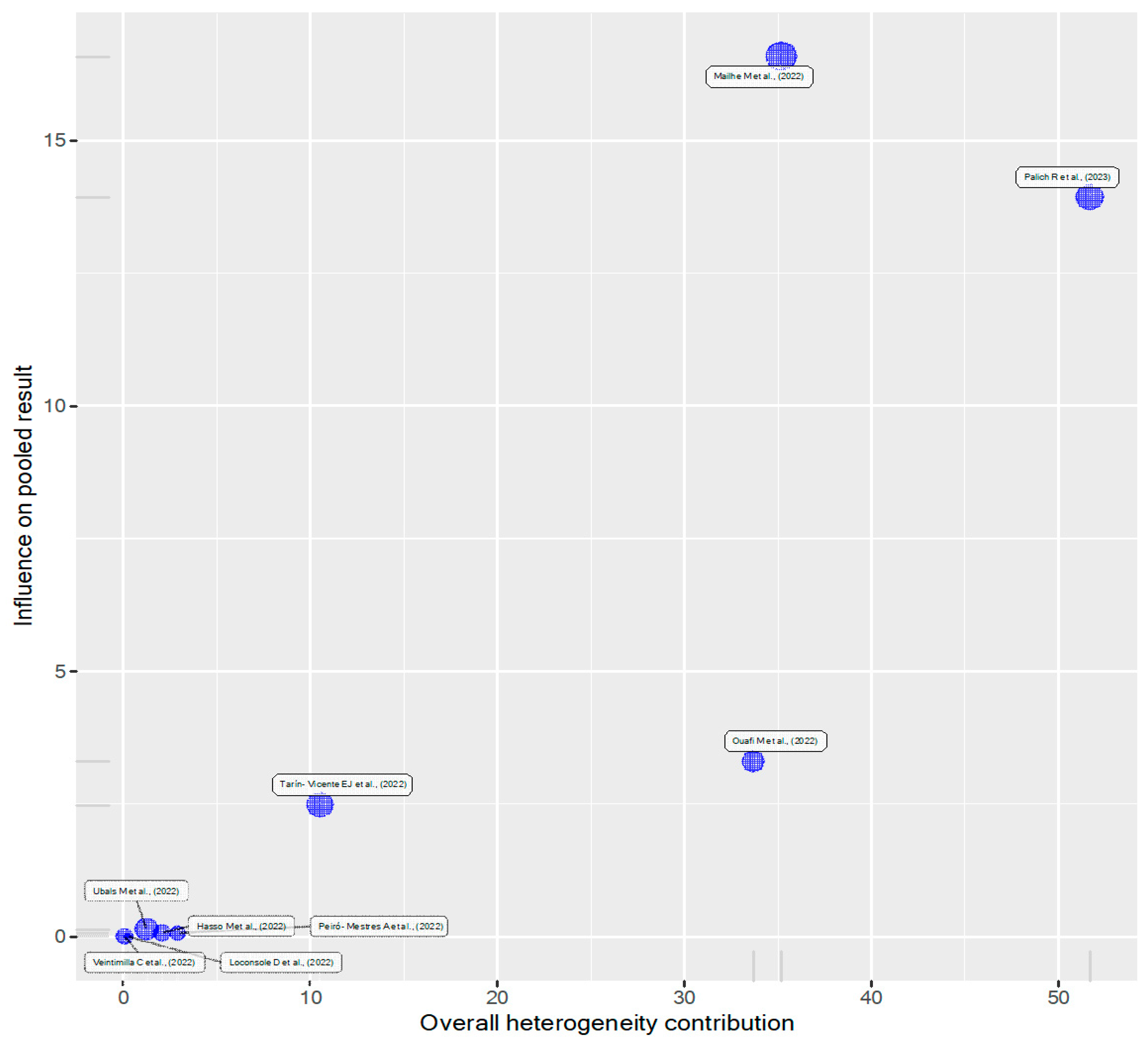
3.3. Heterogeneity Estimation and Exploration
3.4. Influence Assessment
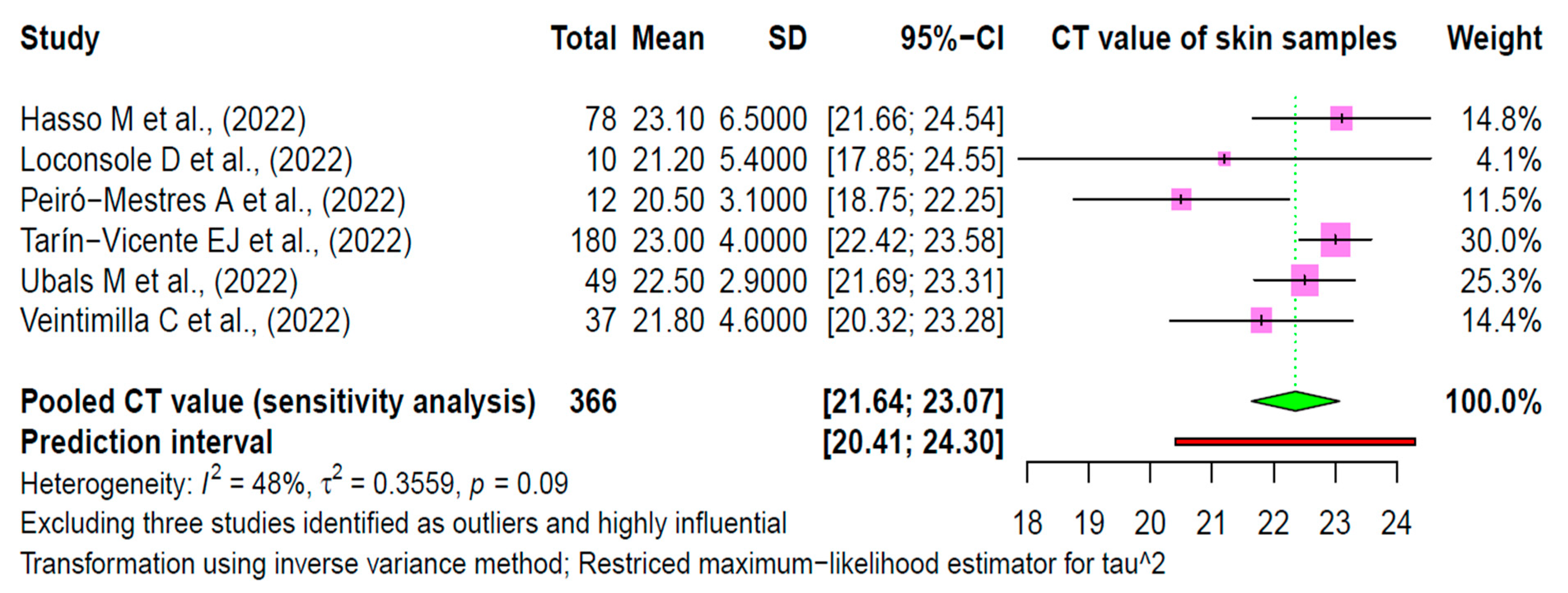
3.5. Sensitivity Analysis
3.6. Publication Bias and Small-Study Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- MacNeil, A.; Reynolds, M.G.; Braden, Z.; Carroll, D.S.; Bostik, V.; Karem, K.; Smith, S.K.; Davidson, W.; Li, Y.; Moundeli, A.; et al. Transmission of Atypical Varicella-Zoster Virus Infections Involving Palm and Sole Manifestations in an Area with Monkeypox Endemicity. Clin. Infect. Dis. 2009, 48, e6–e8. [Google Scholar] [CrossRef] [PubMed]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar] [PubMed]
- Rubins, K.H.; Hensley, L.E.; Relman, D.A.; Brown, P.O. Stunned Silence: Gene Expression Programs in Human Cells Infected with Monkeypox or Vaccinia Virus. PLoS ONE 2011, 6, e15615. [Google Scholar] [CrossRef] [PubMed]
- Shamim, M.A.; Padhi, B.K.; Satapathy, P.; Veeramachaneni, S.D.; Chatterjee, C.; Tripathy, S.; Akhtar, N.; Pradhan, A.; Dwivedi, P.; Mohanty, A.; et al. The use of antivirals in the treatment of human monkeypox outbreaks: A systematic review. Int. J. Infect. Dis. 2023, 127, 150–161. [Google Scholar] [CrossRef] [PubMed]
- CDC. 2022 Monkeypox Outbreak Global Map. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (accessed on 28 February 2023).
- World Health Organization (WHO). Available online: https://www.who.int/emergencies/emergency-events/item/2022-e000121 (accessed on 23 February 2023).
- Huang, Y.; Mu, L.; Wang, W. Monkeypox: Epidemiology, pathogenesis, treatment and prevention. Signal Transduct. Target Ther. 2022, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Karagoz, A.; Tombuloglu, H.; Alsaeed, M.; Tombuloglu, G.; AlRubaish, A.A.; Mahmoud, A.; Smajlović, S.; Ćordić, S.; Rabaan, A.A.; Alsuhaimi, E. Monkeypox (mpox) virus: Classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis. J. Infect. Public Health 2023, 16, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical Characteristics of Human Monkeypox, and Risk Factors for Severe Disease. Clin. Infect. Dis. 2005, 41, 1742–1751. [Google Scholar] [CrossRef]
- Gandhi, P.A.; Patro, S.K.; Sandeep, M.; Satapathy, P.; Shamim, M.A.; Kumar, V.; Aggarwal, A.K.; Padhi, B.K.; Sah, R. Oral manifestation of the monkeypox virus: A systematic review and meta-analysis. eClinicalMedicine 2023, 56, 101817. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Satapathy, P.; Mohanty, P.; Manna, S.; Shamim, M.A.; Rao, P.P.; Aggarwal, A.K.; Khubchandani, J.; Mohanty, A.; Nowrouzi-Kia, B.; Chattu, V.K.; et al. Potentially Asymptomatic Infection of Monkeypox Virus: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 2083. [Google Scholar] [CrossRef]
- Choudhary, O.P.; Priyanka Chopra, H.; Shafaati, M.; Dhawan, M.; Metwally, A.A.; Saied, A.A.; Rabaan, A.A.; Alhumaid, S.; Al Mutair, A. Reverse zoonosis and its relevance to the monkeypox outbreak 2022. New Microbes New Infect. 2022, 49–50, 101049. [Google Scholar] [CrossRef] [PubMed]
- Upadhayay, S.; Arthur, R.; Soni, D.; Yadav, P.; Navik, U.; Singh, R.; Gurjeet Singh, T.; Kumar, P. Monkeypox infection: The past, present, and future. Int. Immunopharmacol. 2022, 113, 109382. [Google Scholar] [CrossRef]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.A.; et al. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Català, A.; Clavo-Escribano, P.; Riera-Monroig, J.; Martín-Ezquerra, G.; Fernandez-Gonzalez, P.; Revelles-Peñas, L.; Simon-Gozalbo, A.; Rodríguez-Cuadrado, F.J.; Castells, V.G.; de la Torre Gomar, F.J.; et al. Monkeypox outbreak in Spain: Clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Br. J. Dermatol. 2022, 187, 765–772. [Google Scholar] [CrossRef]
- Palich, R.; Burrel, S.; Monsel, G.; Nouchi, A.; Bleibtreu, A.; Seang, S.; Bérot, V.; Brin, C.; Gavaud, A.; Wakim, Y.; et al. Viral loads in clinical samples of men with monkeypox virus infection: A French case series. Lancet Infect. Dis. 2023, 23, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Peiró-Mestres, A.; Fuertes, I.; Camprubí-Ferrer, D.; Marcos, M.Á.; Vilella, A.; Navarro, M.; Rodriguez-Elena, L.; Riera, J.; Català, A.; Martínez, M.J.; et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Eurosurveillance 2022, 27, 2200503. [Google Scholar] [CrossRef]
- Hennessee, I.; Shelus, V.; McArdle, C.E.; Wolf, M.; Schatzman, S.; Carpenter, A.; Minhaj, F.S.; Petras, J.K.; Cash-Goldwasser, S.; Maloney, M.; et al. Epidemiologic and Clinical Features of Children and Adolescents Aged <18 Years with Monkeypox—United States, May 17–September 24, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1407–1411. [Google Scholar] [CrossRef]
- Stroup, D.F. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA 2000, 283, 2008. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- WebPlotDigitizer. Web Based Tool to Extract Numerical Data from Plots, Images and Maps. Available online: https://automeris.io/WebPlotDigitizer/ (accessed on 28 February 2023.).
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Luo, D.; Wan, X.; Liu, Y.; Liu, J.; Bian, Z.; Tong, T. Detecting the skewness of data from the sample size and the five-number summary. arXiv 2020, arXiv:2010.05749. [Google Scholar]
- National Institute of Health (NIH). Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 22 March 2023).
- Viechtbauer, W. Bias and Efficiency of Meta-Analytic Variance Estimators in the Random-Effects Model. J. Educ. Behav. Stat. 2005, 30, 261–293. [Google Scholar] [CrossRef]
- Shamim, M.A.; Dwivedi, P.; Padhi, B.K. Beyond the funnel plot: The advantages of Doi plots and prediction intervals in meta-analyses. Asian J. Psychiatry 2023, 84, 103550. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 22 March 2023).
- Gaspari, V.; Rossini, G.; Robuffo, S.; Rapparini, L.; Scagliarini, A.; Mistral De Pascali, A.; Piraccini, B.M.; Lazzarotto, T. Monkeypox Outbreak 2022: Clinical and Virological Features of 30 Patients at the Sexually Transmitted Diseases Centre of Sant’ Orsola Hospital, Bologna, Northeastern Italy. J. Clin. Microbiol. 2023, 61, e0136522. [Google Scholar] [CrossRef]
- Silva, M.S.T.; Coutinho, C.; Torres, T.S.; Peixoto, E.; Ismério, R.; Lessa, F.; Nunes, E.P.; Hoagland, B.; Echeverria Guevara, A.D.; Bastos, M.O.; et al. Ambulatory and hospitalized patients with suspected and confirmed mpox: An observational cohort study from Brazil. Lancet Reg. Health Am. 2023, 17, 100406. [Google Scholar] [CrossRef]
- García-Piqueras, P.; Bergón-Sendín, M.; Córdoba-García-Rayo, M.; Vírseda-González, D.; Medrano-Martínez, N.; Jiménez-Briones, L.; Lacasta-Plasín, C.; Hernández de la Torre-Ruiz, E.; Balaguer-Franch, I.; Pulido-Pérez, A.; et al. Human monkeypox virus in a tertiary hospital in Madrid, Spain: An observational study of the clinical and epidemiological characteristics of 53 cases. Exp. Dermatol. 2022, 32, 198–202. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Palich, R.; Ghosn, J.; Walmsley, S.; Moschese, D.; Cortes, C.P.; Galliez, R.M.; Garlin, A.B.; Nozza, S.; Mitja, O.; et al. Human monkeypox virus infection in women and non-binary individuals during the 2022 outbreaks: A global case series. Lancet 2022, 400, 1953–1965. [Google Scholar] [CrossRef]
- Nörz, D.; Brehm, T.T.; Tang, H.T.; Grewe, I.; Hermanussen, L.; Matthews, H.; Pestel, J.; Degen, O.; Günther, T.; Grundhoff, A.; et al. Clinical characteristics and comparison of longitudinal qPCR results from different specimen types in a cohort of ambulatory and hospitalized patients infected with monkeypox virus. J. Clin. Virol. 2022, 155, 105254. [Google Scholar] [CrossRef]
- Ouafi, M.; Regueme, A.; Alcaraz, I.; Riviere, P.; Bazus, H.; Salmon-Rousseau, A.; Cappeliez, B.; Cartier, N.; Guigon, A.; Lazrek, M.; et al. Oropharyngeal samples versus lesion specimens at diagnosis in patients infected with monkeypox virus in Northern France. J. Med. Virol. 2023, 95, e28276. [Google Scholar] [CrossRef]
- Ubals, M.; Tarín-Vicente, E.J.; Oller, X.; Mendoza, A.; Alemany, A.; Hernández-Rodríguez, Á.; Casañ, C.; Rivero, Á.; Coll, P.; Cabrera, J.M.; et al. Evaluating the Accuracy of Self-Collected Swabs for the Diagnosis of Monkeypox. Clin. Infect. Dis. 2022, 76, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Veintimilla, C.; Catalán, P.; Alonso, R.; García de Viedma, D.; Pérez-Lago, L.; Palomo, M.; Cobos, A.; Aldamiz-Echevarria, T.; Muñoz, P. The relevance of multiple clinical specimens in the diagnosis of monkeypox virus, Spain, June 2022. Eurosurveillance 2022, 27, 2200598. [Google Scholar] [CrossRef] [PubMed]
- Mailhe, M.; Beaumont, A.L.; Thy, M.; Le Pluart, D.; Perrineau, S.; Houhou-Fidouh, N.; Deconinck, L.; Bertin, C.; Ferré, V.M.; Cortier, M.; et al. Clinical characteristics of ambulatory and hospitalized patients with monkeypox virus infection: An observational cohort study. Clin. Microbiol. Infect. 2022, 29, 233–239. [Google Scholar] [CrossRef]
- Loconsole, D.; Sallustio, A.; Centrone, F.; Casulli, D.; Accogli, M.; Saracino, A.; Foti, C.; Grandolfo, M.; Buccoliero, G.B.; Vitale, V.; et al. Monkeypox Virus Infections in Southern Italy: Is There a Risk for Community Spread? Int. J. Environ. Res. Public Health 2022, 19, 11719. [Google Scholar] [CrossRef] [PubMed]
- Tarín-Vicente, E.J.; Alemany, A.; Agud-Dios, M.; Ubals, M.; Suñer, C.; Antón, A.; Arando, M.; Arroyo-Andrés, J.; Calderón-Lozano, L.; Casañ, C.; et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: A prospective observational cohort study. Lancet 2022, 400, 661–669. [Google Scholar] [CrossRef]
- Hasso, M.; Perusini, S.; Eshaghi, A.; Tang, E.; Olsha, R.; Zhang, H.; Lau, E.; Sullivan, A.; Cronin, K.; Lee, S.; et al. Monkeypox Virus Detection in Different Clinical Specimen Types. Emerg. Infect. Dis. 2022, 28, 2513–2515. [Google Scholar] [CrossRef]
- Paran, N.; Yahalom-Ronen, Y.; Shifman, O.; Lazar, S.; Ben-Ami, R.; Yakubovsky, M.; Levy, I.; Wieder-Feinsod, A.; Amit, S.; Katzir, M.; et al. Monkeypox DNA levels correlate with virus infectivity in clinical samples, Israel, 2022. Eurosurveillance 2022, 27, 2200636. [Google Scholar] [CrossRef]
- Lim, C.K.; McKenzie, C.; Deerain, J.; Chow, E.P.F.; Towns, J.; Chen, M.Y.; Fairley, C.K.; Tran, T.; Williamson, D.A. Correlation between monkeypox viral load and infectious virus in clinical specimens. J. Clin. Virol. 2023, 161, 105421. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, B.; Muñoz-Gómez, A.; Sanchiz, A.; Orviz, E.; Valls-Carbo, A.; Sagastagoitia, I.; Ayerdi, O.; Martín, R.; Puerta, T.; Vera, M.; et al. Monitoring monkeypox virus in saliva and air samples in Spain: A cross-sectional study. Lancet Microbe 2023, 4, e21–e28. [Google Scholar] [CrossRef]
- Lapa, D.; Carletti, F.; Mazzotta, V.; Matusali, G.; Pinnetti, C.; Meschi, S.; Gagliardini, R.; Colavita, F.; Mondi, A.; Minosse, C.; et al. Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. Lancet Infect. Dis. 2022, 22, 1267–1269. [Google Scholar] [CrossRef]
- Martins-Filho, P.R.; Tanajura, D.M.; Alves dos Santos, C. Polymerase chain reaction positivity and cycle threshold values in biological samples from patients with monkeypox: A meta-analysis. Travel Med. Infect. Dis. 2022, 50, 102448. [Google Scholar] [CrossRef] [PubMed]
- Noe, S.; Zange, S.; Seilmaier, M.; Antwerpen, M.H.; Fenzl, T.; Schneider, J.; Spinner, C.D.; Bugert, J.J.; Wendtner, C.M.; Wölfel, R. Clinical and virological features of first human monkeypox cases in Germany. Infection 2023, 51, 265–270. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Available online: https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1 (accessed on 28 February 2022).
- Jiang, Z.; Sun, J.; Zhang, L.; Yan, S.; Li, D.; Zhang, C.; Lai, A.; Su, S. Laboratory diagnostics for monkeypox: An overview of sensitivities from various published tests. Travel Med. Infect. Dis. 2022, 49, 102425. [Google Scholar] [CrossRef] [PubMed]
| Result for Subgroup Differences (Common Effect Model) | ||||||
|---|---|---|---|---|---|---|
| Country | k | Mean | 95% CI | Q | I2 | |
| Canada [41] | 1 | 23.1000 | [21.6575; 24.5425] | 0.00 | -- | |
| Italy [39] | 1 | 21.2000 | [17.8531; 24.5469] | 0.00 | -- | |
| France [17,35,38] | 3 | 21.6791 | [21.3551; 22.0031] | 115.99 | 98.3% | |
| Spain [18,36,37,40] | 4 | 22.5948 | [22.1574; 23.0323] | 8.48 | 64.6% | |
| Test for Subgroup Differences (Common Effect Model) | ||||||
| Between groups | Q.d.f | p value | ||||
| 13.26 | 3 | 0.0041 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, I.; Satapathy, P.; Goyal, A.; Shamim, M.A.; Pal, A.; Squitti, R.; Goswami, K.; Pradhan, K.B.; Rustagi, S.; Hermis, A.H.; et al. Viral Loads in Skin Samples of Patients with Monkeypox Virus Infection: A Systematic Review and Meta-Analysis. Viruses 2023, 15, 1386. https://doi.org/10.3390/v15061386
Rani I, Satapathy P, Goyal A, Shamim MA, Pal A, Squitti R, Goswami K, Pradhan KB, Rustagi S, Hermis AH, et al. Viral Loads in Skin Samples of Patients with Monkeypox Virus Infection: A Systematic Review and Meta-Analysis. Viruses. 2023; 15(6):1386. https://doi.org/10.3390/v15061386
Chicago/Turabian StyleRani, Isha, Prakasini Satapathy, Anmol Goyal, Muhammad Aaqib Shamim, Amit Pal, Rosanna Squitti, Kalyan Goswami, Keerti Bhusan Pradhan, Sarvesh Rustagi, Alaa Hamza Hermis, and et al. 2023. "Viral Loads in Skin Samples of Patients with Monkeypox Virus Infection: A Systematic Review and Meta-Analysis" Viruses 15, no. 6: 1386. https://doi.org/10.3390/v15061386
APA StyleRani, I., Satapathy, P., Goyal, A., Shamim, M. A., Pal, A., Squitti, R., Goswami, K., Pradhan, K. B., Rustagi, S., Hermis, A. H., Barboza, J. J., Rodriguez-Morales, A. J., Sah, R., & Padhi, B. K. (2023). Viral Loads in Skin Samples of Patients with Monkeypox Virus Infection: A Systematic Review and Meta-Analysis. Viruses, 15(6), 1386. https://doi.org/10.3390/v15061386










