A Narrative Review of Alternative Symptomatic Treatments for Herpes Simplex Virus
Abstract
1. Introduction
2. Methods
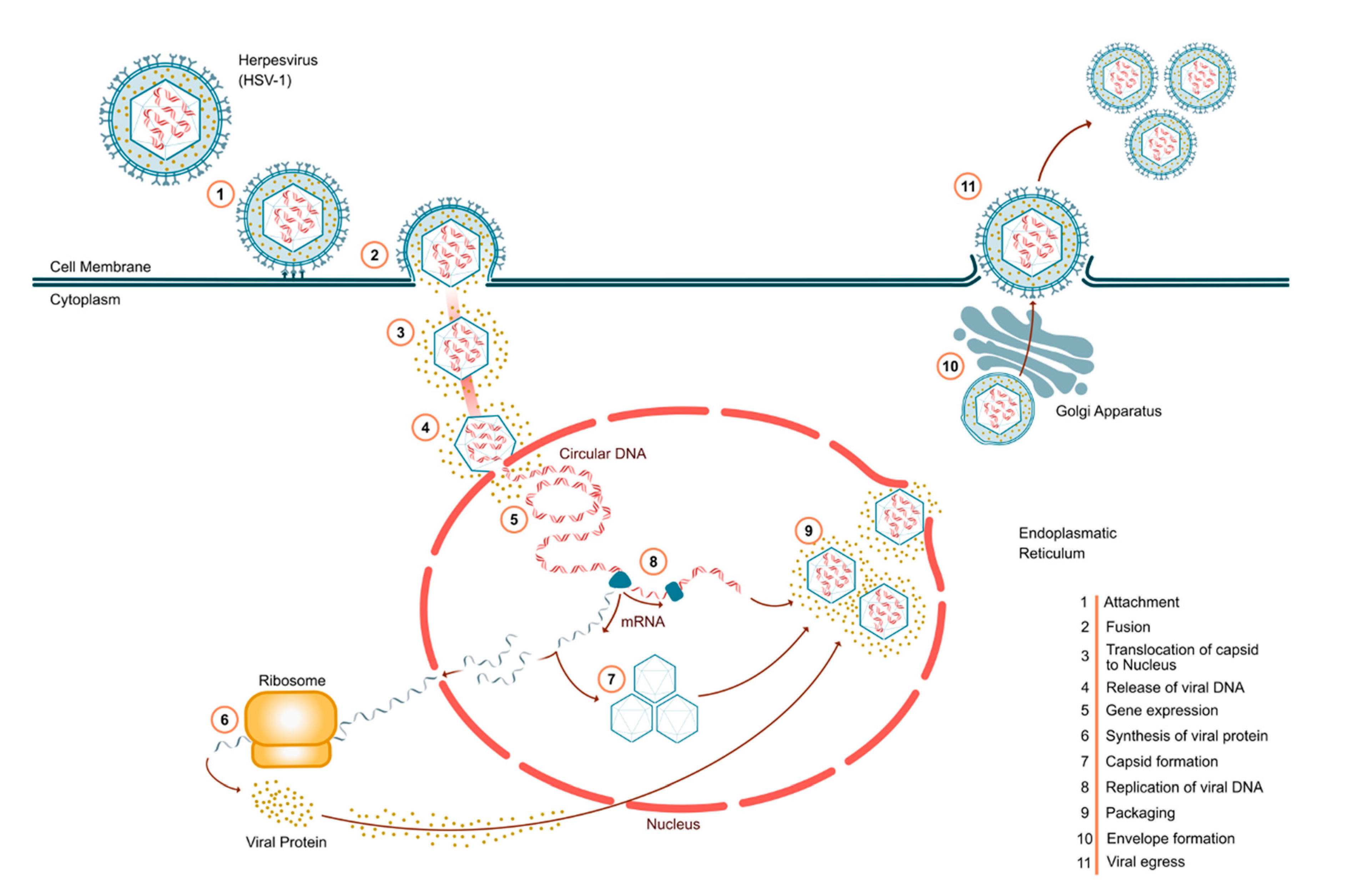
3. HSV Pathogenesis
3.1. Established HSV Treatments: Antivirals
3.2. Resistance
4. Supplements and Natural Products
4.1. Lysine/Arginine
| Natural Substance | Hypothesized Mechanism of Action | Clinical Studies? | Results | Adverse Events | References |
|---|---|---|---|---|---|
| Arginine | Amino acid, enhances viral protein synthesis | Multiple studies | Exacerbated disease | Increased viral replication | [25,28,29] |
| Cannabis | Possible membrane perturbation, may enhance viral DNA synthesis, immunosuppressive | Multiple observational studies | Likely exacerbated disease, enhanced HSV replication, increased viral shedding | Anxiety, potential psychoses, long-term cognitive decline | [30,31,32] |
| Lemon balm | Prevents virus ingress | Phase II and preclinical | Reduced symptom scores in patients; in vitro, shows highly reduced cell death and infection | No known adverse effects | [33,34] |
| Lysine | Amino acid, attenuates viral protein synthesis | Multiple | Decreased outbreak severity and duration; prophylactic | Safe up to 6 g per day | [35] |
| Refined carbohydrates | Multiple | Phase I | Exacerbated disease | Decreased innate immunity | [36,37] |
| Vitamin C | Antioxidant, boosts immune response | Limited | 57% reduced time to remission; reduced HSV keratitis recurrence by 53.2% | Minor effects at very high doses | [38,39] |
| Vitamin D | Largely unknown, but may inhibit Toll-like receptor detrimental inflammation | Observational | Reduced viral titers in a HeLa cells HSV- infection model, reduced Behçet’s disease in an HSV-1 mouse model | Hypercalcemia or GI effects with high doses | [40,41] |
| Vitamin E | Immunomodulation | Preclinical | Alleviated mouse herpes simplex encephalitis | Bleeding at excessively high doses | [42] |
| Zinc (oral) | Antioxidant, direct inhibition of HSV DNA polymerase | Multiple | Reduced number and duration of lesions | Excessive doses (100–300 mg) may elicit copper deficiency | [43] |
| Zinc (topical) | Antioxidant, direct inhibition of HSV DNA polymerase | Multiple | Shortened lesion outbreaks, decreased complications | No observed adverse events | [44,45] |
4.2. Propolis
4.3. Lemon Balm
4.4. Vitamin E
4.5. Zinc
4.6. Vitamin D
4.7. Refined Carbohydrates
4.8. Cannabis
4.9. Other Addictive/Recreational Substances
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef]
- Koelle, D.M. Immunobiology and host response. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Whitley, R.; Baines, J. Clinical management of herpes simplex virus infections: Past, present, and future. F1000Research 2018, 7, 1726. [Google Scholar] [CrossRef] [PubMed]
- Cole, S. Herpes Simplex Virus: Epidemiology, Diagnosis, and Treatment. Nurs. Clin. N. Am. 2020, 55, 337–345. [Google Scholar] [CrossRef]
- Schiffer, J.T.; Corey, L. New concepts in understanding genital herpes. Curr. Infect. Dis. Rep. 2009, 11, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Acosta, E.P.; Fletcher, C.V. Valacyclovir. Ann. Pharm. 1997, 31, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Klysik, K.; Pietraszek, A.; Karewicz, A.; Nowakowska, M. Acyclovir in the Treatment of Herpes Viruses—A Review. Curr. Med. Chem. 2020, 27, 4118–4137. [Google Scholar] [CrossRef]
- Li, F.; Maag, H.; Alfredson, T. Prodrugs of nucleoside analogues for improved oral absorption and tissue targeting. J. Pharm. Sci. 2008, 97, 1109–1134. [Google Scholar] [CrossRef]
- Cowley, N.J.; Owen, A.; Shiels, S.C.; Millar, J.; Woolley, R.; Ives, N.; Osman, H.; Moss, P.; Bion, J.F. Safety and Efficacy of Antiviral Therapy for Prevention of Cytomegalovirus Reactivation in Immunocompetent Critically Ill Patients: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 774–783. [Google Scholar] [CrossRef]
- Darby, G.; Field, H.J.; Salisbury, S.A. Altered substrate specificity of herpes simplex virus thymidine kinase confers acyclovir-resistance. Nature 1981, 289, 81–83. [Google Scholar] [CrossRef]
- Strick, L.B.; Wald, A.; Celum, C. Management of herpes simplex virus type 2 infection in HIV type 1-infected persons. Clin. Infect. Dis. 2006, 43, 347–356. [Google Scholar] [CrossRef]
- Mertz, G.J.; Jones, C.C.; Mills, J.; Fife, K.H.; Lemon, S.M.; Stapleton, J.T.; Hill, E.L.; Davis, L.G. Long-term acyclovir suppression of frequently recurring genital herpes simplex virus infection. A multicenter double-blind trial. JAMA 1988, 260, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Pottage, J.C., Jr.; Kessler, H.A. Herpes simplex virus resistance to acyclovir: Clinical relevance. Infect. Agents Dis. 1995, 4, 115–124. [Google Scholar] [PubMed]
- Schalkwijk, H.H.; Snoeck, R.; Andrei, G. Acyclovir resistance in herpes simplex viruses: Prevalence and therapeutic alternatives. Biochem. Pharm. 2022, 206, 115322. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Resistance of herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef]
- Bacon, T.H.; Levin, M.J.; Leary, J.J.; Sarisky, R.T.; Sutton, D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 2003, 16, 114–128. [Google Scholar] [CrossRef]
- Duan, R.; de Vries, R.D.; Osterhaus, A.D.; Remeijer, L.; Verjans, G.M. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J. Infect. Dis. 2008, 198, 659–663. [Google Scholar] [CrossRef]
- Upadhyayula, S.; Michaels, M.G. Ganciclovir, Foscarnet, and Cidofovir: Antiviral Drugs Not Just for Cytomegalovirus. J. Pediatr. Infect. Dis. Soc. 2013, 2, 286–290. [Google Scholar] [CrossRef]
- Erard, V.; Wald, A.; Corey, L.; Leisenring, W.M.; Boeckh, M. Use of long-term suppressive acyclovir after hematopoietic stem-cell transplantation: Impact on herpes simplex virus (HSV) disease and drug-resistant HSV disease. J. Infect. Dis. 2007, 196, 266–270. [Google Scholar] [CrossRef]
- O'Brien, J.J.; Campoli-Richards, D.M. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1989, 37, 233–309. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.H.; Luo, Z.; Liu, L.F.; Yan, C.; Yan, C.Y.; Chen, G.D.; Gao, H.; Duan, W.J.; Kurihara, H.; et al. Traditional Chinese Medicine as a Potential Source for HSV-1 Therapy by Acting on Virus or the Susceptibility of Host. Int. J. Mol. Sci. 2018, 19, 3266. [Google Scholar] [CrossRef]
- Lin, L.T.; Hsu, W.C.; Lin, C.C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef]
- Bello-Morales, R.; Andreu, S.; Ruiz-Carpio, V.; Ripa, I.; Lopez-Guerrero, J.A. Extracellular Polymeric Substances: Still Promising Antivirals. Viruses 2022, 14, 1337. [Google Scholar] [CrossRef]
- Gaby, A.R. Natural remedies for Herpes simplex. Altern. Med. Rev. 2006, 11, 93–101. [Google Scholar] [PubMed]
- Pedrazini, M.C.; da Silva, M.H.; Groppo, F.C. L-lysine: Its antagonism with L-arginine in controlling viral infection. Narrative literature review. Br. J. Clin. Pharm. 2022, 88, 4708–4723. [Google Scholar] [CrossRef]
- Mailoo, V.J.; Rampes, S. Lysine for Herpes Simplex Prophylaxis: A Review of the Evidence. Integr. Med. 2017, 16, 42–46. [Google Scholar]
- Becker, Y.; Olshevsky, U.; Levitt, J. The role of arginine in the replication of herpes simplex virus. J. Gen. Virol. 1967, 1, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Griffith, R.S.; DeLong, D.C.; Nelson, J.D. Relation of arginine-lysine antagonism to herpes simplex growth in tissue culture. Chemotherapy 1981, 27, 209–213. [Google Scholar] [CrossRef]
- Inglis, V.B. Requirement of arginine for the replication of herpes virus. J. Gen. Virol. 1968, 3, 9–17. [Google Scholar] [CrossRef]
- Rahn, E.J.; Hohmann, A.G. Cannabinoids as pharmacotherapies for neuropathic pain: From the bench to the bedside. Neurotherapeutics 2009, 6, 713–737. [Google Scholar] [CrossRef] [PubMed]
- Reiss, C.S. Cannabinoids and Viral Infections. Pharmaceuticals 2010, 3, 1873–1886. [Google Scholar] [CrossRef]
- Cabral, G.A.; Lockmuller, J.C.; Mishkin, E.M. Delta 9-tetrahydrocannabinol decreases alpha/beta interferon response to herpes simplex virus type 2 in the B6C3F1 mouse. Proc. Soc. Exp. Biol. Med. 1986, 181, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Battinelli, L.; Pompeo, C.; Serrilli, A.M.; Rossi, R.; Sauzullo, I.; Mengoni, F.; Vullo, V. Inhibitory activity of Melissa officinalis L. extract on Herpes simplex virus type 2 replication. Nat. Prod. Res. 2008, 22, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Nolkemper, S.; Reichling, J.; Stintzing, F.C.; Carle, R.; Schnitzler, P. Antiviral effect of aqueous extracts from species of the Lamiaceae family against Herpes simplex virus type 1 and type 2 in vitro. Planta Med. 2006, 72, 1378–1382. [Google Scholar] [CrossRef]
- Hayamizu, K.; Oshima, I.; Fukuda, Z.; Kuramochi, Y.; Nagai, Y.; Izumo, N.; Nakano, M. Safety assessment of L-lysine oral intake: A systematic review. Amino Acids 2019, 51, 647–659. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Sudomova, M.; Masarcikova, R. Herpes simplex virus infection: An overview of the problem, pharmacologic therapy and dietary measures. Ceska Slov. Farm. 2017, 66, 95–102. [Google Scholar]
- Nalder, B.N.; Mahoney, A.W.; Ramakrishnan, R.; Hendricks, D.G. Sensitivity of the immunological response to the nutritional status of rats. J. Nutr. 1972, 102, 535–541. [Google Scholar] [CrossRef]
- Terezhalmy, G.T.; Bottomley, W.K.; Pelleu, G.B. The use of water-soluble bioflavonoid-ascorbic acid complex in the treatment of recurrent herpes labialis. Oral. Surg. Oral. Med. Oral. Pathol. 1978, 45, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.N.; Yoo, W.S.; Park, M.H.; Chung, J.K.; Han, Y.S.; Chung, I.Y.; Seo, S.W.; Yoo, J.M.; Kim, S.J. Clinical Features of Herpes Simplex Keratitis in a Korean Tertiary Referral Center: Efficacy of Oral Antiviral and Ascorbic Acid on Recurrence. Korean J. Ophthalmol. 2018, 32, 353–360. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, M.P.; Kumar, R.S.; Ratho, R.K. 25-Hydroxyvitamin D3 and 1,25 Dihydroxyvitamin D3 as an Antiviral and Immunomodulator Against Herpes Simplex Virus-1 Infection in HeLa Cells. Viral Immunol. 2018, 31, 589–593. [Google Scholar] [CrossRef]
- Choi, B.; Lee, E.S.; Sohn, S. Vitamin D3 ameliorates herpes simplex virus-induced Behcet’s disease-like inflammation in a mouse model through down-regulation of Toll-like receptors. Clin. Exp. Rheumatol. 2011, 29, S13–S19. [Google Scholar]
- Sheridan, P.A.; Beck, M.A. The immune response to herpes simplex virus encephalitis in mice is modulated by dietary vitamin E. J. Nutr. 2008, 138, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Femiano, F.; Gombos, F.; Scully, C. Recurrent herpes labialis: A pilot study of the efficacy of zinc therapy. J. Oral. Pathol. Med. 2005, 34, 423–425. [Google Scholar] [CrossRef]
- Godfrey, H.R.; Godfrey, N.J.; Godfrey, J.C.; Riley, D. A randomized clinical trial on the treatment of oral herpes with topical zinc oxide/glycine. Altern. Health Med. 2001, 7, 49–56. [Google Scholar]
- Mahajan, B.B.; Dhawan, M.; Singh, R. Herpes genitalis—Topical zinc sulfate: An alternative therapeutic and modality. Indian J. Sex. Transm. Dis. AIDS 2013, 34, 32–34. [Google Scholar] [CrossRef]
- Krol, W.; Bankova, V.; Sforcin, J.M.; Szliszka, E.; Czuba, Z.; Kuropatnicki, A.K. Propolis: Properties, application, and its potential. Evid. Based Complement. Altern. Med. 2013, 2013, 807578. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Duran, G.G.; Duran, N.; Jenedi, K.; Bolgul, B.S.; Miraloglu, M.; Muz, M. Antiviral Activity of Hatay Propolis Against Replication of Herpes Simplex Virus Type 1 and Type 2. Med. Sci. Monit. 2016, 22, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharm. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother. Res. 2010, 24 (Suppl. 1), S20–S28. [Google Scholar] [CrossRef]
- Amoros, M.; Lurton, E.; Boustie, J.; Girre, L.; Sauvager, F.; Cormier, M. Comparison of the anti-herpes simplex virus activities of propolis and 3-methyl-but-2-enyl caffeate. J. Nat. Prod. 1994, 57, 644–647. [Google Scholar] [CrossRef]
- Bankova, V.; Galabov, A.S.; Antonova, D.; Vilhelmova, N.; Di Perri, B. Chemical composition of Propolis Extract ACF(R) and activity against herpes simplex virus. Phytomedicine 2014, 21, 1432–1438. [Google Scholar] [CrossRef]
- Rocha, M.P.; Amorim, J.M.; Lima, W.G.; Brito, J.C.M.; da Cruz Nizer, W.S. Effect of honey and propolis, compared to acyclovir, against Herpes Simplex Virus (HSV)-induced lesions: A systematic review and meta-analysis. J. Ethnopharmacol. 2022, 287, 114939. [Google Scholar] [CrossRef] [PubMed]
- Lemon Balm. In Drugs and Lactation Database (LactMed (R)); National Institute of Child Health and Human Development: Bethesda, MD, USA, 2006.
- Astani, A.; Navid, M.H.; Schnitzler, P. Attachment and penetration of acyclovir-resistant herpes simplex virus are inhibited by Melissa officinalis extract. Phytother. Res. 2014, 28, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Koytchev, R.; Alken, R.G.; Dundarov, S. Balm mint extract (Lo-701) for topical treatment of recurring herpes labialis. Phytomedicine 1999, 6, 225–230. [Google Scholar] [CrossRef]
- Wolbling, R.H.; Leonhardt, K. Local therapy of herpes simplex with dried extract from Melissa officinalis. Phytomedicine 1994, 1, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- Sheridan, P.A.; Beck, M.A. The dendritic and T cell responses to herpes simplex virus-1 are modulated by dietary vitamin E. Free. Radic. Biol. Med. 2009, 46, 1581–1588. [Google Scholar] [CrossRef]
- Starasoler, S.; Haber, G.S. Use of vitamin E oil in primary herpes gingivostomatitis in an adult. N. Y. State Dent. J. 1978, 44, 382–383. [Google Scholar]
- Cohen, B. Treatment of herpes simplex by alphatocopheral (vitamin E). Br. Dent. J. 1980, 149, 69. [Google Scholar] [CrossRef]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef]
- Tuerk, M.J.; Fazel, N. Zinc deficiency. Curr. Opin. Gastroenterol. 2009, 25, 136–143. [Google Scholar] [CrossRef]
- Arens, M.; Travis, S. Zinc salts inactivate clinical isolates of herpes simplex virus in vitro. J. Clin. Microbiol. 2000, 38, 1758–1762. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Rapp, F. Effect of zinc ions on synthesis of herpes simplex virus type 2-induced polypeptides. Proc. Soc. Exp. Biol. Med. 1976, 152, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, B.; Chejanovsky, N.; Becker, Y. Selective inhibition of herpes simplex virus type 1 DNA polymerase by zinc ions. Virology 1978, 84, 551–554. [Google Scholar] [CrossRef]
- Kumel, G.; Schrader, S.; Zentgraf, H.; Daus, H.; Brendel, M. The mechanism of the antiherpetic activity of zinc sulphate. J. Gen. Virol. 1990, 71, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Fitzherbert, J.C. Genital herpes and zinc. Med. J. Aust. 1979, 1, 399. [Google Scholar] [CrossRef]
- Ranjbar, Z.; Zahed, M.; Ranjbar, M.A.; Shirmardan, Z. Comparative study of serum zinc concentration in recurrent herpes labialis patients and healthy individuals. BMC Oral. Health 2020, 20, 296. [Google Scholar] [CrossRef]
- Tavakoli, A.; Ataei-Pirkooh, A.; Mm Sadeghi, G.; Bokharaei-Salim, F.; Sahrapour, P.; Kiani, S.J.; Moghoofei, M.; Farahmand, M.; Javanmard, D.; Monavari, S.H. Polyethylene glycol-coated zinc oxide nanoparticle: An efficient nanoweapon to fight against herpes simplex virus type 1. Nanomedicine 2018, 13, 2675–2690. [Google Scholar] [CrossRef]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Ren. Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Chan, M.; Zhou, S.; Wang, J.; Reed, G.; Bronson, R.; Arnold, M.M.; Knipe, D.M.; Finberg, R.W. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 2004, 101, 1315–1320. [Google Scholar] [CrossRef]
- Finberg, R.W.; Knipe, D.M.; Kurt-Jones, E.A. Herpes simplex virus and toll-like receptors. Viral Immunol. 2005, 18, 457–465. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.; Wang, M.; Lin, S. The association between serum 25-hydroxyvitamin D and the prevalence of herpes simplex virus. J. Med. Virol. 2023, 95, e28297. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Chang, W.-C.; Moyo, P.; White, M.R.; Meelu, P.; Verma, A.; Stahl, G.L.; Hartshorn, K.L.; Yajnik, V. Dietary sugars inhibit biologic functions of the pattern recognition molecule, mannose-binding lectin. Open. J. Immunol. 2011, 1, 41–49. [Google Scholar] [CrossRef]
- Shan, T.; Huang, Y.; Zhao, Z.; Li, F.; Wang, Y.; Ye, C.; Zheng, K.; Ren, Z. Ketogenic diet restrains herpes simplex encephalitis via gut microbes. Microbes Infect. 2023, 25, 105061. [Google Scholar] [CrossRef] [PubMed]
- Spindler, M.P.; Ho, A.M.; Tridgell, D.; McCulloch-Olson, M.; Gersuk, V.; Ni, C.; Greenbaum, C.; Sanda, S. Acute hyperglycemia impairs IL-6 expression in humans. Immun. Inflamm. Dis. 2016, 4, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.J.; Barrientos, R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav. Immun. 2020, 87, 53–54. [Google Scholar] [CrossRef]
- Woelfle, T.; Linkohr, B.; Waterboer, T.; Thorand, B.; Seissler, J.; Chadeau-Hyam, M.; Peters, A. Health impact of seven herpesviruses on (pre)diabetes incidence and HbA(1c): Results from the KORA cohort. Diabetologia 2022, 65, 1328–1338. [Google Scholar] [CrossRef]
- Cabral, G.A.; McNerney, P.J.; Mishkin, E.M. Delta-9-tetrahydrocannabinol enhances release of herpes simplex virus type 2. J. Gen. Virol. 1986, 67, 2017–2022. [Google Scholar] [CrossRef]
- Karmaus, P.W.; Chen, W.; Kaplan, B.L.; Kaminski, N.E. Delta9-tetrahydrocannabinol suppresses cytotoxic T lymphocyte function independent of CB1 and CB 2, disrupting early activation events. J. Neuroimmune Pharm. 2012, 7, 843–855. [Google Scholar] [CrossRef]
- Massi, P.; Sacerdote, P.; Ponti, W.; Fuzio, D.; Manfredi, B.; Vigano, D.; Rubino, T.; Bardotti, M.; Parolaro, D. Immune function alterations in mice tolerant to delta9-tetrahydrocannabinol: Functional and biochemical parameters. J. Neuroimmunol. 1998, 92, 60–66. [Google Scholar] [CrossRef]
- Maggirwar, S.B.; Khalsa, J.H. The Link between Cannabis Use, Immune System, and Viral Infections. Viruses 2021, 13, 1099. [Google Scholar] [CrossRef]
- Khoury, M.; Cohen, I.; Bar-Sela, G. “The Two Sides of the Same Coin”-Medical Cannabis, Cannabinoids and Immunity: Pros and Cons Explained. Pharmaceutics 2022, 14, 389. [Google Scholar] [CrossRef]
- Lancz, G.; Specter, S.; Brown, H.K. Suppressive effect of delta-9-tetrahydrocannabinol on herpes simplex virus infectivity in vitro. Proc. Soc. Exp. Biol. Med. 1991, 196, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Maor, Y.; Yu, J.; Kuzontkoski, P.M.; Dezube, B.J.; Zhang, X.; Groopman, J.E. Cannabidiol inhibits growth and induces programmed cell death in kaposi sarcoma-associated herpesvirus-infected endothelium. Genes. Cancer 2012, 3, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.I.; Toyang, N.J.; McLaughlin, W. Potential of Cannabidiol for the Treatment of Viral Hepatitis. Pharmacogn. Res. 2017, 9, 116–118. [Google Scholar] [CrossRef]
- Mieres-Castro, D.; Ahmar, S.; Shabbir, R.; Mora-Poblete, F. Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses. Pharmaceuticals (Basel) 2021, 14, 1210. [Google Scholar] [CrossRef]
- Sea, Y.L.; Gee, Y.J.; Lal, S.K.; Choo, W.S. Cannabis as antivirals. J. Appl. Microbiol. 2023, 134, lxac036. [Google Scholar] [CrossRef]
- Mojadadi, S.; Jamali, A.; Khansarinejad, B.; Soleimanjahi, H.; Bamdad, T. Acute morphine administration reduces cell-mediated immunity and induces reactivation of latent herpes simplex virus type 1 in BALB/c mice. Cell. Mol. Immunol. 2009, 6, 111–116. [Google Scholar] [CrossRef]
- Jamali, A.; Bamdad, T.; Soleimanjahi, H.; Pakdel, F.G.; Arefian, E. Acute morphine administration reduces white blood cells' capability to induce innate resistance against HSV-1 infection in BALB/c mice. Neuroimmunomodulation 2007, 14, 16–23. [Google Scholar] [CrossRef]
- Swanson, J.M.; Remy, L.; Chenitz, W.C.; Chastain, R.L.; Trocki, K.F. Illicit drug use among young adults with genital herpes. Public. Health Nurs. 1993, 10, 197–203. [Google Scholar] [CrossRef]
- Crone, L.A.; Conly, J.M.; Clark, K.M.; Crichlow, A.C.; Wardell, G.C.; Zbitnew, A.; Rea, L.M.; Cronk, S.L.; Anderson, C.M.; Tan, L.K.; et al. Recurrent herpes simplex virus labialis and the use of epidural morphine in obstetric patients. Anesth. Analg. 1988, 67, 318–323. [Google Scholar] [CrossRef]
- Cook, R.L.; Pollock, N.K.; Rao, A.K.; Clark, D.B. Increased prevalence of herpes simplex virus type 2 among adolescent women with alcohol use disorders. J. Adolesc. Health 2002, 30, 169–174. [Google Scholar] [CrossRef]
- Szabo, A. Psychedelics and Immunomodulation: Novel Approaches and Therapeutic Opportunities. Front. Immunol. 2015, 6, 358. [Google Scholar] [CrossRef] [PubMed]
- Rudin, D.; Areesanan, A.; Liechti, M.E.; Grundemann, C. Classic psychedelics do not affect T cell and monocyte immune responses. Front. Psychiatry 2023, 14, 1042440. [Google Scholar] [CrossRef] [PubMed]
- van de Sand, L.; Bormann, M.; Schmitz, Y.; Heilingloh, C.S.; Witzke, O.; Krawczyk, A. Antiviral Active Compounds Derived from Natural Sources against Herpes Simplex Viruses. Viruses 2021, 13, 1386. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.Y.; Balch, C.; Puccio, J.; Oh, H.S. A Narrative Review of Alternative Symptomatic Treatments for Herpes Simplex Virus. Viruses 2023, 15, 1314. https://doi.org/10.3390/v15061314
Chang JY, Balch C, Puccio J, Oh HS. A Narrative Review of Alternative Symptomatic Treatments for Herpes Simplex Virus. Viruses. 2023; 15(6):1314. https://doi.org/10.3390/v15061314
Chicago/Turabian StyleChang, Jane Y., Curt Balch, Joseph Puccio, and Hyung S. Oh. 2023. "A Narrative Review of Alternative Symptomatic Treatments for Herpes Simplex Virus" Viruses 15, no. 6: 1314. https://doi.org/10.3390/v15061314
APA StyleChang, J. Y., Balch, C., Puccio, J., & Oh, H. S. (2023). A Narrative Review of Alternative Symptomatic Treatments for Herpes Simplex Virus. Viruses, 15(6), 1314. https://doi.org/10.3390/v15061314





