Integration of Cellular and Humoral Immune Responses as an Immunomonitoring Tool for SARS-CoV-2 Vaccination in Healthy and Fragile Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. Assessment of SARS-CoV-2 Immune Responses
2.3. Statistical Analysis
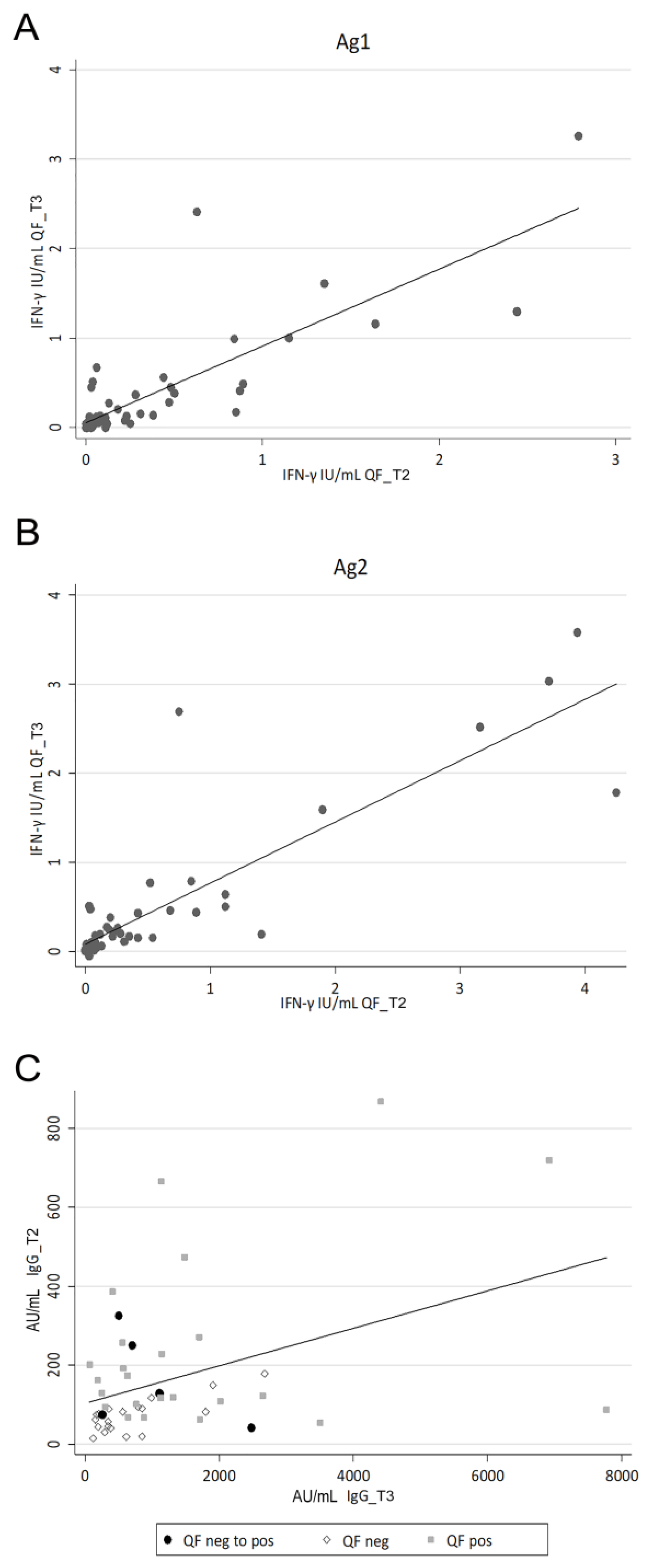
3. Results
3.1. Study Population and Clinical Characteristics
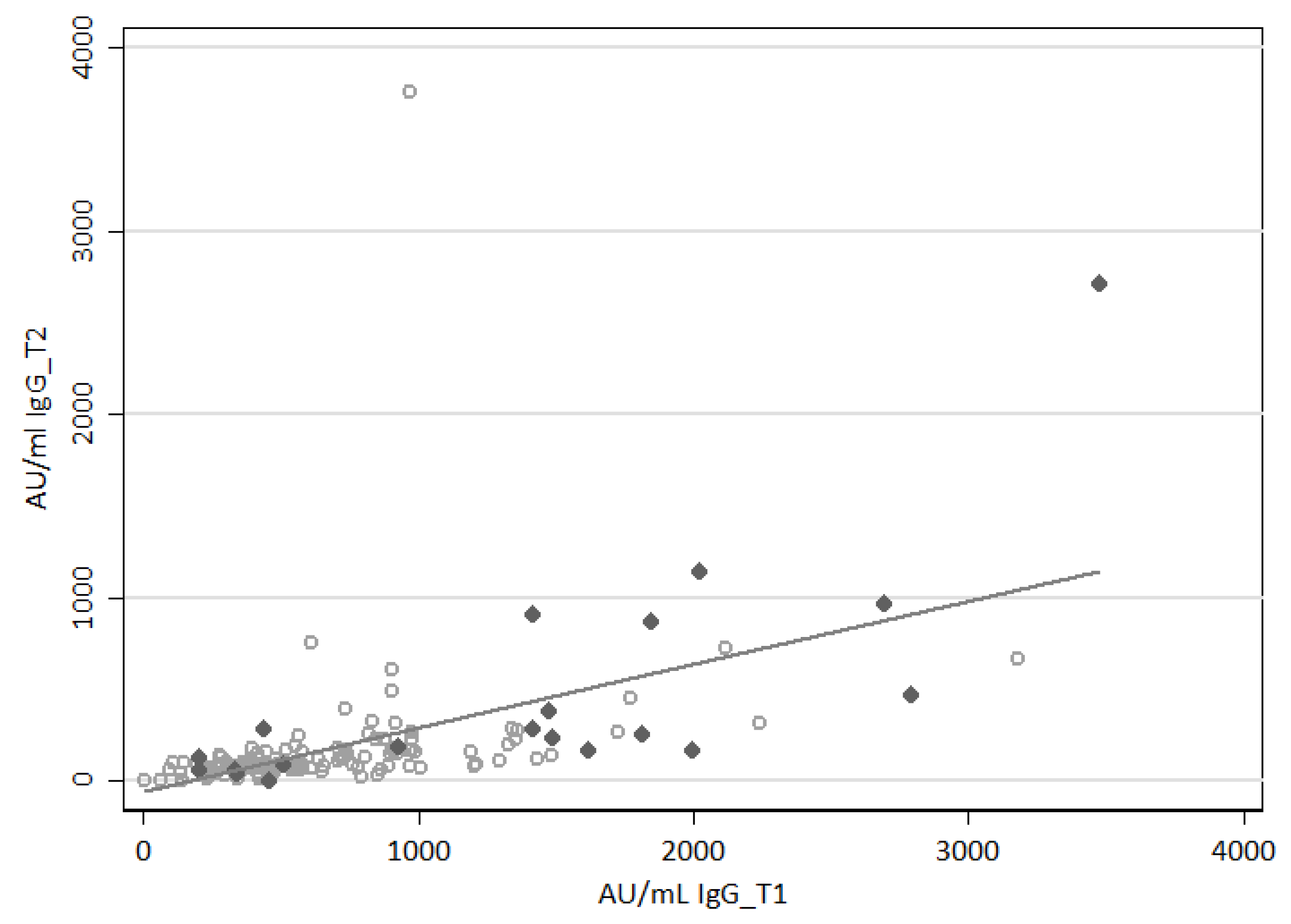
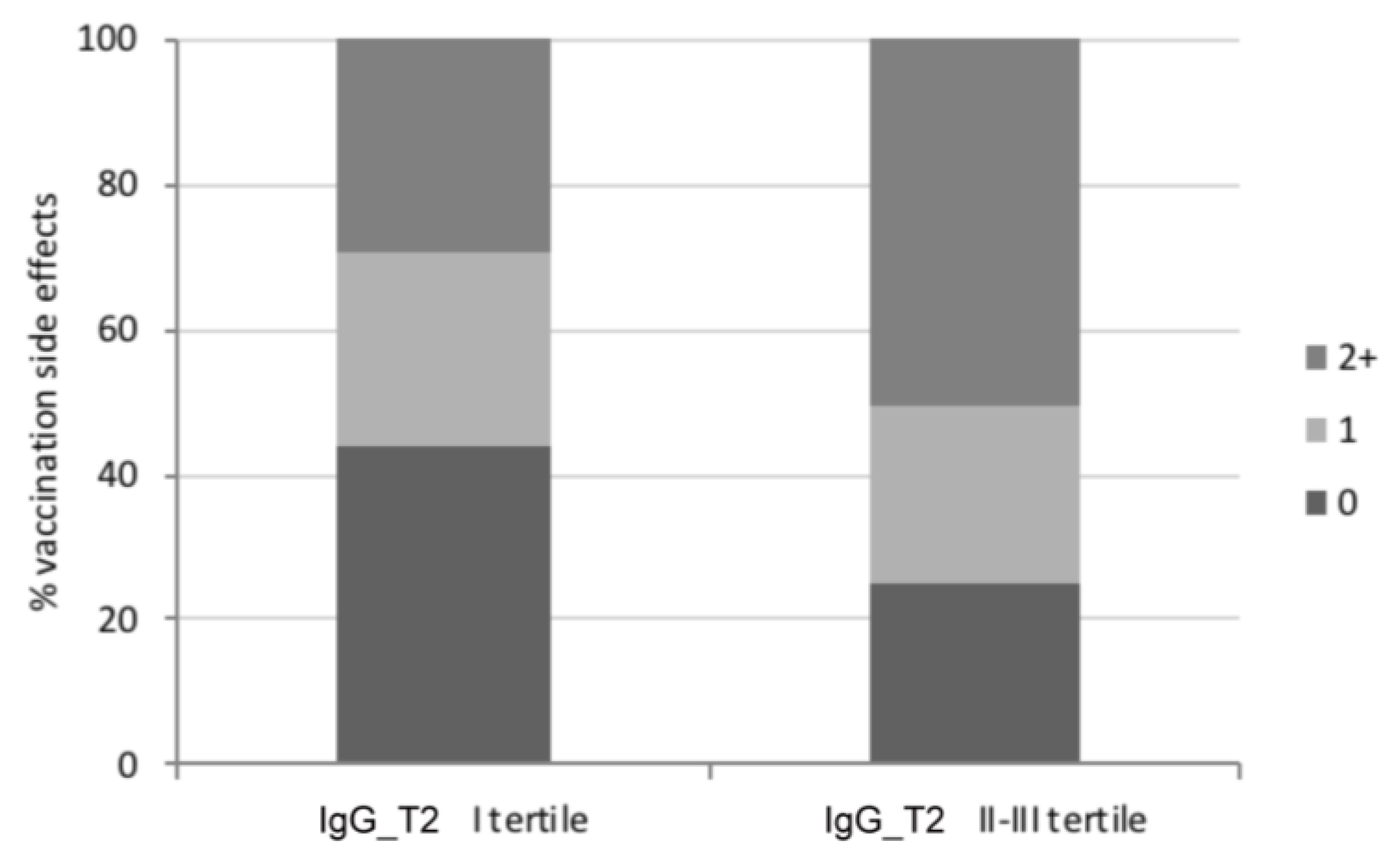
3.2. Assessment of the SARS-CoV-2-Specific Humoral Immune Response
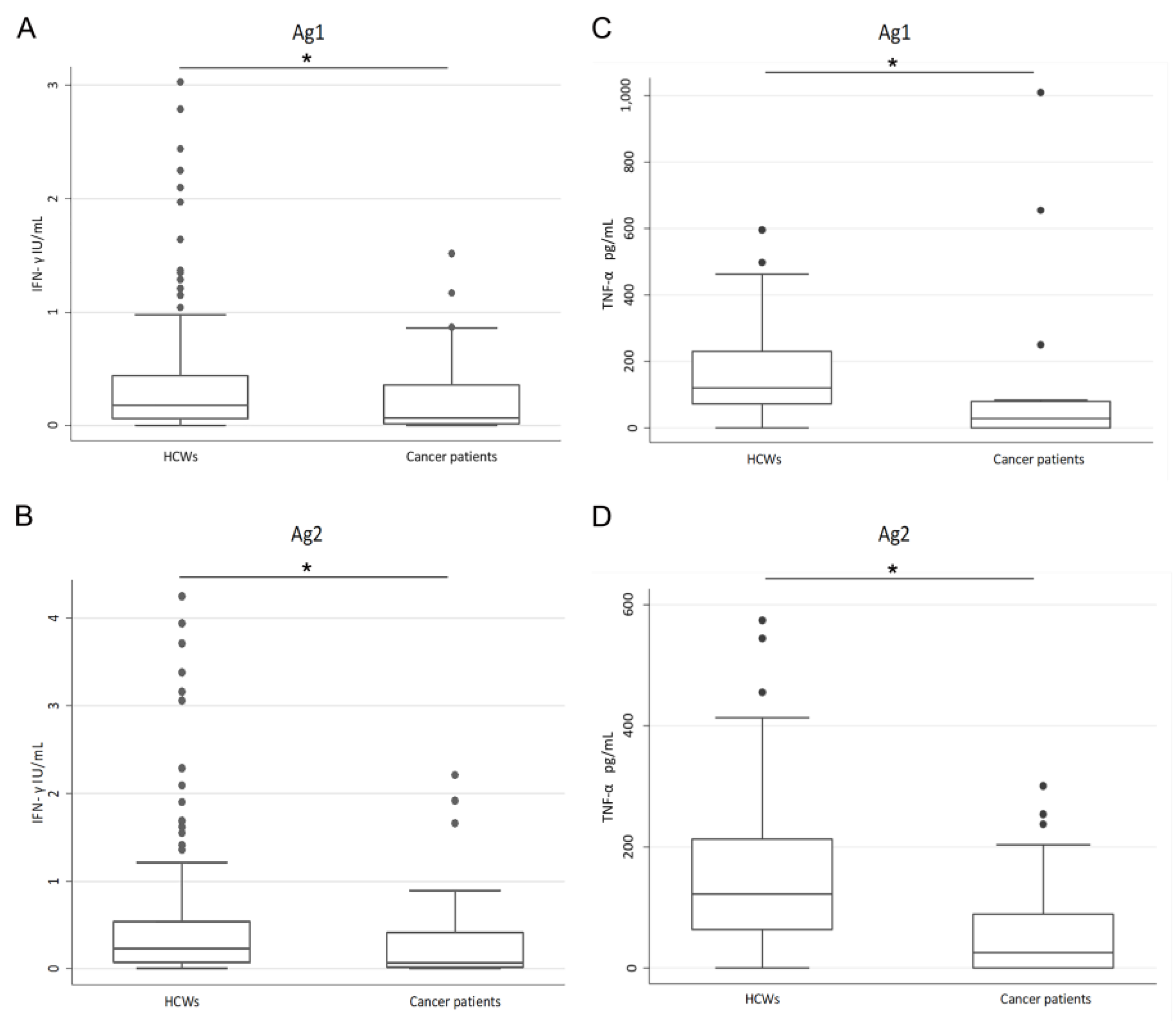
3.3. Evaluation of the SARS-CoV-2-Specific Cellular Immune Response
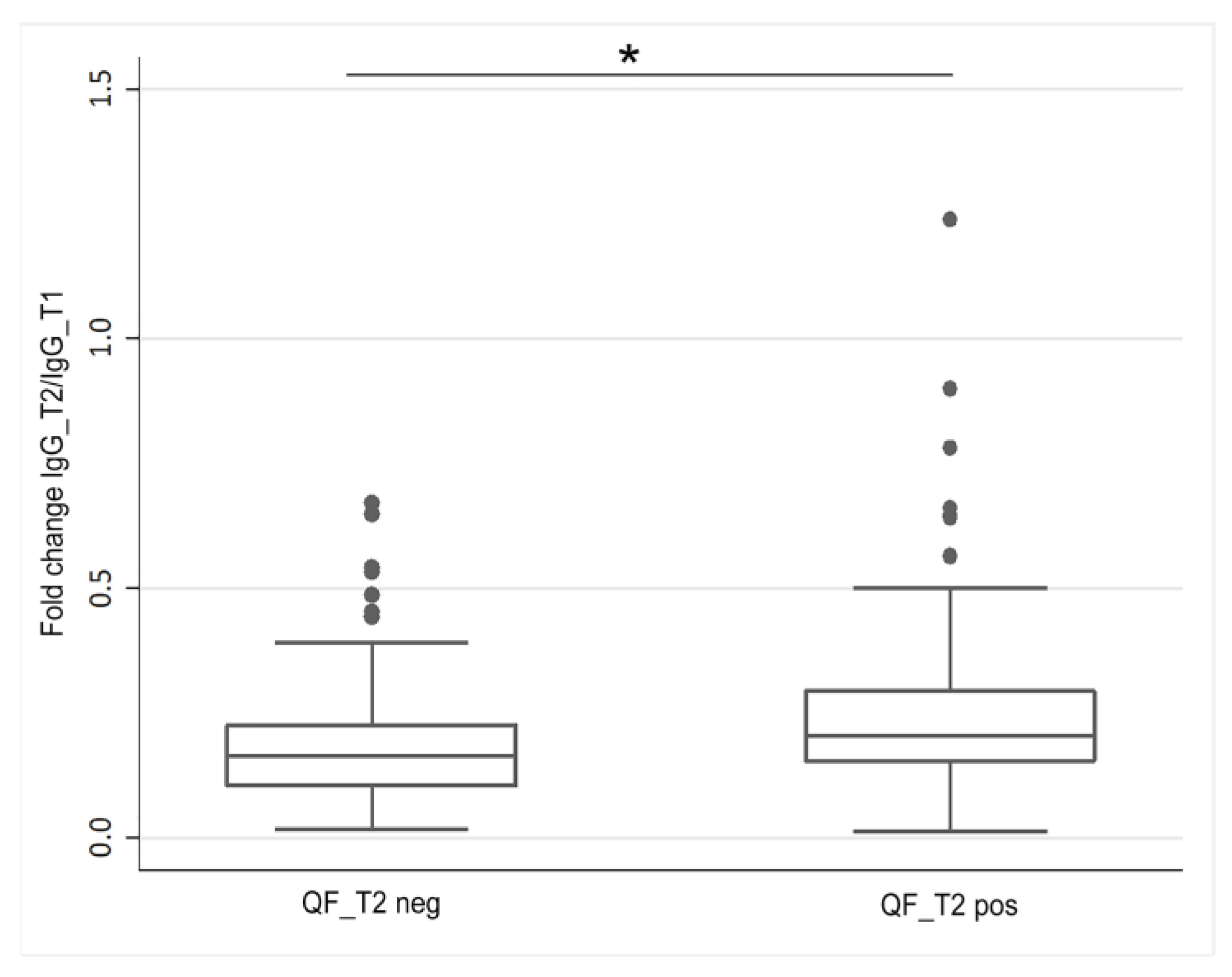
3.4. Evaluation of the SARS-CoV-2-Specific Humoral and Cellular Immune Response after Vaccination Boost in HCWs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breton, G.; Mendoza, P.; Hägglöf, T.; Oliveira, T.Y.; Schaefer-Babajew, D.; Gaebler, C.; Turroja, M.; Hurley, A.; Caskey, M.; Nussenzweig, M.C. Persistent Cellular Immunity to SARS-CoV-2 Infection. J. Exp. Med. 2021, 218, e20202515. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Ye, F.; Cheng, M.-L.; Feng, Y.; Deng, Y.-Q.; Zhao, H.; Wei, P.; Ge, J.; Gou, M.; Li, X.; et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 2020, 52, 971–977.e3. [Google Scholar] [CrossRef] [PubMed]
- Moga, E.; Lynton-Pons, E.; Domingo, P. The Robustness of Cellular Immunity Determines the Fate of SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 904686. [Google Scholar] [CrossRef]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid Induction of Antigen-Specific CD4+ T Cells Is Associated with Coordinated Humoral and Cellular Immunity to SARS-CoV-2 MRNA Vaccination. Immunity 2021, 54, 2133–2142.e3. [Google Scholar] [CrossRef] [PubMed]
- Bonifacius, A.; Tischer-Zimmermann, S.; Dragon, A.C.; Gussarow, D.; Vogel, A.; Krettek, U.; Gödecke, N.; Yilmaz, M.; Kraft, A.R.M.; Hoeper, M.M.; et al. COVID-19 Immune Signatures Reveal Stable Antiviral T Cell Function despite Declining Humoral Responses. Immunity 2021, 54, 340–354.e6. [Google Scholar] [CrossRef]
- Aiello, A.; Coppola, A.; Vanini, V.; Petrone, L.; Cuzzi, G.; Salmi, A.; Altera, A.M.G.; Tortorella, C.; Gualano, G.; Gasperini, C.; et al. Accuracy of QuantiFERON SARS-CoV-2 RUO Assay and Characterization of the CD4+ and CD8+ T-Cell-SARS-CoV-2 Response: Comparison with a Homemade IFN-γ Release Assay. Int. J. Infect. Dis. 2022, 122, 841–849. [Google Scholar] [CrossRef]
- Channappanavar, R.; Zhao, J.; Perlman, S. T Cell-Mediated Immune Response to Respiratory Coronaviruses. Immunol. Res. 2014, 59, 118–128. [Google Scholar] [CrossRef]
- Ng, O.-W.; Chia, A.; Tan, A.T.; Jadi, R.S.; Leong, H.N.; Bertoletti, A.; Tan, Y.-J. Memory T Cell Responses Targeting the SARS Coronavirus Persist up to 11 Years Post-Infection. Vaccine 2016, 34, 2008–2014. [Google Scholar] [CrossRef]
- Petrone, L.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; Najafi Fard, S.; Alonzi, T.; Castilletti, C.; Palmieri, F.; Gualano, G.; Vittozzi, P.; et al. A Whole Blood Test to Measure SARS-CoV-2-Specific Response in COVID-19 Patients. Clin. Microbiol. Infect. 2021, 27, e7–e286. [Google Scholar] [CrossRef]
- Lee, E.; Sandgren, K.; Duette, G.; Stylianou, V.V.; Khanna, R.; Eden, J.-S.; Blyth, E.; Gottlieb, D.; Cunningham, A.L.; Palmer, S. Identification of SARS-CoV-2 Nucleocapsid and Spike T-Cell Epitopes for Assessing T-Cell Immunity. J. Virol. 2021, 95, e02002–e02020. [Google Scholar] [CrossRef]
- Nelde, A.; Bilich, T.; Heitmann, J.S.; Maringer, Y.; Salih, H.R.; Roerden, M.; Lübke, M.; Bauer, J.; Rieth, J.; Wacker, M.; et al. SARS-CoV-2-Derived Peptides Define Heterologous and COVID-19-Induced T Cell Recognition. Nat. Immunol. 2021, 22, 74–85. [Google Scholar] [CrossRef]
- Murugesan, K.; Jagannathan, P.; Pham, T.D.; Pandey, S.; Bonilla, H.F.; Jacobson, K.; Parsonnet, J.; Andrews, J.R.; Weiskopf, D.; Sette, A.; et al. Interferon-γ Release Assay for Accurate Detection of Severe Acute Respiratory Syndrome Coronavirus 2 T-Cell Response. Clin. Infect. Dis. 2021, 73, e3130–e3132. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Scozzari, G.; Migliore, E.; Galassi, C.; Ciccone, G.; Ricciardelli, G.; Scarmozzino, A.; Angelone, L.; Cassoni, P.; Cavallo, R.; et al. Cellular Immune Response to BNT162b2 MRNA COVID-19 Vaccine in a Large Cohort of Healthcare Workers in a Tertiary Care University Hospital. Vaccines 2022, 10, 1031. [Google Scholar] [CrossRef] [PubMed]
- Desmecht, S.; Tashkeev, A.; El Moussaoui, M.; Marechal, N.; Perée, H.; Tokunaga, Y.; Fombellida-Lopez, C.; Polese, B.; Legrand, C.; Wéry, M.; et al. Kinetics and Persistence of the Cellular and Humoral Immune Responses to BNT162b2 MRNA Vaccine in SARS-CoV-2-Naive and -Experienced Subjects: Impact of Booster Dose and Breakthrough Infections. Front. Immunol. 2022, 13, 863554. [Google Scholar] [CrossRef] [PubMed]
- Brisotto, G.; Muraro, E.; Montico, M.; Corso, C.; Evangelista, C.; Casarotto, M.; Caffau, C.; Vettori, R.; Cozzi, M.R.; Zanussi, S.; et al. IgG Antibodies against SARS-CoV-2 Decay but Persist 4 Months after Vaccination in a Cohort of Healthcare Workers. Clin. Chim. Acta 2021, 523, 476–482. [Google Scholar] [CrossRef]
- Ciabattini, A.; Pastore, G.; Fiorino, F.; Polvere, J.; Lucchesi, S.; Pettini, E.; Auddino, S.; Rancan, I.; Durante, M.; Miscia, M.; et al. Evidence of SARS-CoV-2-Specific Memory B Cells Six Months After Vaccination With the BNT162b2 MRNA Vaccine. Front. Immunol. 2021, 12, 740708. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA 2021, 325, 1784–1786. [Google Scholar] [CrossRef]
- Del Poeta, G.; Bomben, R.; Polesel, J.; Rossi, F.M.; Pozzo, F.; Zaina, E.; Cattarossi, I.; Varaschin, P.; Nanni, P.; Boschian Boschin, R.; et al. COVID-19 Vaccination: Evaluation of Risk for Protection Failure in Chronic Lymphocytic Leukemia Patients. Hematol. Oncol. 2021, 39, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Palich, R.; Veyri, M.; Marot, S.; Vozy, A.; Gligorov, J.; Maingon, P.; Marcelin, A.-G.; Spano, J.-P. Weak Immunogenicity after a Single Dose of SARS-CoV-2 MRNA Vaccine in Treated Cancer Patients. Ann. Oncol. 2021, 32, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Agrati, C.; Di Cosimo, S.; Fenoglio, D.; Apolone, G.; Ciceri, F.; Ciliberto, G.; Baldanti, F.; Costantini, M.; Giannarelli, D.; Ippolito, G.; et al. COVID-19 Vaccination in Fragile Patients: Current Evidence and an Harmonized Transdisease Trial. Front. Immunol. 2021, 12, 704110. [Google Scholar] [CrossRef] [PubMed]
- Corradini, P.; Agrati, C.; Apolone, G.; Mantovani, A.; Giannarelli, D.; Marasco, V.; Bordoni, V.; Sacchi, A.; Matusali, G.; Salvarani, C.; et al. Humoral and T-Cell Immune Response after Three Doses of MRNA SARS-CoV-2 Vaccines in Fragile Patients: The Italian VAX4FRAIL Study. Clin. Infect. Dis. 2023, 76, e426–e438. [Google Scholar] [CrossRef]
- Fiorino, F.; Ciabattini, A.; Sicuranza, A.; Pastore, G.; Santoni, A.; Simoncelli, M.; Polvere, J.; Galimberti, S.; Baratè, C.; Sammartano, V.; et al. The Third Dose of MRNA SARS-CoV-2 Vaccines Enhances the Spike-Specific Antibody and Memory B Cell Response in Myelofibrosis Patients. Front. Immunol. 2022, 13, 1017863. [Google Scholar] [CrossRef]
- Pettini, E.; Ciabattini, A.; Pastore, G.; Polvere, J.; Lucchesi, S.; Fiorino, F.; Montagnani, F.; Bucalossi, A.; Tozzi, M.; Marotta, G.; et al. A Third Dose of MRNA-1273 Vaccine Improves SARS-CoV-2 Immunity in HCT Recipients with Low Antibody Response after 2 Doses. Blood Adv. 2022, 6, 2247–2249. [Google Scholar] [CrossRef]
- Sugihara, K.; Wakiya, R.; Shimada, H.; Kameda, T.; Nakashima, S.; Kato, M.; Miyagi, T.; Mizusaki, M.; Mino, R.; Nomura, Y.; et al. Immunogenicity against the BNT162b2 MRNA COVID-19 Vaccine in Rheumatic Disease Patients Receiving Immunosuppressive Therapy. Intern. Med. 2022, 61, 1953–1958. [Google Scholar] [CrossRef]
- Porru, S.; Monaco, M.G.L.; Carta, A.; Spiteri, G.; Parpaiola, M.; Battaggia, A.; Galligioni, G.; Ferrazzi, B.; Lo Cascio, G.; Gibellini, D.; et al. SARS-CoV-2 Infection in Health Workers: Analysis from Verona SIEROEPID Study during the Pre-Vaccination Era. Int. J. Environ. Res. Public Health 2021, 18, 6446. [Google Scholar] [CrossRef]
- EpiCentro Coronavirus | Istituto Superiore di Sanità. Available online: https://www.epicentro.iss.it/coronavirus/ (accessed on 8 May 2023).
- Petrone, D.; Mateo-Urdiales, A.; Sacco, C.; Riccardo, F.; Bella, A.; Ambrosio, L.; Lo Presti, A.; Di Martino, A.; Ceccarelli, E.; Del Manso, M.; et al. Reduction of the Risk of Severe COVID-19 Due to Omicron Compared to Delta Variant in Italy (November 2021–February 2022). Int. J. Infect. Dis. 2023, 129, 135–141. [Google Scholar] [CrossRef]
- Governo Italiano—Report Vaccini Anti COVID-19. Available online: https://www.governo.it/it/cscovid19/report-vaccini/ (accessed on 8 May 2023).
- Zamagni, G.; Armocida, B.; Abbafati, C.; Ronfani, L.; Monasta, L. COVID-19 Vaccination Coverage in Italy: How Many Hospitalisations and Related Costs Could Have Been Saved If We Were All Vaccinated? Front. Public Health 2022, 10, 825416. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. MRNA Vaccines Induce Durable Immune Memory to SARS-CoV-2 and Variants of Concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Chapin-Bardales, J.; Gee, J.; Myers, T. Reactogenicity Following Receipt of MRNA-Based COVID-19 Vaccines. JAMA 2021, 325, 2201–2202. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.L.; Yasawardene, P.; Wijerathne, W.; Somawardana, B. COVID-19 Vaccination in Cancer Patients: A Narrative Review. J. Int. Med. Res. 2022, 50, 3000605221086155. [Google Scholar] [CrossRef] [PubMed]
- Parés-Badell, O.; Zules-Oña, R.; Armadans, L.; Pinós, L.; Borrás-Bermejo, B.; Otero, S.; Rodrigo-Pendás, J.Á.; Vivet-Escalé, M.; Cossio-Gil, Y.; Agustí, A.; et al. Reactogenicity to the MRNA-1273 Booster According to Previous MRNA COVID-19 Vaccination. Vaccines 2022, 10, 1217. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M.; Baggs, J.; Gee, J.; Marquez, P.; Myers, T.R.; Shimabukuro, T.T.; Shay, D.K. Safety Monitoring of an Additional Dose of COVID-19 Vaccine—United States, August 12–September 19, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1379–1384. [Google Scholar] [CrossRef]
- Sugiyama, A.; Kurisu, A.; Nagashima, S.; Hando, K.; Saipova, K.; Akhmedova, S.; Abe, K.; Imada, H.; Hussain, M.R.A.; Ouoba, S.; et al. Seroepidemiological Study of Factors Affecting Anti-Spike IgG Antibody Titers after a Two-Dose MRNA COVID-19 Vaccination in 3744 Healthy Japanese Volunteers. Sci. Rep. 2022, 12, 16294. [Google Scholar] [CrossRef]
- Coggins, S.A.; Laing, E.D.; Olsen, C.H.; Goguet, E.; Moser, M.; Jackson-Thompson, B.M.; Samuels, E.C.; Pollett, S.D.; Tribble, D.R.; Davies, J.; et al. Adverse Effects and Antibody Titers in Response to the BNT162b2 MRNA COVID-19 Vaccine in a Prospective Study of Healthcare Workers. Open Forum. Infect. Dis. 2022, 9, ofab575. [Google Scholar] [CrossRef]
- Takeuchi, M.; Higa, Y.; Esaki, A.; Nabeshima, Y.; Nakazono, A. Does Reactogenicity after a Second Injection of the BNT162b2 Vaccine Predict Spike IgG Antibody Levels in Healthy Japanese Subjects? PLoS ONE 2021, 16, e0257668. [Google Scholar] [CrossRef]
- Uwamino, Y.; Kurafuji, T.; Sato, Y.; Tomita, Y.; Shibata, A.; Tanabe, A.; Yatabe, Y.; Noguchi, M.; Arai, T.; Ohno, A.; et al. Young Age, Female Sex, and Presence of Systemic Adverse Reactions Are Associated with High Post-Vaccination Antibody Titer after Two Doses of BNT162b2 MRNA SARS-CoV-2 Vaccination: An Observational Study of 646 Japanese Healthcare Workers and University Staff. Vaccine 2022, 40, 1019–1025. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Song, K.-H.; Choi, Y.; Go, S.; Choi, S.-J.; Jung, J.; Kang, C.K.; Choe, P.G.; Kim, N.-J.; Park, W.B.; et al. Can Reactogenicity Predict Immunogenicity after COVID-19 Vaccination? Korean J. Intern. Med. 2021, 36, 1486–1491. [Google Scholar] [CrossRef]
- Maeda, K.; Amano, M.; Uemura, Y.; Tsuchiya, K.; Matsushima, T.; Noda, K.; Shimizu, Y.; Fujiwara, A.; Takamatsu, Y.; Ichikawa, Y.; et al. Correlates of Neutralizing/SARS-CoV-2-S1-Binding Antibody Response with Adverse Effects and Immune Kinetics in BNT162b2-Vaccinated Individuals. Sci. Rep. 2021, 11, 22848. [Google Scholar] [CrossRef] [PubMed]
- Rechavi, Y.; Shashar, M.; Lellouche, J.; Yana, M.; Yakubovich, D.; Sharon, N. Occurrence of BNT162b2 Vaccine Adverse Reactions Is Associated with Enhanced SARS-CoV-2 IgG Antibody Response. Vaccines 2021, 9, 977. [Google Scholar] [CrossRef] [PubMed]
- Debes, A.K.; Xiao, S.; Colantuoni, E.; Egbert, E.R.; Caturegli, P.; Gadala, A.; Milstone, A.M. Association of Vaccine Type and Prior SARS-CoV-2 Infection with Symptoms and Antibody Measurements Following Vaccination Among Health Care Workers. JAMA Intern. Med. 2021, 181, 1660–1662. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of Antibody Response to BNT162b2 Vaccine after Six Months: A Longitudinal Prospective Study. Lancet Reg. Health Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Kobashi, Y.; Shimazu, Y.; Kawamura, T.; Nishikawa, Y.; Omata, F.; Kaneko, Y.; Kodama, T.; Tsubokura, M. Factors Associated with Anti-Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein Antibody Titer and Neutralizing Activity among Healthcare Workers Following Vaccination with the BNT162b2 Vaccine. PLoS ONE 2022, 17, e0269917. [Google Scholar] [CrossRef]
- Levy, I.; Levin, E.G.; Olmer, L.; Regev-Yochay, G.; Agmon-Levin, N.; Wieder-Finesod, A.; Indenbaum, V.; Herzog, K.; Doolman, R.; Asraf, K.; et al. Correlation between Adverse Events and Antibody Titers among Healthcare Workers Vaccinated with BNT162b2 MRNA COVID-19 Vaccine. Vaccines 2022, 10, 1220. [Google Scholar] [CrossRef]
- Bauernfeind, S.; Salzberger, B.; Hitzenbichler, F.; Scigala, K.; Einhauser, S.; Wagner, R.; Gessner, A.; Koestler, J.; Peterhoff, D. Association between Reactogenicity and Immunogenicity after Vaccination with BNT162b2. Vaccines 2021, 9, 1089. [Google Scholar] [CrossRef]
- Primorac, D.; Brlek, P.; Matišić, V.; Molnar, V.; Vrdoljak, K.; Zadro, R.; Parčina, M. Cellular Immunity-The Key to Long-Term Protection in Individuals Recovered from SARS-CoV-2 and after Vaccination. Vaccines 2022, 10, 442. [Google Scholar] [CrossRef]
- Choi, H.; Lee, S.-M.; Lim, S.; Shin, K.-H.; Kim, T.; Kim, W.-J.; Yun, M.; Oh, S.-H. Immunogenicity after Second ChAdOx1 NCoV-19 (AZD1222) Vaccination According to the Individual Reactogenicity, Health Status and Lifestyle. Vaccines 2021, 9, 1473. [Google Scholar] [CrossRef]
- Takano, T.; Morikawa, M.; Adachi, Y.; Kabasawa, K.; Sax, N.; Moriyama, S.; Sun, L.; Isogawa, M.; Nishiyama, A.; Onodera, T.; et al. Distinct Immune Cell Dynamics Correlate with the Immunogenicity and Reactogenicity of SARS-CoV-2 MRNA Vaccine. Cell Rep. Med. 2022, 3, 100631. [Google Scholar] [CrossRef]
- Lasagna, A.; Lilleri, D.; Agustoni, F.; Percivalle, E.; Borgetto, S.; Alessio, N.; Comolli, G.; Sarasini, A.; Bergami, F.; Sammartino, J.C.; et al. Analysis of the Humoral and Cellular Immune Response after a Full Course of BNT162b2 Anti-SARS-CoV-2 Vaccine in Cancer Patients Treated with PD-1/PD-L1 Inhibitors with or without Chemotherapy: An Update after 6 Months of Follow-Up. ESMO Open 2022, 7, 100359. [Google Scholar] [CrossRef] [PubMed]
- Bordry, N.; Addeo, A.; Jaksic, C.; Dutoit, V.; Roux-Lombard, P.; Shah, D.P.; Shah, P.K.; Gayet-Ageron, A.; Friedlaender, A.; Bugeia, S.; et al. Humoral and Cellular Immunogenicity Two Months after SARS-CoV-2 Messenger RNA Vaccines in Patients with Cancer. iScience 2022, 25, 103699. [Google Scholar] [CrossRef] [PubMed]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Byrne, F.; Cerrone, M.; Schmitt, A.M.; Joharatnam-Hogan, N.; Shum, B.; et al. Adaptive Immunity and Neutralizing Antibodies against SARS-CoV-2 Variants of Concern Following Vaccination in Patients with Cancer: The CAPTURE Study. Nat. Cancer 2021, 2, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, C.; Pagoni, M.; Rosati, M.; Angel, M.; Tzannou, I.; Vlachou, M.; Darmani, I.; Ullah, A.; Bear, J.; Devasundaram, S.; et al. Reduced Antibodies and Innate Cytokine Changes in SARS-CoV-2 BNT162b2 MRNA Vaccinated Transplant Patients With Hematological Malignancies. Front. Immunol. 2022, 13, 899972. [Google Scholar] [CrossRef] [PubMed]
- Farroni, C.; Picchianti-Diamanti, A.; Aiello, A.; Nicastri, E.; Laganà, B.; Agrati, C.; Castilletti, C.; Meschi, S.; Colavita, F.; Cuzzi, G.; et al. Kinetics of the B- and T-Cell Immune Responses After 6 Months From SARS-CoV-2 MRNA Vaccination in Patients With Rheumatoid Arthritis. Front. Immunol. 2022, 13, 846753. [Google Scholar] [CrossRef]
- Pri-Paz Basson, Y.; Tayer-Shifman, O.E.; Naser, R.; Tartakover Matalon, S.; Kimhi, O.; Gepstein, R.; Halperin, T.; Ziv-Baran, T.; Ziv, A.; Parikh, R.; et al. Immunogenicity and Safety of the MRNA-Based BNT162b2 Vaccine in Systemic Autoimmune Rheumatic Diseases Patients. Clin. Rheumatol. 2022, 41, 3879–3885. [Google Scholar] [CrossRef]
- Andreica, I.; Blazquez-Navarro, A.; Sokolar, J.; Anft, M.; Kiltz, U.; Pfaender, S.; Vidal Blanco, E.; Westhoff, T.; Babel, N.; Stervbo, U.; et al. Different Humoral but Similar Cellular Responses of Patients with Autoimmune Inflammatory Rheumatic Diseases under Disease-Modifying Antirheumatic Drugs after COVID-19 Vaccination. RMD Open 2022, 8, e002293. [Google Scholar] [CrossRef]
- Agrati, C.; Castilletti, C.; Goletti, D.; Meschi, S.; Sacchi, A.; Matusali, G.; Bordoni, V.; Petrone, L.; Lapa, D.; Notari, S.; et al. Coordinate Induction of Humoral and Spike Specific T-Cell Response in a Cohort of Italian Health Care Workers Receiving BNT162b2 MRNA Vaccine. Microorganisms 2021, 9, 1315. [Google Scholar] [CrossRef]
- Holder, K.A.; Ings, D.P.; Harnum, D.O.A.; Russell, R.S.; Grant, M.D. Moderate to Severe SARS-CoV-2 Infection Primes Vaccine-Induced Immunity More Effectively than Asymptomatic or Mild Infection. NPJ Vaccines 2022, 7, 122. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zhang, X.; Lin, Y.; Liu, D.; Xun, J.; Wang, Z.; Gu, L.; Li, Q.; Yin, D.; et al. Decline in Neutralising Antibody Responses, but Sustained T-Cell Immunity, in COVID-19 Patients at 7 Months Post-Infection. Clin. Transl. Immunol. 2021, 10, e1319. [Google Scholar] [CrossRef]
- Waickman, A.T.; Lu, J.; Chase, C.; Fang, H.; McDowell, E.; Bingham, E.; Bogart, J.; Graziano, S.; Thomas, S.J.; Gentile, T. Systemic Cancer Therapy Does Not Significantly Impact Early Vaccine-Elicited SARS-CoV-2 Immunity in Patients with Solid Tumors. Vaccines 2022, 10, 738. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, S.J.; Yu, J.; Olson, D.; Zha, Y.; Pezeshk, A.; Cabanov, A.; Pyzer, A.R.; Trujillo, J.; Derman, B.A.; O’Donnell, P.; et al. Antibody and T Cell Responses to COVID-19 Vaccination in Patients Receiving Anticancer Therapies. J. Immunother. Cancer 2022, 10, e004766. [Google Scholar] [CrossRef] [PubMed]
- Tychala, A.; Meletis, G.; Katsimpourlia, E.; Gkeka, I.; Dimitriadou, R.; Sidiropoulou, E.; Skoura, L. Evaluation of the QuantiFERON SARS-CoV-2 Assay to Assess Cellular Immunogenicity of the BNT162b2 MRNA COVID-19 Vaccine in Individuals with Low and High Humoral Response. Hum. Vaccines Immunother. 2021, 17, 5148–5149. [Google Scholar] [CrossRef] [PubMed]
| HCWs | Cancer Patients | p-Value * | |
|---|---|---|---|
| Age, median (IQR) | 44 (34–54) | 56 (49–64) | p < 0.001 |
| Sex, n (%) | |||
| Female | 101 (64.7) | 24 (68.6) | p = 0.667 |
| Male | 55 (35.3) | 11 (31.4) | |
| Vaccination schedule, n (%) | |||
| Only BNT162b2 | 140 (89.7) | 20 (57.1) | p < 0.001 |
| Only mRNA-1273 | 6 (3.9) | 1 (2.9) | |
| Infection and mRNA vaccination | 10 (6.4) | 3 (8.6) | |
| Unknown | 0 (0.0) | 11 (31.4) | |
| Autoimmune diseases, n (%) | |||
| No | 139 (89.1) | 34 (97.1) | p = 0.204 |
| Yes | 17 (10.9) | 1 (2.9) | |
| Anti-inflammatory therapies during vaccination, n (%) | |||
| No | 148 (94.9) | 31 (88.6) | p = 0.238 |
| Yes | 8 (5.1) | 4 (11.4) | |
| Anti-inflammatory therapies during immune response evaluation, n (%) | |||
| No | 144 (92.3) | 32 (91.4) | p = 0.741 |
| Yes | 12 (7.7) | 3 (8.6) | |
| Previous SARS-CoV-2 infection, n (%) | |||
| No | 134 (85.9) | 31 (88.6) | p = 0.791 |
| Yes | 22 (14.1) | 4 (11.4) | |
| Vaccination side effects, n (%) | |||
| None | 31 (22.1) | 11 (47.8) | p = 0.072 |
| Only after 1st dose | 11 (7.9) | 2 (8.7) | |
| Only after 2nd dose | 27 (19.3) | 2 (8.7) | |
| After both doses | 71 (50.7) | 8 (34.8) |
| IgG Levels (AU/mL) | p-Value * | |
|---|---|---|
| IgG_T1, median (IQR) | 568.2 (361.1–924.1) | - |
| IgG_T2, median (IQR) | 98.7 (70.4–179.4) | - |
| IgG_T3, median (IQR) | 779.0 (345.7–1704.0) | - |
| Vaccination side effects, median IgG_T1 (IQR) | ||
| No | 362.1 (258.4–539.4) | p < 0.001 |
| Yes | 728.6 (420.6–976.1) | |
| Vaccination side effects, median IgG_T2 (IQR) | ||
| No | 84.2 (53.3–99.2) | p < 0.001 |
| Yes | 118.6 (74.5–250.5) | |
| Vaccination side effects, median IgG_T3 (IQR) | ||
| No | 575.8 (290.9–852.7) | p = 0.032 |
| Yes | 1048.3 (594.1–1750.5) | |
| Previous SARS-CoV-2 infection, median IgG_T2 (IQR) | ||
| No | 94.3 (70.0–161.8) | p < 0.001 |
| Yes | 248.1 (130.1–868.0) |
| QF Qualitative Results | |||
|---|---|---|---|
| Global | Ag1 | Ag2 | |
| IgG_T1, median (IQR) * | |||
| HCWs | |||
| QF_T2 Neg | 419.8 (304.5–736.0) | 426.3 (298.8–735.3) | 452.0 (315.8–751.6) |
| QF_T2 Pos | 705.9 (415.4–1193.1) | 778.6 (453.1–1355.0) | 642.0 (412.6–1193.1) |
| p-value | p < 0.001 | p < 0.001 | p < 0.001 |
| Infection-naïve HCWs | |||
| QF_T2 Neg | 432.7 (298.8–736.0) | 436.0 (290.7–732.5) | 458.4 (304.5–741.0) |
| QF_T2 Pos | 609.0 (389.8–970.4) | 677.8 (415.4–976.1) | 579.1 (389.4–970.1) |
| p-value | p = 0.004 | p < 0.001 | p = 0.015 |
| IgG_T2, median (IQR)* | |||
| HCWs | |||
| QF_T2 Neg | 81.3 (44.5–139.2) | 84.7 (47.1–130.1) | 81.9 (45.0–139.2) |
| QF_T2 Pos | 125.6 (85.2–247.0) | 146.0 (85.7–274.0) | 129.8 (85.2–248.1) |
| p-value | p < 0.001 | p < 0.001 | p < 0.001 |
| Infection-naïve HCWs | |||
| QF_T2 Neg | 81.6 (44.0–133.7) | 84.5 (47.1–128.1) | 82.4 (45.5–133.7) |
| QF_T2 Pos | 114.9 (80.5–173.2) | 118.4 (80.5–228.1) | 116.9 (78.3–173.2) |
| p-value | p = 0.004 | p < 0.001 | p = 0.002 |
| Cancer patients | |||
| QF_T2 Neg | 30.4 (12.6–123.3) | 36.3 (12.9–102.7) | 30.4 (12.6–123.3) |
| QF_T2 Pos | 79.0 (41.3–715.2) | 126.4 (36.2–935.1) | 79.1 (41.3–715.2) |
| p-value | p = 0.069 | p = 0.082 | p = 0.069 |
| IgG_T3, median (IQR) * | |||
| HCWs | |||
| QF_T3 Neg | 475.3 (250.1–850.3) | 569.6 (290.9–852.7) | 475.2 (256.1–847.9) |
| QF_T3 Pos | 1120.0 (555.1–2018.1) | 1136.1 (633.1–1711.5) | 1132.6 (562.3–2249.9) |
| p-value ** | p = 0.021 | p = 0.036 | p = 0.008 |
| Infection-naïve HCWs | |||
| QF_T3 Neg | 475.3 (250.1–850.3) | 569.6 (290.9–852.7) | 475.3 (256.1–847.9) |
| QF_T3 Pos | 1120 (569.6–2018.1) | 1136.1 (638.9–1711.5) | 1132.6 (601.4–2249.9) |
| p-value ** | p = 0.027 | p = 0.042 | p = 0.010 |
| Adjusted Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|
| IgG_T1 (HCWs) | |||
| I tertile | ref | ||
| II-III tertiles | 2.29 | 1.10–4.73 | p = 0.026 |
| Age | 0.99 | 0.97–1.03 | p = 0.894 |
| IgG_T2 (HCWs) | |||
| I tertile | ref | ||
| II-III tertiles | 3.81 | 1.70–8.53 | p = 0.001 |
| Age | 1.01 | 0.98–1.04 | p = 0.429 |
| IgG_T2 (Cancer patients) | |||
| I tertile | ref | ||
| II-III tertiles | 2.41 | 0.56–10.44 | p = 0.239 |
| Age | 0.92 | 0.84–1.01 | p = 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brisotto, G.; Montico, M.; Turetta, M.; Zanussi, S.; Cozzi, M.R.; Vettori, R.; Boschian Boschin, R.; Vinante, L.; Matrone, F.; Revelant, A.; et al. Integration of Cellular and Humoral Immune Responses as an Immunomonitoring Tool for SARS-CoV-2 Vaccination in Healthy and Fragile Subjects. Viruses 2023, 15, 1276. https://doi.org/10.3390/v15061276
Brisotto G, Montico M, Turetta M, Zanussi S, Cozzi MR, Vettori R, Boschian Boschin R, Vinante L, Matrone F, Revelant A, et al. Integration of Cellular and Humoral Immune Responses as an Immunomonitoring Tool for SARS-CoV-2 Vaccination in Healthy and Fragile Subjects. Viruses. 2023; 15(6):1276. https://doi.org/10.3390/v15061276
Chicago/Turabian StyleBrisotto, Giulia, Marcella Montico, Matteo Turetta, Stefania Zanussi, Maria Rita Cozzi, Roberto Vettori, Romina Boschian Boschin, Lorenzo Vinante, Fabio Matrone, Alberto Revelant, and et al. 2023. "Integration of Cellular and Humoral Immune Responses as an Immunomonitoring Tool for SARS-CoV-2 Vaccination in Healthy and Fragile Subjects" Viruses 15, no. 6: 1276. https://doi.org/10.3390/v15061276
APA StyleBrisotto, G., Montico, M., Turetta, M., Zanussi, S., Cozzi, M. R., Vettori, R., Boschian Boschin, R., Vinante, L., Matrone, F., Revelant, A., Palazzari, E., Innocente, R., Fanetti, G., Gerratana, L., Garutti, M., Lisanti, C., Bolzonello, S., Nicoloso, M. S., Steffan, A., & Muraro, E. (2023). Integration of Cellular and Humoral Immune Responses as an Immunomonitoring Tool for SARS-CoV-2 Vaccination in Healthy and Fragile Subjects. Viruses, 15(6), 1276. https://doi.org/10.3390/v15061276








