The S2 Subunit of Infectious Bronchitis Virus Affects Abl2-Mediated Syncytium Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids, Cells, and Viruses
2.2. Biological Properties of Recombinant Strains
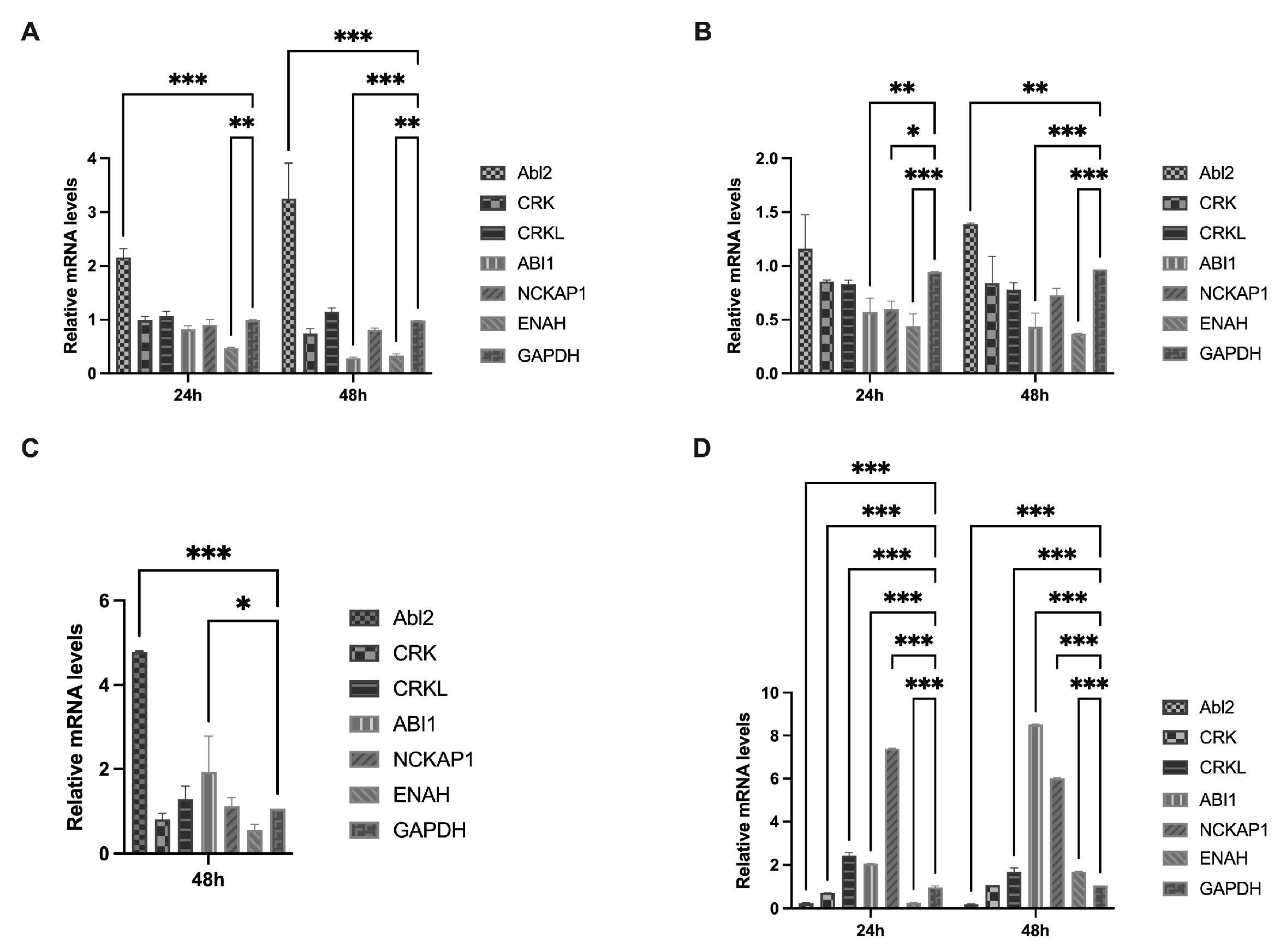
2.3. Analysis of the Relative Transcript Levels of Host Factors
2.4. Transfection of S2 Protein
2.5. RNA Interfering Assay
2.6. Abl2-Mediated Pathways in Mutated Strains
2.7. Affinity Purification and Mass Spectrometry
2.8. Statistical Analysis
3. Results
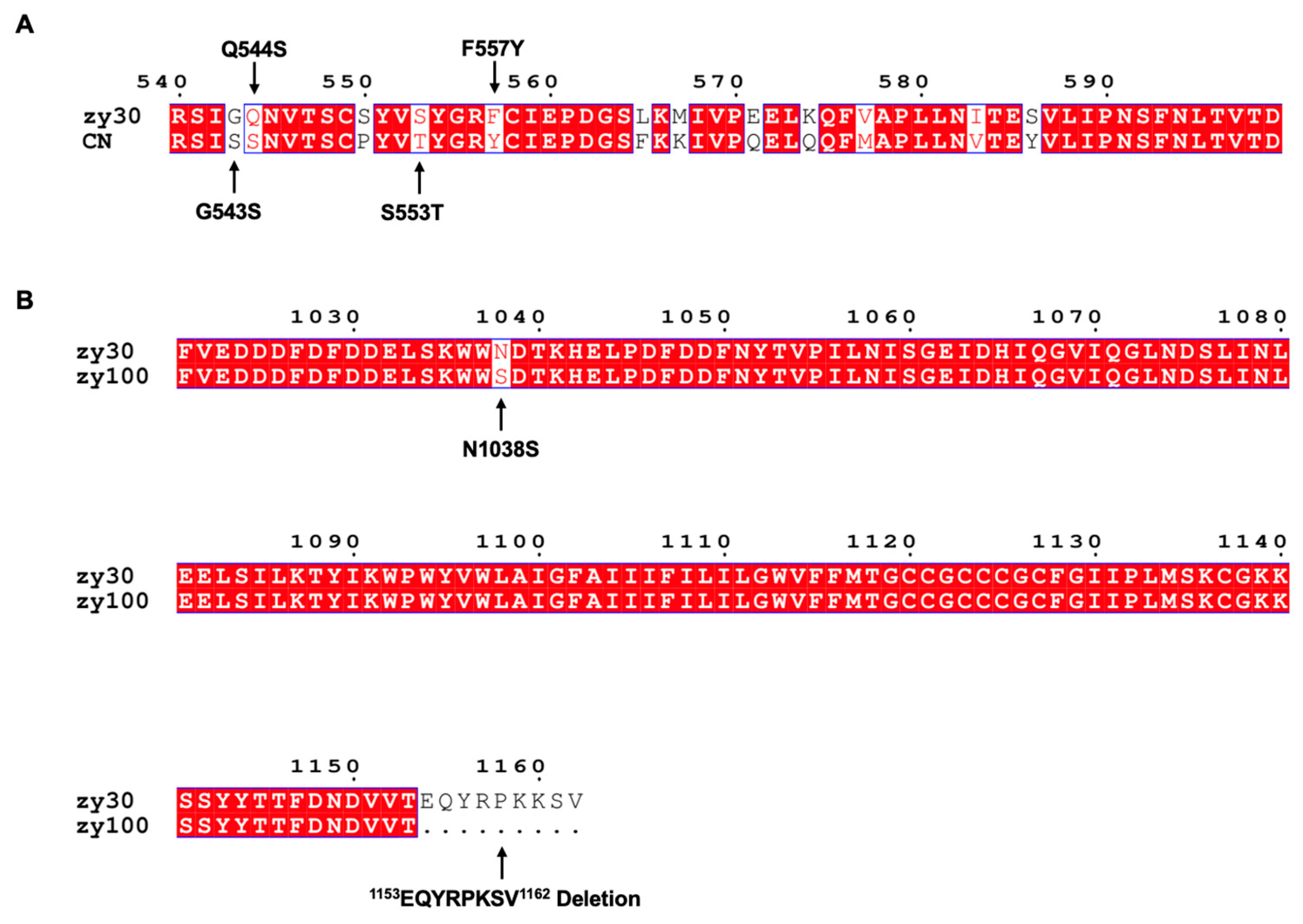
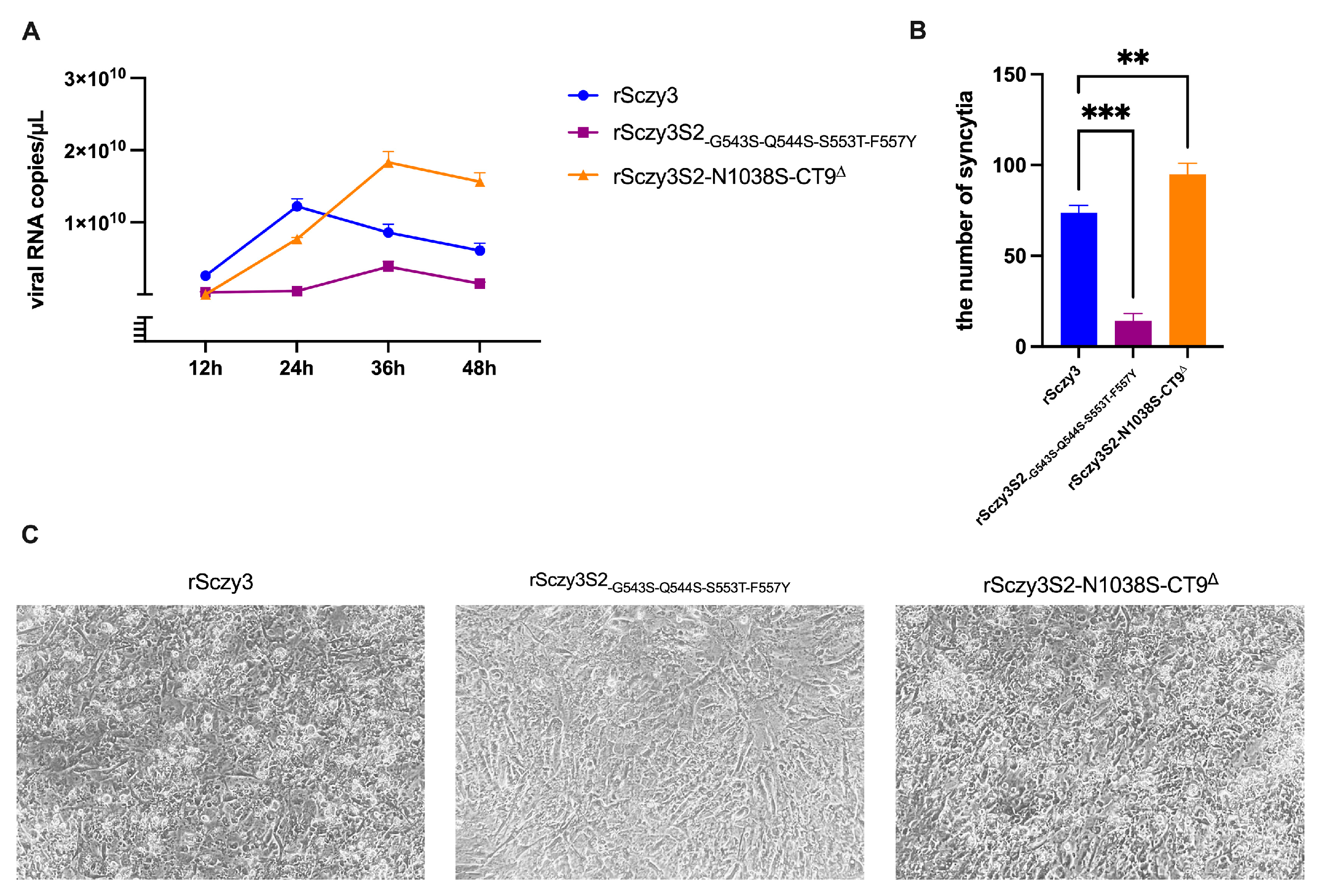
3.1. Mutated Strains Have Different Biological Properties
3.2. IBV Infection and Expression of S2 Protein Activate Abl2
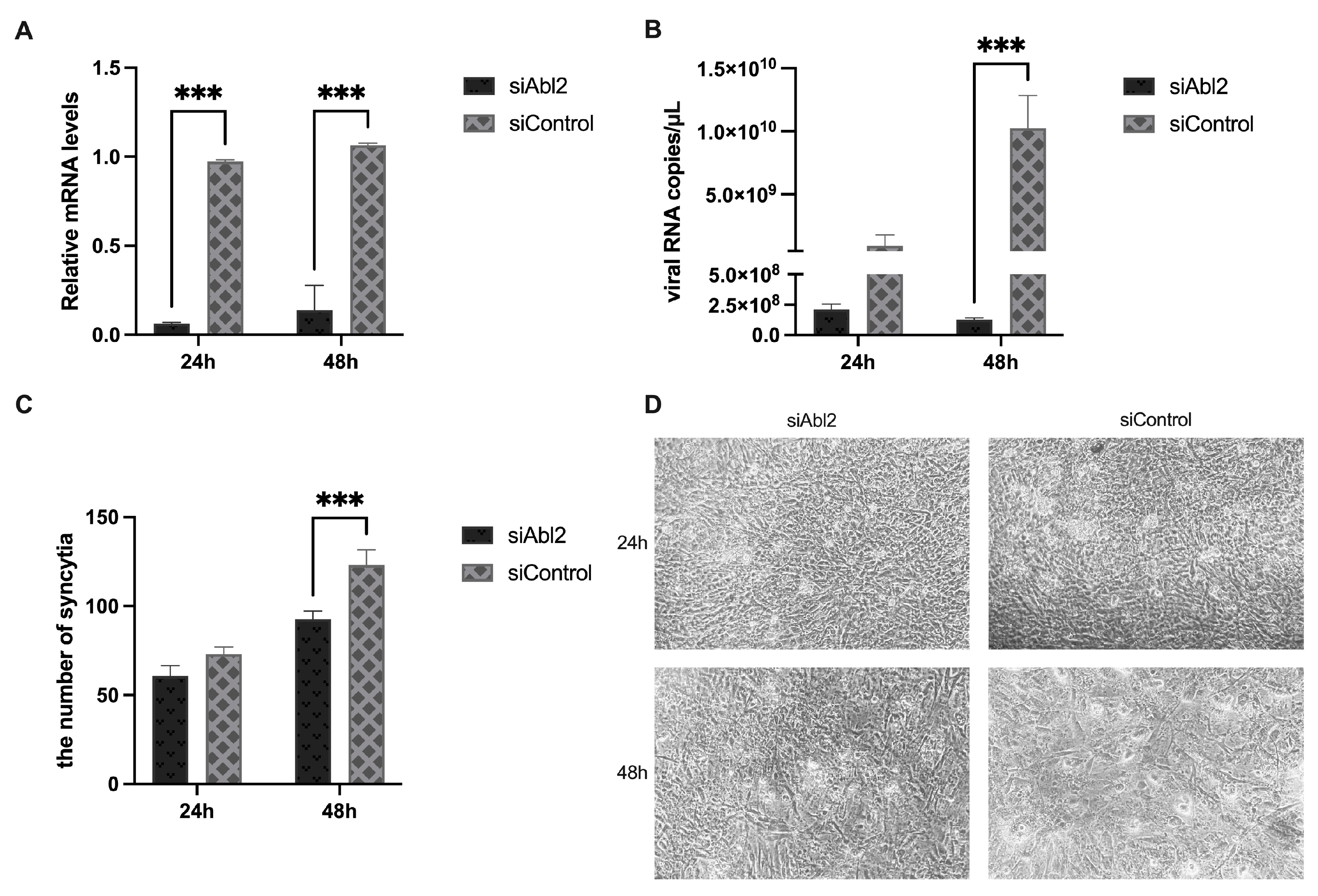
3.3. Abl2 Knockdown Inhibits the Production of Viral Particles and Syncytium
3.4. Multiple Abl2 Signaling Pathways Are Activated in CEKs
3.5. Bioinformatics Analysis of Mass Spectrometry Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wickramasinghe, I.N.; van Beurden, S.J.; Weerts, E.A.; Verheije, M.H. The avian coronavirus spike protein. Virus Res. 2014, 194, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Soma, J.; Takahashi, S.; Matsune, K.; Ono, M.; Oosumi, T. Detection and isolation of QX-like infectious bronchitis virus in Japan. J. Vet. Med. Sci. 2022, 84, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, E.; Paldurai, A.; Manoharan, V.K.; Varghese, B.P.; Samal, S.K. A Recombinant Newcastle Disease Virus (NDV) Expressing S Protein of Infectious Bronchitis Virus (IBV) Protects Chickens against IBV and NDV. Sci. Rep. 2018, 8, 11951. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhao, Y.; Xu, G.; Zhang, K.; Jia, W.; Sun, Y.; Zhao, J.; Xue, J.; Hu, Y.; Zhang, G. The S2 Subunit of QX-type Infectious Bronchitis Coronavirus Spike Protein Is an Essential Determinant of Neurotropism. Viruses 2019, 11, 972. [Google Scholar] [CrossRef]
- Li, S.Y.; Shen, Y.X.; Xiang, X.L.; Li, Y.X.; Li, N.L.; Wang, A.D.; Cui, M.; Han, X.F.; Huang, Y.; Xia, J. The conserved L1089 in the S2 subunit of avian infectious bronchitis virus determines viral kidney tropism by disrupting virus-cell fusion. Vet. Microbiol. 2023, 277, 109619. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Sun, Y.; Mao, X.; Meng, C.; Tan, L.; Song, C.; Qiu, X.; Ding, C.; Liao, Y. Infectious bronchitis virus entry mainly depends on clathrin mediated endocytosis and requires classical endosomal/lysosomal system. Virology 2019, 528, 118–136. [Google Scholar] [CrossRef]
- Bickerton, E.; Maier, H.J.; Stevenson-Leggett, P.; Armesto, M.; Britton, P. The S2 Subunit of Infectious Bronchitis Virus Beaudette Is a Determinant of Cellular Tropism. J. Virol. 2018, 92, e01044-18. [Google Scholar] [CrossRef]
- Li, S.; Fan, S.; Li, N.; Shen, Y.; Xiang, X.; Chen, W.; Xia, J.; Han, X.; Cui, M.; Huang, Y. The N1038S Substitution and (1153)EQTRPKKSV(1162) Deletion of the S2 Subunit of QX-Type Avian Infectious Bronchitis Virus Can Synergistically Enhance Viral Proliferation. Front. Microbiol. 2022, 13, 829218. [Google Scholar] [CrossRef]
- Liu, C.; Ma, Y.; Yang, Y.; Zheng, Y.; Shang, J.; Zhou, Y.; Jiang, S.; Du, L.; Li, J.; Li, F. Cell Entry of Porcine Epidemic Diarrhea Coronavirus Is Activated by Lysosomal Proteases. J. Biol. Chem. 2016, 291, 24779–24786. [Google Scholar] [CrossRef]
- Hashimoto, R.; Sakamoto, A.; Deguchi, S.; Yi, R.; Sano, E.; Hotta, A.; Takahashi, K.; Yamanaka, S.; Takayama, K. Dual inhibition of TMPRSS2 and Cathepsin Bprevents SARS-CoV-2 infection in iPS cells. Mol. Ther. Nucleic Acids 2021, 26, 1107–1114. [Google Scholar] [CrossRef]
- Li, H.; Ni, R.; Wang, K.; Tian, Y.; Gong, H.; Yan, W.; Tang, Y.; Lei, C.; Wang, H.; Yang, X. Chicken interferon-induced transmembrane protein 1 promotes replication of coronavirus infectious bronchitis virus in a cell-specific manner. Vet. Microbiol. 2022, 275, 109597. [Google Scholar] [CrossRef] [PubMed]
- Strobelt, R.; Adler, J.; Paran, N.; Yahalom-Ronen, Y.; Melamed, S.; Politi, B.; Shulman, Z.; Schmiedel, D.; Shaul, Y. Imatinib inhibits SARS-CoV-2 infection by an off-target-mechanism. Sci. Rep. 2022, 12, 5758. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.M.; Sisk, J.M.; Mingo, R.M.; Nelson, E.A.; White, J.M.; Frieman, M.B. Abelson Kinase Inhibitors Are Potent Inhibitors of Severe Acute Respiratory Syndrome Coronavirus and Middle East Respiratory Syndrome Coronavirus Fusion. J. Virol. 2016, 90, 8924–8933. [Google Scholar] [CrossRef] [PubMed]
- Sisk, J.M.; Frieman, M.B.; Machamer, C.E. Coronavirus S protein-induced fusion is blocked prior to hemifusion by Abl kinase inhibitors. J. Gen. Virol. 2018, 99, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Bannach, C.; Brinkert, P.; Kuhling, L.; Greune, L.; Schmidt, M.A.; Schelhaas, M. Epidermal Growth Factor Receptor and Abl2 Kinase Regulate Distinct Steps of Human Papillomavirus 16 Endocytosis. J. Virol. 2020, 94, e02143-19. [Google Scholar] [CrossRef] [PubMed]
- Bamia, A.; Marcato, V.; Boissiere, M.; Mansuroglu, Z.; Tamietti, C.; Romani, M.; Simon, D.; Tian, G.; Niedergang, F.; Panthier, J.J.; et al. The NSs Protein Encoded by the Virulent Strain of Rift Valley Fever Virus Targets the Expression of Abl2 and the Actin Cytoskeleton of the Host, Affecting Cell Mobility, Cell Shape, and Cell-Cell Adhesion. J. Virol. 2020, 95, e01768-20. [Google Scholar] [CrossRef]
- Walsh, D.; Naghavi, M.H. Exploitation of Cytoskeletal Networks during Early Viral Infection. Trends Microbiol. 2019, 27, 39–50. [Google Scholar] [CrossRef]
- Wu, K.; Wu, H.; Lyu, W.; Kim, Y.; Furdui, C.M.; Anderson, K.S.; Koleske, A.J. Platelet-derived growth factor receptor beta activates Abl2 via direct binding and phosphorylation. J. Biol. Chem. 2021, 297, 100883. [Google Scholar] [CrossRef]
- Colicelli, J. ABL tyrosine kinases: Evolution of function, regulation, and specificity. Sci. Signal 2010, 3, re6. [Google Scholar] [CrossRef]
- Sato, M.; Maruoka, M.; Takeya, T. Functional mechanisms and roles of adaptor proteins in abl-regulated cytoskeletal actin dynamics. J. Signal Transduct. 2012, 2012, 414913. [Google Scholar] [CrossRef]
- Hossain, S.; Dubielecka, P.M.; Sikorski, A.F.; Birge, R.B.; Kotula, L. Crk and ABI1: Binary molecular switches that regulate abl tyrosine kinase and signaling to the cytoskeleton. Genes Cancer 2012, 3, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, D. The adaptor protein Crk in immune response. Immunol. Cell Biol. 2014, 92, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Quiles, N.; Feuerbacher, L.A.; Benito-Leon, M.; Hardwidge, P.R. Contribution of Crk adaptor proteins to host cell and bacteria interactions. Biomed. Res. Int. 2014, 2014, 372901. [Google Scholar] [CrossRef] [PubMed]
- Park, T. Crk and CrkL as Therapeutic Targets for Cancer Treatment. Cells 2021, 10, 739. [Google Scholar] [CrossRef]
- Wang, T.; Cleary, R.A.; Wang, R.; Tang, D.D. Role of the adapter protein Abi1 in actin-associated signaling and smooth muscle contraction. J. Biol. Chem. 2013, 288, 20713–20722. [Google Scholar] [CrossRef]
- Sekino, S.; Kashiwagi, Y.; Kanazawa, H.; Takada, K.; Baba, T.; Sato, S.; Inoue, H.; Kojima, M.; Tani, K. The NESH/Abi-3-based WAVE2 complex is functionally distinct from the Abi-1-based WAVE2 complex. Cell Commun. Signal 2015, 13, 41. [Google Scholar] [CrossRef]
- Whitelaw, J.A.; Swaminathan, K.; Kage, F.; Machesky, L.M. The WAVE Regulatory Complex Is Required to Balance Protrusion and Adhesion in Migration. Cells 2020, 9, 1635. [Google Scholar] [CrossRef]
- Chen, J.; Ge, J.; Zhang, W.; Xie, X.; Zhong, X.; Tang, S. NCKAP1 is a Prognostic Biomarker for Inhibition of Cell Growth in Clear Cell Renal Cell Carcinoma. Front. Genet 2022, 13, 764957. [Google Scholar] [CrossRef]
- Kwon, M.R.; Lee, J.H.; Park, J.; Park, S.S.; Ju, E.J.; Ko, E.J.; Shin, S.H.; Son, G.W.; Lee, H.W.; Kim, Y.J.; et al. NCK-associated protein 1 regulates metastasis and is a novel prognostic marker for colorectal cancer. Cell Death Discov. 2023, 9, 7. [Google Scholar] [CrossRef]
- Yamamoto, A.; Suzuki, T.; Sakaki, Y. Isolation of hNap1BP which interacts with human Nap1 (NCKAP1) whose expression is down-regulated in Alzheimer’s disease. Gene 2001, 271, 159–169. [Google Scholar] [CrossRef]
- Young, L.E.; Latario, C.J.; Higgs, H.N. Roles for Ena/VASP proteins in FMNL3-mediated filopodial assembly. J. Cell Sci. 2018, 131, jcs220814. [Google Scholar] [CrossRef] [PubMed]
- Skruber, K.; Warp, P.V.; Shklyarov, R.; Thomas, J.D.; Swanson, M.S.; Henty-Ridilla, J.L.; Read, T.A.; Vitriol, E.A. Arp2/3 and Mena/VASP Require Profilin 1 for Actin Network Assembly at the Leading Edge. Curr. Biol. 2020, 30, 2651–2664.e5. [Google Scholar] [CrossRef] [PubMed]
- Zou, N.L.; Zhao, F.F.; Wang, Y.P.; Liu, P.; Cao, S.J.; Wen, X.T.; Huang, Y. Genetic analysis revealed LX4 genotype strains of avian infectious bronchitis virus became predominant in recent years in Sichuan area, China. Virus Genes 2010, 41, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; He, X.; Du, L.J.; Liu, Y.Y.; You, G.J.; Li, S.Y.; Liu, P.; Cao, S.J.; Han, X.F.; Huang, Y. Preparation and protective efficacy of a chicken embryo kidney cell-attenuation GI-19/QX-like avian infectious bronchitis virus vaccine. Vaccine 2018, 36, 4087–4094. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Watanabe, Y.; Moriya, Y.; Kawano, S.; Yamamoto, T.; Matsumoto, M.; Takami, T.; Kobayashi, D.; Araki, N.; Yoshizawa, A.C.; et al. jPOSTrepo: An international standard data repository for proteomes. Nucleic Acids Res. 2017, 45, D1107–D1111. [Google Scholar] [CrossRef] [PubMed]
- de Wit, J.J.S.; Cook, J.K.A. Spotlight on avian pathology: Infectious bronchitis virus. Avian Pathol. 2019, 48, 393–395. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, M.; Zhao, J.; Wu, Y. Avian Infectious Bronchitis in China: Epidemiology, Vaccination, and Control. Avian Dis. 2021, 65, 652–656. [Google Scholar] [CrossRef]
- Chen, D.; Xu, L.; Li, X.; Chu, Y.; Jiang, M.; Xu, B.; Zhao, M.; Wang, W.; Wang, H.; Kang, H.; et al. Enah overexpression is correlated with poor survival and aggressive phenotype in gastric cancer. Cell Death Dis. 2018, 9, 998. [Google Scholar] [CrossRef]
- Kapoor, M.; Chinnathambi, S. TGF-beta1 signalling in Alzheimer’s pathology and cytoskeletal reorganization: A specialized Tau perspective. J. Neuroinflamm. 2023, 20, 72. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Laufer, S. Kinases as Potential Therapeutic Targets for Anti-coronaviral Therapy. J. Med. Chem. 2022, 65, 955–982. [Google Scholar] [CrossRef]
- Cagno, V.; Magliocco, G.; Tapparel, C.; Daali, Y. The tyrosine kinase inhibitor nilotinib inhibits SARS-CoV-2 in vitro. Basic Clin. Pharmacol. Toxicol. 2021, 128, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Wang, J.; Pendergast, A.M. Multifunctional Abl kinases in health and disease. J. Cell Sci. 2016, 129, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mooren, O.L.; Galletta, B.J.; Cooper, J.A. Roles for actin assembly in endocytosis. Annu. Rev. Biochem. 2012, 81, 661–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y. The capable ABL: What is its biological function? Mol. Cell Biol. 2014, 34, 1188–1197. [Google Scholar] [CrossRef]
- Narvaez-Ortiz, H.Y.; Nolen, B.J. Unconcerted conformational changes in Arp2/3 complex integrate multiple activating signals to assemble functional actin networks. Curr. Biol. 2022, 32, 975–987.e6. [Google Scholar] [CrossRef]
- Rottner, K.; Stradal, T.E.B.; Chen, B. WAVE regulatory complex. Curr. Biol. 2021, 31, R512–R517. [Google Scholar] [CrossRef]
- Burke, T.A.; Christensen, J.R.; Barone, E.; Suarez, C.; Sirotkin, V.; Kovar, D.R. Homeostatic actin cytoskeleton networks are regulated by assembly factor competition for monomers. Curr. Biol. 2014, 24, 579–585. [Google Scholar] [CrossRef]
- Chen, X.J.; Squarr, A.J.; Stephan, R.; Chen, B.; Higgins, T.E.; Barry, D.J.; Martin, M.C.; Rosen, M.K.; Bogdan, S.; Way, M. Ena/VASP proteins cooperate with the WAVE complex to regulate the actin cytoskeleton. Dev. Cell 2014, 30, 569–584. [Google Scholar] [CrossRef]
- Shang, J.; Zheng, Y.; Yang, Y.; Liu, C.; Geng, Q.; Luo, C.; Zhang, W.; Li, F. Cryo-EM structure of infectious bronchitis coronavirus spike protein reveals structural and functional evolution of coronavirus spike proteins. PLoS Pathog. 2018, 14, e1007009. [Google Scholar] [CrossRef]
- Suarez, C.; Carroll, R.T.; Burke, T.A.; Christensen, J.R.; Bestul, A.J.; Sees, J.A.; James, M.L.; Sirotkin, V.; Kovar, D.R. Profilin regulates F-actin network homeostasis by favoring formin over Arp2/3 complex. Dev. Cell 2015, 32, 43–53. [Google Scholar] [CrossRef]
- Kadzik, R.S.; Homa, K.E.; Kovar, D.R. F-Actin Cytoskeleton Network Self-Organization Through Competition and Cooperation. Annu. Rev. Cell Dev. Biol. 2020, 36, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Vehlow, A.; Soong, D.; Vizcay-Barrena, G.; Bodo, C.; Law, A.L.; Perera, U.; Krause, M. Endophilin, Lamellipodin, and Mena cooperate to regulate F-actin-dependent EGF-receptor endocytosis. EMBO J. 2013, 32, 2722–2734. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Luo, Y.; Zhang, Y.; Zhang, J.; He, Z. Enabled homolog (ENAH) regulated by RNA binding protein splicing factor 3b subunit 4 (SF3B4) exacerbates the proliferation, invasion and migration of hepatocellular carcinoma cells via Notch signaling pathway. Bioengineered 2022, 13, 2194–2206. [Google Scholar] [CrossRef] [PubMed]
- Havrylenko, S.; Noguera, P.; Abou-Ghali, M.; Manzi, J.; Faqir, F.; Lamora, A.; Guerin, C.; Blanchoin, L.; Plastino, J. WAVE binds Ena/VASP for enhanced Arp2/3 complex-based actin assembly. Mol. Biol. Cell 2015, 26, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Rotty, J.D.; Wu, C.; Haynes, E.M.; Suarez, C.; Winkelman, J.D.; Johnson, H.E.; Haugh, J.M.; Kovar, D.R.; Bear, J.E. Profilin-1 serves as a gatekeeper for actin assembly by Arp2/3-dependent and -independent pathways. Dev. Cell 2015, 32, 54–67. [Google Scholar] [CrossRef]


| Gene | Accession No. | Forward 5′→3′ | Reverse 5′→3′ |
|---|---|---|---|
| CTSL | AA495703.1 | TGGCTTTGTTGACATCCCTC | AGTGTCCTGCATCAATAGCAA |
| CTSB | NM_205371.3 | CAAGCACTACGGCATCACA | TCCTTCAACTGGGCCGTTC |
| Abl1 | XM_040685978.2 | CTGGCAAGAACCTCTACACC | CTTATTGATGGCCTCCCGAA |
| Abl2 | XM_040677820.2 | GACAACACGCTCAGTATCACC | CCCTGCCCATTCTTCGAAC |
| IFITM3 | KC876032.1 | CGCTGTACGCCAACGTCTGCT | CCGAGGACTTTGCGATCCCT |
| ADAM17 | NM_001008682.2 | AGCTCCAAAGATACCTGCAA | CTGCTCTATTTGTATGCCGTA |
| TMPRSS2 | XM_416737.8 | ACAGCAATATCTTCCTGCAAC | ACTTCCGTTTTATGTCGCATC |
| NRP1 | NM_204782.1 | CCCCACTGATGTTGTTTACAGA | TTCAAATCTCAGCGATACACC |
| CD74 | NM_001001613.2 | AAGAACGAGCCATTAATGCTT | CTTCAACCTACACAGGGGTCA |
| GAPDH | NC_052532.1 | GGTGGTGCTAAGCGTGTTA | CCCTCCACAATGCCAA |
| CRK | NM_001353939.2 | CACTCCGCTCCCTAACCTTC | GGCTGTCTTGTCGTAGGCAT |
| CRKL | XM_415233.8 | CATCCACGCAGAACGGACCA | ATCTCCAACCTCTAATGCCAGT |
| ABI1 | NM_001039281.3 | GTGCCCATTTCTTCAGGCCAT | TGCCATCAGGTAAGACATACTGC |
| NCKAP1 | XM_040676557.2 | GCAGCTTTATTTACCATCCAC | AGTAGTTTTGTCCGTCTCCTG |
| ENAH | NM_204300.2 | CCCTAATGCCTCGACTCCCTC | CTGCCTGCAAGACTGGCTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Shen, Y.; Li, S.; Xiang, X.; Li, N.; Li, Y.; Xu, J.; Cui, M.; Han, X.; Xia, J.; et al. The S2 Subunit of Infectious Bronchitis Virus Affects Abl2-Mediated Syncytium Formation. Viruses 2023, 15, 1246. https://doi.org/10.3390/v15061246
Fan S, Shen Y, Li S, Xiang X, Li N, Li Y, Xu J, Cui M, Han X, Xia J, et al. The S2 Subunit of Infectious Bronchitis Virus Affects Abl2-Mediated Syncytium Formation. Viruses. 2023; 15(6):1246. https://doi.org/10.3390/v15061246
Chicago/Turabian StyleFan, Shunyi, Yuxi Shen, Shuyun Li, Xuelian Xiang, Nianling Li, Yongxin Li, Jing Xu, Min Cui, Xinfeng Han, Jing Xia, and et al. 2023. "The S2 Subunit of Infectious Bronchitis Virus Affects Abl2-Mediated Syncytium Formation" Viruses 15, no. 6: 1246. https://doi.org/10.3390/v15061246
APA StyleFan, S., Shen, Y., Li, S., Xiang, X., Li, N., Li, Y., Xu, J., Cui, M., Han, X., Xia, J., & Huang, Y. (2023). The S2 Subunit of Infectious Bronchitis Virus Affects Abl2-Mediated Syncytium Formation. Viruses, 15(6), 1246. https://doi.org/10.3390/v15061246





