Changes in HRSV Epidemiology but Not Circulating Variants in Hospitalized Children due to the Emergence of SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Preparation, Nucleic Acid Extraction, and Diagnostic Real-Time RT-PCR
2.3. HRSV Hypervariable G Gene Region Nested RT-PCR and Sanger Sequencing
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L.; et al. ICTV Virus Taxonomy Profile: Pneumoviridae. J. Gen. Virol. 2017, 98, 2912–2913. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Goya, S.; Galiano, M.; Nauwelaers, I.; Trento, A.; Openshaw, P.J.; Mistchenko, A.S.; Zambon, M.; Viegas, M. Toward unified molecular surveillance of RSV: A proposal for genotype definition. Influenza Other Respir. Viruses 2020, 14, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Eshaghi, A.; Duvvuri, V.R.; Lai, R.; Nadarajah, J.T.; Li, A.; Patel, S.N.; Low, D.E.; Gubbay, J.B. Genetic variability of human respiratory syncytial virus A strains circulating in Ontario: A novel genotype with a 72 nucleotide G gene duplication. PLoS ONE 2012, 7, e32807. [Google Scholar] [CrossRef] [PubMed]
- Trento, A.; Galiano, M.; Videla, C.; Carballal, G.; Garcia-Barreno, B.; Melero, J.A.; Palomo, C. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. J. Gen. Virol. 2003, 84, 3115–3120. [Google Scholar] [CrossRef] [PubMed]
- Morley, C.; Grimwood, K.; Maloney, S.; Ware, R.S. Meteorological factors and respiratory syncytial virus seasonality in subtropical Australia. Epidemiol. Infect. 2018, 146, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Nenna, R.; Evangelisti, M.; Frassanito, A.; Scagnolari, C.; Pierangeli, A.; Antonelli, G.; Nicolai, A.; Arima, S.; Moretti, C.; Papoff, P.; et al. Respiratory syncytial virus bronchiolitis, weather conditions and air pollution in an Italian urban area: An observational study. Environ. Res. 2017, 158, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Ursic, T.; Kogoj, R.; Erzar, K.; Virant, M.J.; Petrovec, M. Respiratory Viruses in Hospitalized Patients before and after SARS-CoV-2. J. Biomed. Res. Environ. Sci. 2022, 3, 703–711. [Google Scholar] [CrossRef]
- Trento, A.; Casas, I.; Calderon, A.; Garcia-Garcia, M.L.; Calvo, C.; Perez-Brena, P.; Melero, J.A. Ten years of global evolution of the human respiratory syncytial virus BA genotype with a 60-nucleotide duplication in the G protein gene. J. Virol. 2010, 84, 7500–7512. [Google Scholar] [CrossRef] [PubMed]
- Cane, P.A. Molecular epidemiology of respiratory syncytial virus. Rev. Med. Virol. 2001, 11, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Jevsnik Virant, M.; Ursic, T.; Kogoj, R.; Korva, M.; Petrovec, M.; Avsic-Zupanc, T. Evaluation of Two Broadly Used Commercial Methods for Detection of Respiratory Viruses with a Recently Added New Target for Detection of SARS-CoV-2. Viruses 2022, 14, 1530. [Google Scholar] [CrossRef] [PubMed]
- Slovic, A.; Ivancic-Jelecki, J.; Ljubin-Sternak, S.; Galinovic, G.M.; Forcic, D. A molecular epidemiological study of human respiratory syncytial virus in Croatia, 2011–2014. Infect. Genet. Evol. 2016, 44, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Aksamentov, I.; Roemer, C.; Hodcroft, E.B.; Neher, R.A. Nextclade: Clade assignment, mutation calling and quality control for viral genomes. J. Open Source Softw. 2021, 6, 3773. [Google Scholar] [CrossRef]
- Dolores, A.; Stephanie, G.; Mercedes, S.N.; Erica, G.; Mistchenko, A.S.; Mariana, V. RSV reemergence in Argentina since the SARS-CoV-2 pandemic. J. Clin. Virol. 2022, 149, 105126. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.A.; Phuong, L.K.; Peplinski, J.; Lim, S.M.; Lee, W.H.; Farhat, A.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; et al. Examining the interseasonal resurgence of respiratory syncytial virus in Western Australia. Arch. Dis. Child. 2022, 107, e7. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, L.; Sheppard, M.; Smith, A.; Dietz, S.; Jayanthi, P.; Yuan, Y.; Bull, L.; Wotiz, S.; Schwarze, T.; Azondekon, R.; et al. Changes in Seasonal Respiratory Illnesses in the United States During the Coronavirus Disease 2019 (COVID-19) Pandemic. Clin. Infect. Dis. 2021, 73, S110–S117. [Google Scholar] [CrossRef] [PubMed]
- Eden, J.S.; Sikazwe, C.; Xie, R.; Deng, Y.M.; Sullivan, S.G.; Michie, A.; Levy, A.; Cutmore, E.; Blyth, C.C.; Britton, P.N.; et al. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat. Commun. 2022, 13, 2884. [Google Scholar] [CrossRef] [PubMed]
- Rabbone, I.; Monzani, A.; Scaramuzza, A.E.; Cavalli, C. See-sawing COVID-19 and RSV bronchiolitis in children under 2 years of age in Northern Italy. Acta Paediatr. 2022, 111, 2174–2175. [Google Scholar] [CrossRef] [PubMed]
- Comas-Garcia, A.; Noyola, D.E.; Cadena-Mota, S.; Rico-Hernandez, M.; Bernal-Silva, S. Respiratory Syncytial Virus-A ON1 Genotype Emergence in Central Mexico in 2009 and Evidence of Multiple Duplication Events. J. Infect. Dis. 2018, 217, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Kamau, E.; Otieno, J.R.; Lewa, C.S.; Mwema, A.; Murunga, N.; Nokes, D.J.; Agoti, C.N. Evolution of respiratory syncytial virus genotype BA in Kilifi, Kenya, 15 years on. Sci. Rep. 2020, 10, 21176. [Google Scholar] [CrossRef] [PubMed]
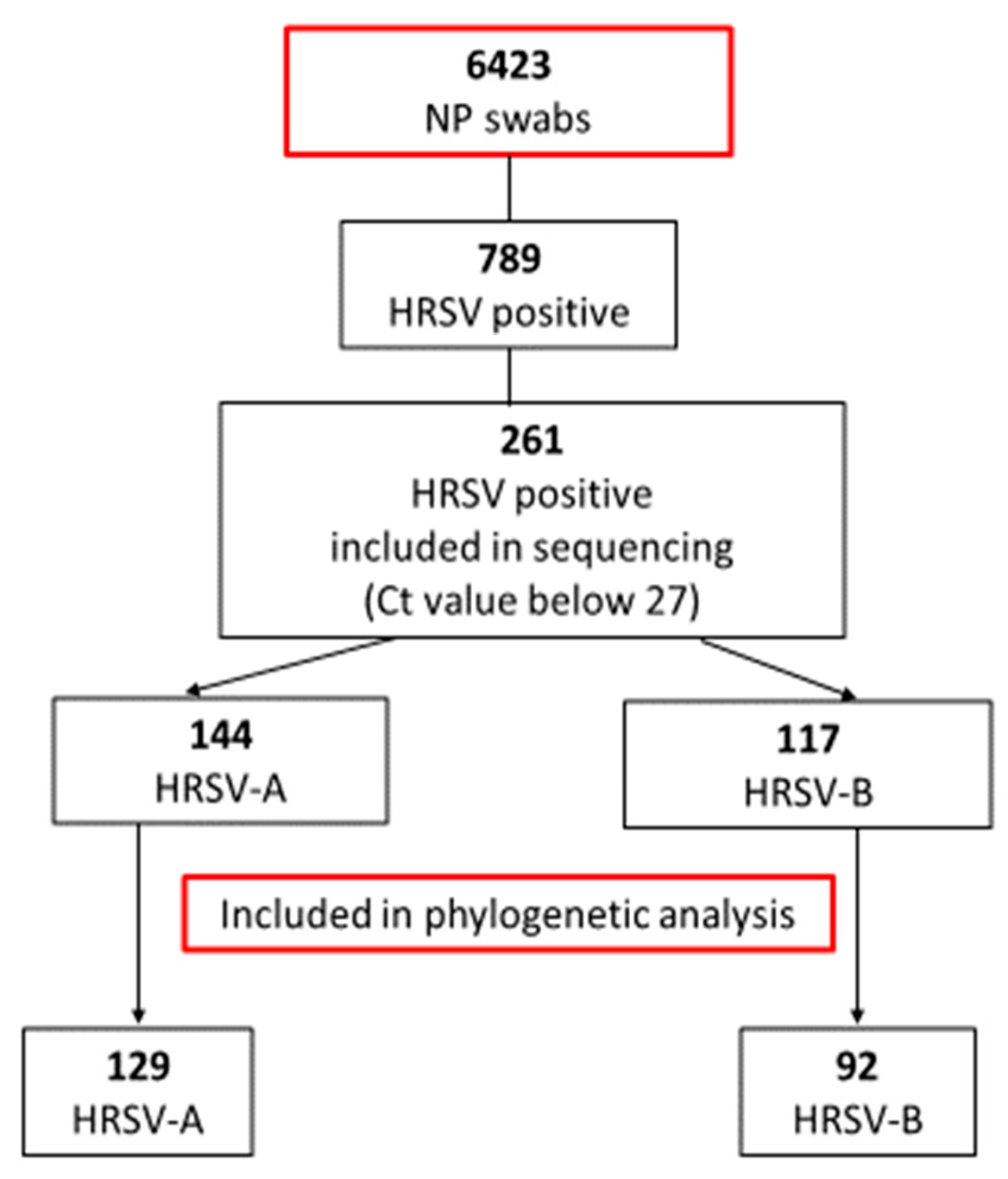
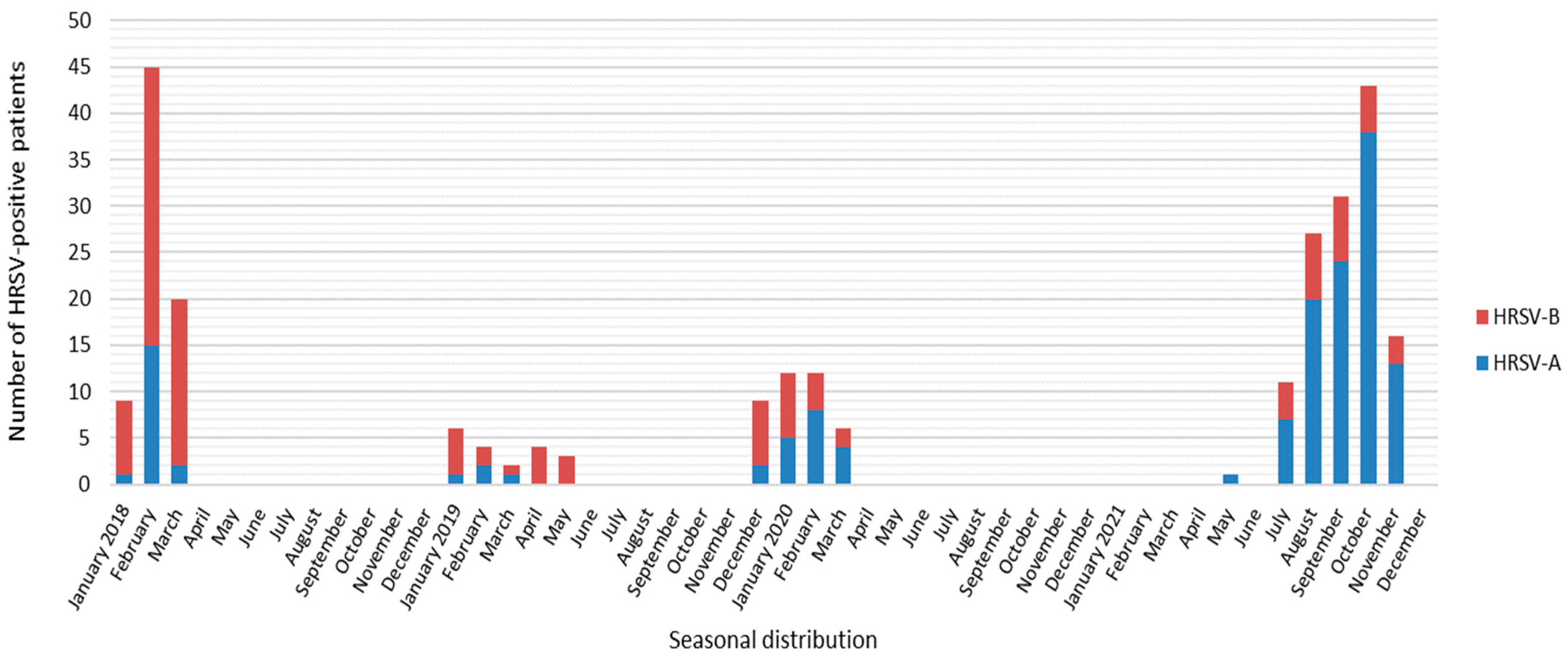

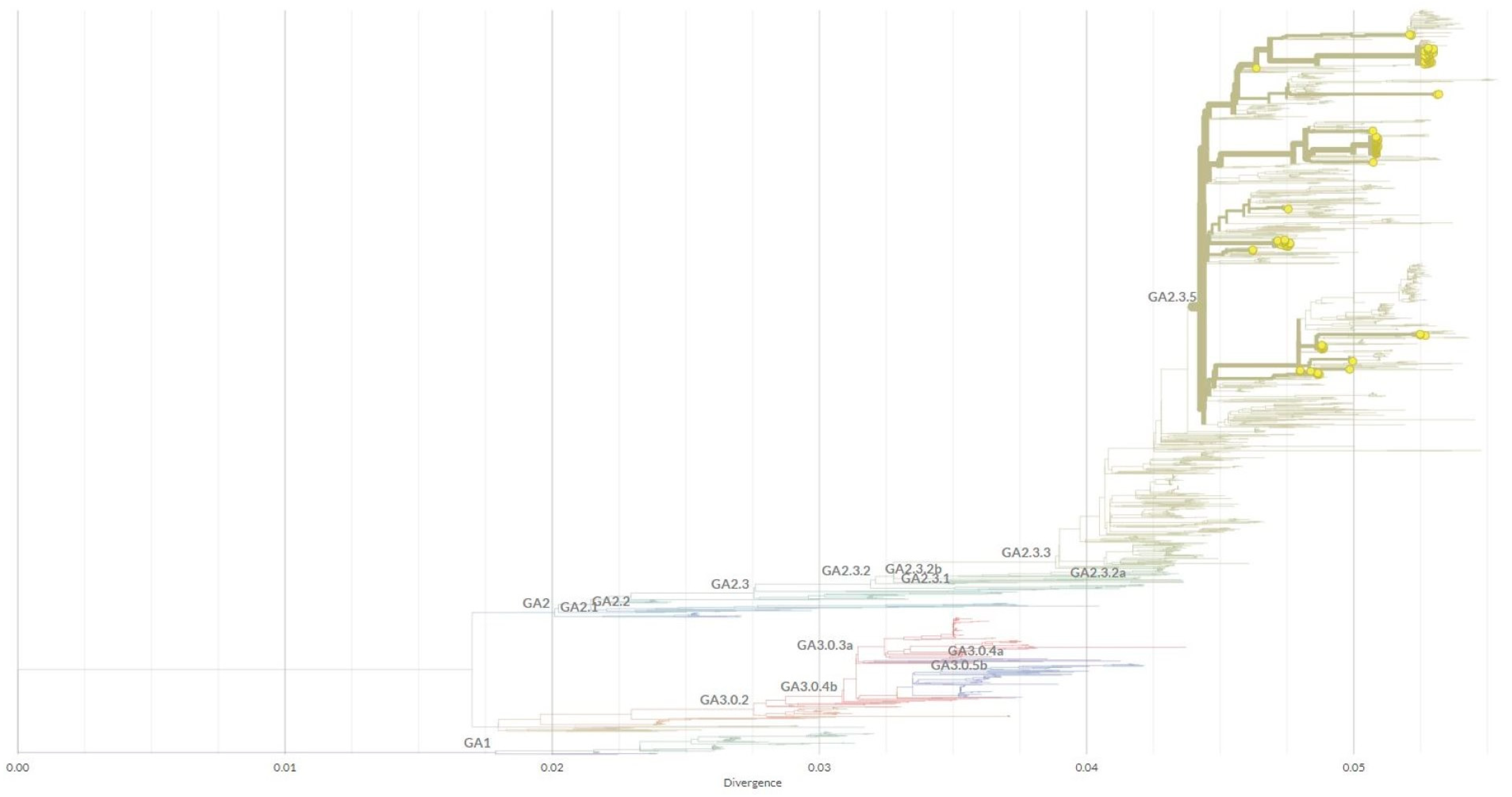
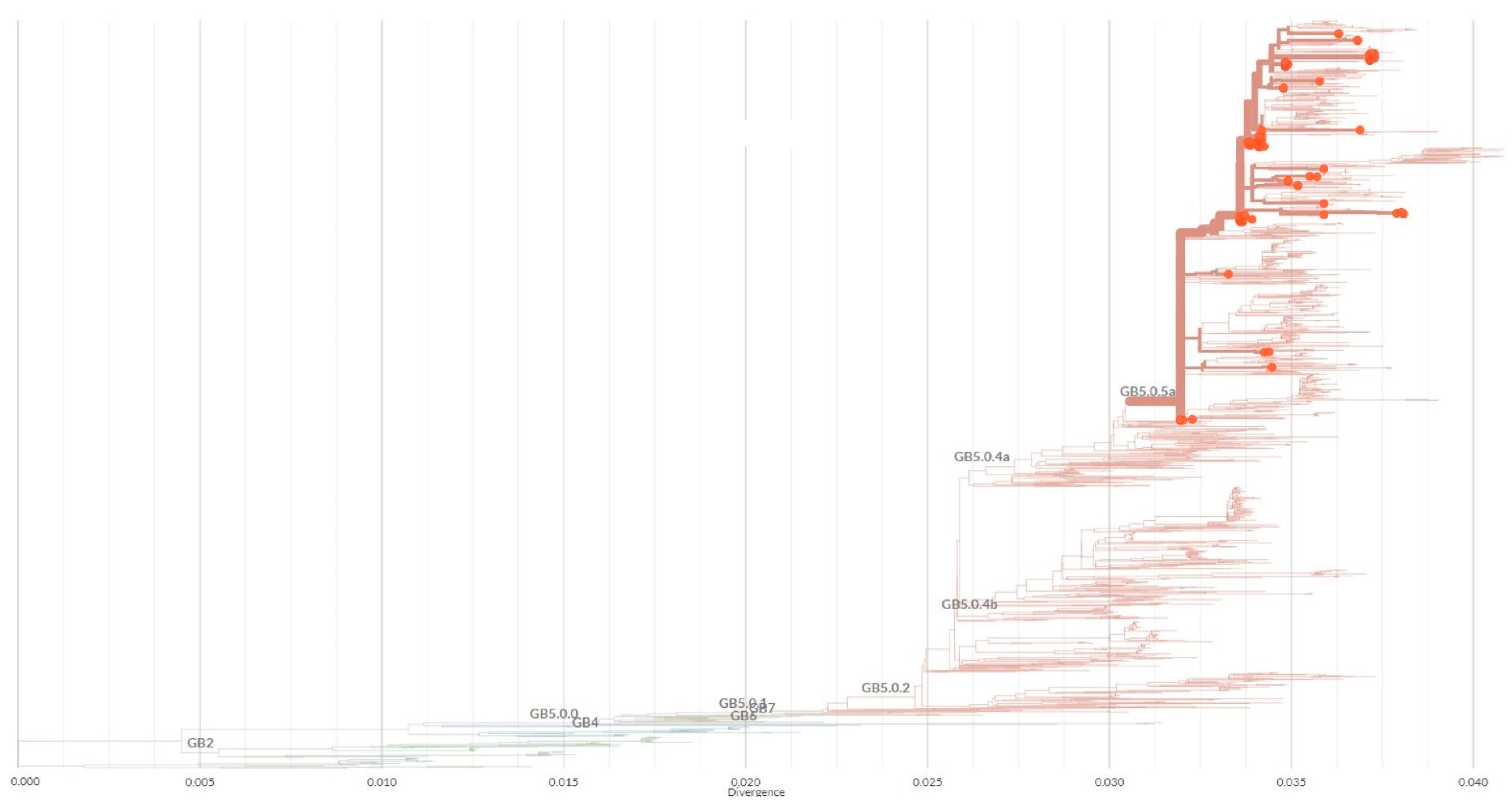


| Year | Total Samples | HRSV Positive [%] | No. Genotyped Samples [%] | Subgroup | No. Samples | Genetic Lineage |
|---|---|---|---|---|---|---|
| 2018 | 1791 | 262 [14.6] | 59 [22.5] | A | 15 | GA2.3.5 |
| B | 44 | GB5.0.5a | ||||
| 2019 | 1316 | 116 [8.8] | 24 [20.7] | A | 7 | GA2.3.5 |
| B | 17 | GB5.0.5a | ||||
| 2020 | 1241 | 86 [6.9] | 25 [29.1] | A | 14 | GA2.3.5 |
| B | 11 | GB5.0.5a | ||||
| 2021 | 2075 | 325 [15.7] | 113 [34.8] | A | 93 | GA2.3.5 |
| B | 20 | GB5.0.5a | ||||
| Total | 6423 | 789 [12.3] | 221 [28.0] | A | 129 | GA2.3.5 |
| B | 92 | GB5.0.5a |
| AA Position † | Relative Position in Rep. ‡ Region | AA ON1 | Mut. * | No. [%] Samples | AA Position † | AA ON1 | Mut. * | No. [%] Samples |
|---|---|---|---|---|---|---|---|---|
| 261 | 1 | Q | N/A | 285 | Q | K | 2 [2.3] | |
| 262 | 2 | E | K | 3 [2.3] | 286 | E | K | 2 [2.3] |
| V | 1 [0.8] | |||||||
| 263 | 3 | E | K | 3 [2.3] | 287 | E | N/A | |
| 264 | 4 | T | N/A | 288 | T | N/A | ||
| 265 | 5 | L | N/A | 289 | L | P | 1 [0.8] | |
| I | 1 [0.8] | |||||||
| 266 | 6 | H | L | 46 [35.6] | 290 | H | N/A | |
| 267 | 7 | S | N/A | 291 | S | N/A | ||
| 268 | 8 | T | N/A | 292 | T | N/A | ||
| 269 | 9 | T | N/A | 293 | T | A | 1 [0.8] | |
| 270 | 10 | S | N/A | 294 | S | N/A | ||
| 271 | 11 | E | K | 10 [7.5] | 295 | E | V | 4 [3.1] |
| 272 | 12 | G | N/A | 296 | G | D | 1 [0.8] | |
| S | 2 [2.3] | |||||||
| 273 | 13 | Y | H | 1 [0.8) | 297 | Y | H | 20 [15.5] |
| 274 | 14 | L | P | 112 [86.8] | 298 | L | P | 71 [55.0] |
| 275 | 15 | S | G | 1 [0.8] | 299 | S | G | 1 [0.8] |
| N | 4 [3.1] | |||||||
| 276 | 16 | P | Q | 13 [10.1] | 300 | P | S | 5 [3.9] |
| L | 3 [2.3] | |||||||
| 277 | 17 | S | N/A | 301 | S | N/A | ||
| 278 | 18 | Q | N/A | 302 | Q | P | 1 [0.8] | |
| K | 1 [0.8] | |||||||
| 279 | 19 | V | I | 4 [3.1] | 303 | V | A | 40 [31.0] |
| 280 | 20 | Y | H | 13 [10.1] | 304 | Y | H | 66 [51.2] |
| 281 | 21 | T | N/A | 305 | T | N/A | ||
| 282 | 22 | T | N/A | 306 | T | N/A | ||
| 283 | 23 | S | P | 2 [1.5] | 307 | S | P | 6 [4.6] |
| AA Position † | Relative Position in Rep. ‡ Region | AA BA | Mut. * | No. [%] Samples | AA Position † | AA BA | Mut. * | No. [%] Samples |
|---|---|---|---|---|---|---|---|---|
| 240 | 1 | T | A | 1 [1.1] | 260 | T | A | 17 [18.5] |
| V | 2 [2.2] | |||||||
| E | 1 [1.1] | |||||||
| 241 | 2 | E | N/A | 261 | E | N/A | ||
| 242 | 3 | R | N/A | 262 | R | N/A | ||
| 243 | 4 | D | N/A | 263 | D | E | 1 [1.1] | |
| 244 | 5 | T | N/A | 264 | T | I | 2 [2.2] | |
| 245 | 6 | S | N/A | 265 | S | N/A | ||
| 246 | 7 | T | S | 2 [2.2] | 266 | T | I | 2 [2.2] |
| 247 | 8 | S | P | 92 [100] | 267 | S | N/A | |
| 248 | 9 | Q | N/A | 268 | Q | N/A | ||
| 249 | 10 | S | P | 1 [1.1] | 269 | S | P | 1 [1.1] |
| 250 | 11 | T | N/A | 270 | T | I | 88 [95.6] | |
| 251 | 12 | V | A | 1 [1.1] | 271 | V | A | 89 [96.7] |
| E | 1 [1.1] | |||||||
| 252 | 13 | L | N/A | 272 | L | P | 1 [1.1] | |
| 253 | 14 | D | N/A | 273 | D | N/A | ||
| 254 | 15 | T | I | 76 [82.6] | 274 | T | N/A | |
| 255 | 16 | T | N/A | 275 | T | N/A | ||
| 256 | 17 | T | S | 2 [2.2] | 276 | T | N/A | |
| 257 | 18 | S | N/A | 277 | S | N/A | ||
| 258 | 19 | K | E | 2 [2.2] | 278 | K | N/A | |
| 259 | 20 | H | N/A | 279 | H | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virant, M.J.; Luštrek, M.; Kogoj, R.; Petrovec, M.; Uršič, T. Changes in HRSV Epidemiology but Not Circulating Variants in Hospitalized Children due to the Emergence of SARS-CoV-2. Viruses 2023, 15, 1218. https://doi.org/10.3390/v15061218
Virant MJ, Luštrek M, Kogoj R, Petrovec M, Uršič T. Changes in HRSV Epidemiology but Not Circulating Variants in Hospitalized Children due to the Emergence of SARS-CoV-2. Viruses. 2023; 15(6):1218. https://doi.org/10.3390/v15061218
Chicago/Turabian StyleVirant, Monika Jevšnik, Manca Luštrek, Rok Kogoj, Miroslav Petrovec, and Tina Uršič. 2023. "Changes in HRSV Epidemiology but Not Circulating Variants in Hospitalized Children due to the Emergence of SARS-CoV-2" Viruses 15, no. 6: 1218. https://doi.org/10.3390/v15061218
APA StyleVirant, M. J., Luštrek, M., Kogoj, R., Petrovec, M., & Uršič, T. (2023). Changes in HRSV Epidemiology but Not Circulating Variants in Hospitalized Children due to the Emergence of SARS-CoV-2. Viruses, 15(6), 1218. https://doi.org/10.3390/v15061218





