Viral Co-Infections and Antiviral Immunity in Honey Bees
Abstract
1. Introduction
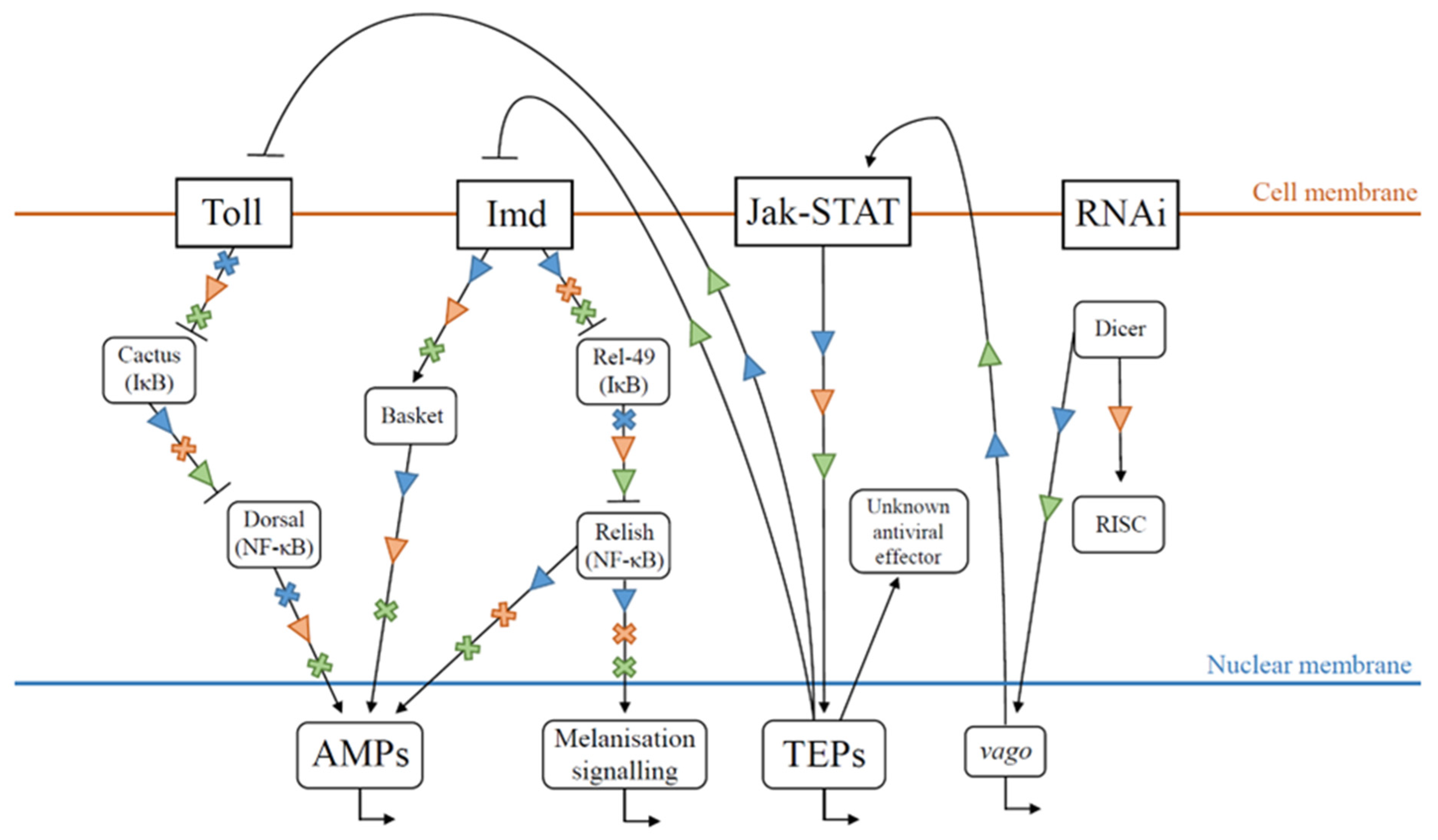
2. Honey Bee Antiviral Immunity
2.1. Vitellogenin
2.2. RNA Interference
2.3. Humoral Immunity
2.4. Melanisation
2.5. Social Immunity
2.6. Immune Priming
2.7. Factors Altering Immune Function
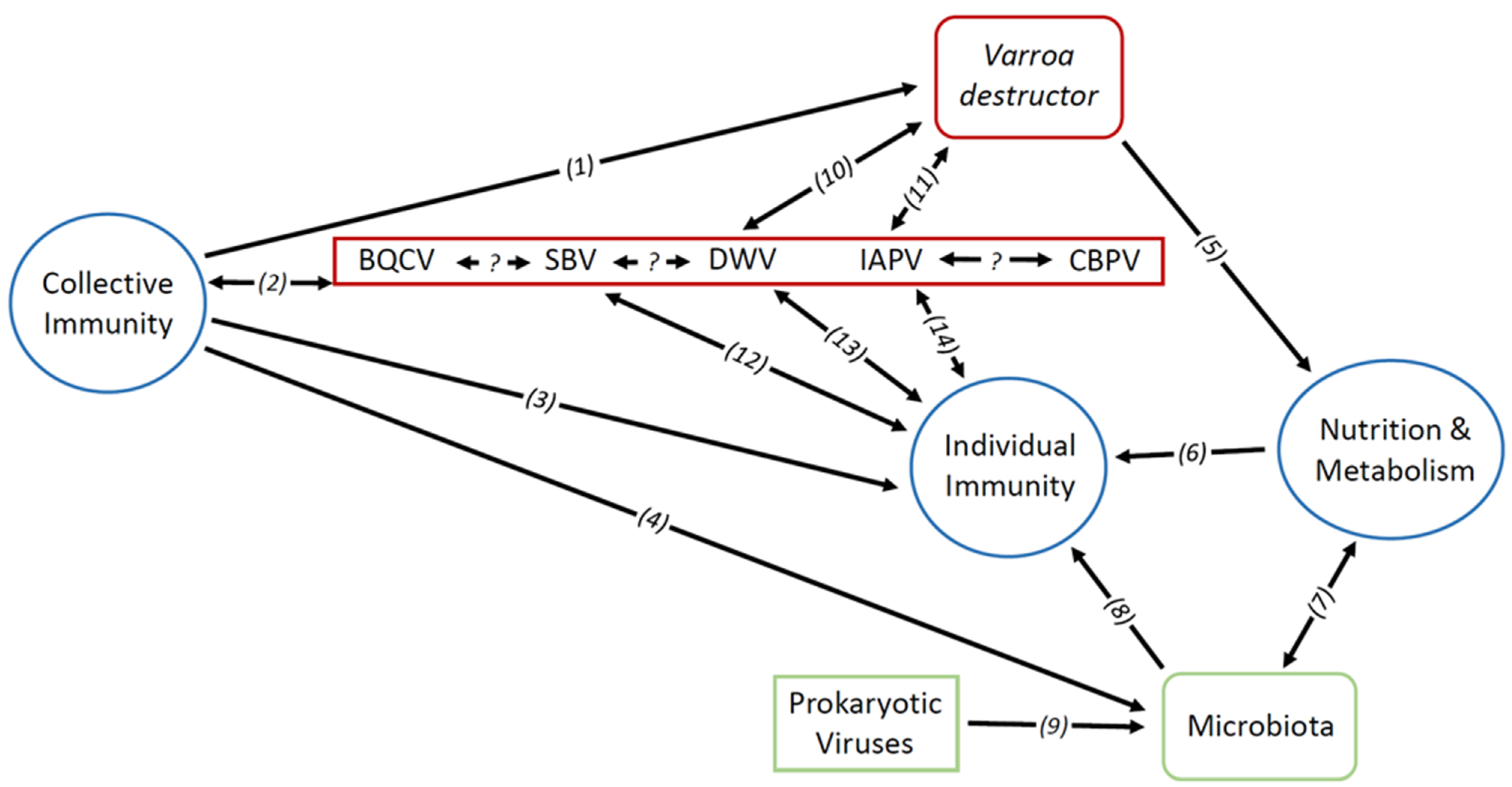
3. Viral Interactions
3.1. DWV-A and DWV-B
3.2. DWV and SBV
3.3. SBV and BQCV
3.4. Viruses from the AKI Complex and Other Viruses
4. Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of Pollinators in Changing Landscapes for World Crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of bee pollination and its economic value for crop production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Coulon, M.; Dalmon, A.; Di Prisco, G.; Prado, A.; Arban, F.; Dubois, E.; Ribière-Chabert, M.; Alaux, C.; Thiéry, R.; Le Conte, Y. Interactions Between Thiamethoxam and Deformed Wing Virus Can Drastically Impair Flight Behavior of Honey Bees. Front. Microbiol. 2020, 11, 766. [Google Scholar] [CrossRef]
- O’Neal, S.T.; Reeves, A.M.; Fell, R.D.; Brewster, C.C.; Anderson, T.D. Chlorothalonil Exposure Alters Virus Susceptibility and Markers of Immunity, Nutrition, and Development in Honey Bees. J. Insect Sci. 2019, 19, 14. [Google Scholar] [CrossRef]
- Cohen, H.; Smith, G.P.; Sardiñas, H.; Zorn, J.F.; McFrederick, Q.S.; Woodard, S.H.; Ponisio, L.C. Mass-Flowering Monoculture Attracts Bees, Amplifying Parasite Prevalence. Proc. R. Soc. B Boil. Sci. 2021, 288, 20211369. [Google Scholar] [CrossRef]
- Gisder, S.; Aumeier, P.; Genersch, E. Deformed Wing Virus: Replication and Viral Load in Mites (Varroa destructor). J. Gen. Virol. 2009, 90, 463–467. [Google Scholar] [CrossRef]
- Hedtke, K.; Jensen, P.M.; Jensen, A.B.; Genersch, E. Evidence for Emerging Parasites and Pathogens Influencing Outbreaks of Stress-Related Diseases like Chalkbrood. J. Invertebr. Pathol. 2011, 108, 167–173. [Google Scholar] [CrossRef]
- Remnant, E.J.; Mather, N.; Gillard, T.L.; Yagound, B.; Beekman, M. Direct Transmission by Injection Affects Competition among RNA Viruses in Honeybees. Proc. R. Soc. B Boil. Sci. 2019, 286, 20182452. [Google Scholar] [CrossRef]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid Pesticides and Nutritional Stress Synergistically Reduce Survival in Honey Bees. Proc. R. Soc. B Boil. Sci. 2017, 284, 20171711. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y. Nutrition, Immunity and Viral Infections in Honey Bees. Curr. Opin. Insect Sci. 2015, 10, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Tritschler, M.; Vollmann, J.J.; Yañez, O.; Chejanovsky, N.; Crailsheim, K.; Neumann, P. Protein Nutrition Governs within-Host Race of Honey Bee Pathogens. Sci. Rep. 2017, 7, 14988. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Q.; Gong, H.R.; Huang, S.K.; Sohr, A.; Hu, F.L.; Chen, Y.P. Evidence of the Synergistic Interaction of Honey Bee Pathogens Nosema ceranae and Deformed wing virus. Vet. Microbiol. 2015, 177, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gajda, A.M.; Mazur, E.D.; Bober, A.M.; Czopowicz, M. Nosema ceranae Interactions with Nosema apis and Black Queen Cell Virus. Agriculture 2021, 11, 963. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Ahmed, H.R.; El-Wahed, A.A.A.; Saeed, A.; Algethami, A.F.; Attia, N.F.; Guo, Z.; Musharraf, S.G.; Khatib, A.; Alsharif, S.M.; et al. Bee stressors from an immunological perspective and strategies to improve bee health. Vet. Sci. 2022, 9, 199. [Google Scholar] [CrossRef]
- Beaurepaire, A.; Piot, N.; Doublet, V.; Antunez, K.; Campbell, E.; Chantawannakul, P.; Chejanovsky, N.; Gajda, A.; Heerman, M.; Panziera, D.; et al. Diversity and Global Distribution of Viruses of the Western Honey Bee, Apis mellifera. Insects 2020, 11, 239. [Google Scholar] [CrossRef]
- Méthot, P.O.; Alizon, S. What Is a Pathogen? Toward a Process View of Host-Parasite Interactions. Virulence 2014, 5, 775–785. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. The Damage-Response Framework of Microbial Pathogenesis. Nat. Rev. Microbiol. 2003, 1, 17–24. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. Host-Pathogen Interactions: Redefining the Basic Concepts of Virulence and Pathogenicity. Infect. Immun. 1999, 67, 3703–3713. [Google Scholar] [CrossRef]
- Hosokawa, T.; Ishii, Y.; Nikoh, N.; Fujie, M.; Satoh, N.; Fukatsu, T. Obligate Bacterial Mutualists Evolving from Environmental Bacteria in Natural Insect Populations. Nat. Microbiol. 2016, 1, 15011. [Google Scholar] [CrossRef]
- Roossinck, M.J. The Good Viruses: Viral Mutualistic Symbioses. Nat. Rev. Microbiol. 2011, 9, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Belden, L.K.; Harris, R.N. Infectious Diseases in Wildlife: The Community Ecology Context. Front. Ecol. Environ. 2007, 5, 533–539. [Google Scholar] [CrossRef]
- Daskin, J.H.; Alford, R.A. Context-Dependent Symbioses and Their Potential Roles in Wildlife Diseases. Proc. R. Soc. B Boil. Sci. 2012, 279, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Dancing with the Stars: How Choreographed Bacterial Interactions Dictate Nososymbiocity and Give Rise to Keystone Pathogens, Accessory Pathogens, and Pathobionts. Trends Microbiol. 2016, 24, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Schurr, F.; Tison, A.; Militano, L.; Cheviron, N.; Sircoulomb, F.; Rivière, M.P.; Ribière-Chabert, M.; Thiéry, R.; Dubois, E. Validation of Quantitative Real-Time RT-PCR Assays for the Detection of Six Honeybee Viruses. J. Virol. Methods 2019, 270, 70–78. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Genersch, E. Deformed Wing Virus. J. Invertebr. Pathol. 2010, 103, S48–S61. [Google Scholar] [CrossRef]
- Cooper, D.; Cory, J.S.; Theilmann, D.A.; Myers, J.H. Nucleopolyhedroviruses of Forest and Western Tent Caterpillars: Cross-Infectivity and Evidence for Activation of Latent Virus in High-Density Field Populations. Ecol. Entomol. 2003, 28, 41–50. [Google Scholar] [CrossRef]
- Boots, M.; Greenman, J.; Ross, D.; Norman, R.; Hails, R.; Sait, S. The Population Dynamical Implications of Covert Infections in Host-Microparasite Interactions. J. Anim. Ecol. 2003, 72, 1064–1072. [Google Scholar] [CrossRef]
- Bailey, L.; Gibbs, A.J.; Woods, R.D. Sacbrood Virus of the Larval Honey Bee (Apis mellifera Linnaeus). Virology 1964, 23, 425–429. [Google Scholar] [CrossRef]
- Wei, R.; Cao, L.; Feng, Y.; Chen, Y.; Chen, G.; Zheng, H. Sacbrood Virus: A Growing Threat to Honeybees and Wild Pollinators. Viruses 2022, 14, 1871. [Google Scholar] [CrossRef]
- Bailey, L.; Woods, R.D. Two More Small RNA Viruses from Honey Bees and Further Observations on Sacbrood and Acute Bee-Paralysis Viruses. J. Gen. Virol. 1977, 37, 175–182. [Google Scholar] [CrossRef]
- Spurny, R.; Pridal, A. Virion Structure of Black Queen Cell Virus, a Common Honeybee Pathogen. J. Virol. 2017, 91, e02100-16. [Google Scholar] [CrossRef] [PubMed]
- Paxton, R.J.; Schäfer, M.O.; Nazzi, F.; Zanni, V.; Annoscia, D.; Marroni, F.; Bigot, D.; Laws-Quinn, E.R.; Panziera, D.; Jenkins, C.; et al. Epidemiology of a Major Honey Bee Pathogen, Deformed Wing Virus: Potential Worldwide Replacement of Genotype A by Genotype B. Int. J. Parasitol. Parasites Wildl. 2022, 18, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L. Paralysis of the Honey Bee, Apis mellifera Linnaeus. J. Invertebr. Pathol. 1965, 7, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ribière, M.; Olivier, V.; Blanchard, P. Chronic Bee Paralysis: A Disease and a Virus like No Other? J. Invertebr. Pathol. 2010, 103, S120–S131. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.; Gibbs, A.J.; Woods, R.D. Two Viruses from Adult Honey Bees (Apis mellifera Linnaeus). Virology 1963, 21, 390–395. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Cordoni, G.; Budge, G. The Acute Bee Paralysis Virus-Kashmir Bee Virus-Israeli Acute Paralysis Virus Complex. J. Invertebr. Pathol. 2010, 103, S30–S47. [Google Scholar] [CrossRef]
- Maebe, K.; Vereecken, N.J.; Piot, N.; Reverté, S.; Cejas, D.; Michez, D.; Vandamme, P.; Smagghe, G. The Holobiont as a Key to the Adaptation and Conservation of Wild Bees in the Anthropocene. Front. Ecol. Evol. 2021, 9, 781470. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Theis, K.R. Host Biology in Light of the Microbiome: Ten Principles of Holobionts and Hologenomes. PLoS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef]
- Theis, K.R.; Dheilly, N.M.; Klassen, J.L.; Brucker, R.M.; Baines, J.F.; Bosch, T.C.G.; Cryan, J.F.; Gilbert, S.F.; Goodnight, C.J.; Lloyd, E.A.; et al. Getting the Hologenome Concept Right: An Eco-Evolutionary Framework for Hosts and Their Microbiomes. Msystems 2016, 1, e00028-16. [Google Scholar] [CrossRef]
- Schwarz, R.S.; Huang, Q.; Evans, J.D. Hologenome Theory and the Honey Bee Pathosphere. Curr. Opin. Insect Sci. 2015, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tecon, R.; Mitri, S.; Ciccarese, D.; Or, D.; van der Meer, J.R.; Johnson, D.R. Bridging the Holistic-Reductionist Divide in Microbial Ecology. mSystems 2019, 4, e00265-18. [Google Scholar] [CrossRef]
- Fang, F.C.; Casadevall, A. Reductionistic and Holistic Science. Infect. Immun. 2011, 79, 1401–1404. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Antiviral Defense Mechanisms in Honey Bees. Curr. Opin. Insect Sci. 2015, 10, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Doublet, V.; Poeschl, Y.; Gogol-Döring, A.; Alaux, C.; Annoscia, D.; Aurori, C.; Barribeau, S.M.; Bedoya-Reina, O.C.; Brown, M.J.F.; Bull, J.C.; et al. Unity in Defence: Honeybee Workers Exhibit Conserved Molecular Responses to Diverse Pathogens. BMC Genom. 2017, 18, 207. [Google Scholar] [CrossRef]
- Negri, P.; Maggi, M.; Ramirez, L.; Szawarski, N.; De Feudis, L.; Lamattina, L.; Eguaras, M. Cellular Immunity in Apis mellifera: Studying Hemocytes Brings Light about Bees Skills to Confront Threats. Apidologie 2016, 47, 379–388. [Google Scholar] [CrossRef]
- Simone-Finstrom, M. Social Immunity and the Superorganism: Behavioural Defenses Protecting Honey Bee Colonies from Pathogens and Parasites. Bee World 2017, 94, 21–29. [Google Scholar] [CrossRef]
- Cohen, A.A.; Martin, L.B.; Wingfield, J.C.; McWilliams, S.R.; Dunne, J.A. Physiological Regulatory Networks: Ecological Roles and Evolutionary Constraints. Trends Ecol. Evol. 2012, 27, 428–435. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, P.; Sirisena, P.D.N.N.; Dubey, S.K.; Kumar, R.; Shrinet, J.; Sunil, S. Mosquito innate immunity. Insects 2018, 9, 95. [Google Scholar] [CrossRef]
- Mondet, F.; Alaux, C.; Severac, D.; Rohmer, M.; Mercer, A.R.; Le Conte, Y. Antennae Hold a Key to Varroa-Sensitive Hygiene Behaviour in Honey Bees. Sci. Rep. 2015, 5, 10454. [Google Scholar] [CrossRef] [PubMed]
- Mondet, F.; Kim, S.H.; De Miranda, J.R.; Beslay, D.; Le Conte, Y.; Mercer, A.R. Specific Cues Associated with Honey Bee Social Defence against Varroa destructor Infested Brood. Sci. Rep. 2016, 6, 25444. [Google Scholar] [CrossRef] [PubMed]
- Vung, N.N.; Choi, Y.S.; Kim, I. High Resistance to Sacbrood Virus Disease in Apis cerana (Hymenoptera: Apidae) Colonies Selected for Superior Brood Viability and Hygienic Behavior. Apidologie 2020, 51, 61–74. [Google Scholar] [CrossRef]
- Schöning, C.; Gisder, S.; Geiselhardt, S.; Kretschmann, I.; Bienefeld, K.; Hilker, M.; Genersch, E. Evidence for Damage-Dependent Hygienic Behaviour towards Varroa destructor-Parasitised Brood in the Western Honey Bee, Apis mellifera. J. Exp. Biol. 2012, 215, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.S.; Evans, E.C.; Pizzorno, M.C. Localization of Deformed Wing Virus (DWV) in the Brains of the Honeybee, Apis mellifera Linnaeus. Virol. J. 2009, 6, 182. [Google Scholar] [CrossRef]
- Lin, Z.; Page, P.; Li, L.; Qin, Y.; Zhang, Y.; Hu, F.; Neumann, P.; Zheng, H.; Dietemann, V. Go East for Better Honey Bee Health: Apis cerana Is Faster at Hygienic Behavior than A. mellifera. PLoS ONE 2016, 11, e0162647. [Google Scholar] [CrossRef]
- Rinderer, T.E.; Rothenbuhler, W.C. Characteristic Field Symptoms Comprising Honeybee Hairless-Black Syndrome Induced in the Laboratory by a Virus. J. Invertebr. Pathol. 1976, 27, 215–219. [Google Scholar] [CrossRef]
- Richard, F.J.; Aubert, A.; Grozinger, C.M. Modulation of Social Interactions by Immune Stimulation in Honey Bee, Apis mellifera, Workers. BMC Biol. 2008, 6, 50. [Google Scholar] [CrossRef]
- Baracchi, D.; Fadda, A.; Turillazzi, S. Evidence for Antiseptic Behaviour towards Sick Adult Bees in Honey Bee Colonies. J. Insect Physiol. 2012, 58, 1589–1596. [Google Scholar] [CrossRef]
- Geffre, A.C.; Gernat, T.; Harwood, G.P.; Jones, B.M.; Gysi, D.M.; Hamilton, A.R.; Bonning, B.C.; Toth, A.L.; Robinson, G.E.; Dolezal, A.G. Honey Bee Virus Causes Context-Dependent Changes in Host Social Behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 10406–10413. [Google Scholar] [CrossRef]
- Amiri, E.; Seddon, G.; Smith, W.Z.; Strand, M.K.; Tarpy, D.R.; Rueppell, O. Israeli acute paralysis virus: Honey bee queen–worker interaction and potential virus transmission pathways. Insects 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Salmela, H.; Amdam, G.V.; Freitak, D. Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin. PLoS Pathog. 2015, 11, e1005015. [Google Scholar] [CrossRef] [PubMed]
- Maori, E.; Garbian, Y.; Kunik, V.; Mozes-Koch, R.; Malka, O.; Kalev, H.; Sabath, N.; Sela, I.; Shafir, S. A Transmissible RNA Pathway in Honey Bees. Cell Rep. 2019, 27, 1949–1959.e6. [Google Scholar] [CrossRef] [PubMed]
- López, J.H.; Schuehly, W.; Crailsheim, K.; Riessberger-Gallé, U. Trans-Generational Immune Priming in Honeybees. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140454. [Google Scholar] [CrossRef]
- De Graaf, D.C.; Laget, D.; De Smet, L.; Claeys Boúúaert, D.; Brunain, M.; Veerkamp, R.F.; Brascamp, E.W. Heritability Estimates of the Novel Trait ‘Suppressed in Ovo Virus Infection’ in Honey Bees (Apis mellifera). Sci. Rep. 2020, 10, 14310. [Google Scholar] [CrossRef]
- Leponiemi, M.; Amdam, G.V.; Freitak, D. Exposure to Inactivated Deformed Wing Virus Leads to Trans-Generational Costs but Not Immune Priming in Honeybees (Apis mellifera). Front. Ecol. Evol. 2021, 9, 626670. [Google Scholar] [CrossRef]
- Lang, S.; Simone-Finstrom, M.; Healy, K. Context-Dependent Viral Transgenerational Immune Priming in Honey Bees (Hymenoptera: Apidae). J. Insect Sci. 2022, 22, 19. [Google Scholar] [CrossRef]
- Saelao, P.; Borba, R.S.; Ricigliano, V.; Spivak, M.; Simone-Finstrom, M. Honeybee microbiome is stabilized in the presence of propolis. Biol. Lett. 2020, 16, 2–6. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor Feeds Primarily on Honey Bee Fat Body Tissue and Not Hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef]
- Aronstein, K.A.; Saldivar, E.; Vega, R.; Westmiller, S.; Douglas, A.E. How Varroa Parasitism Affects the Immunological and Nutritional Status of the Honey Bee, Apis mellifera. Insects 2012, 3, 601–615. [Google Scholar] [CrossRef]
- Annoscia, D.; Brown, S.P.; Di Prisco, G.; De Paoli, E.; Del Fabbro, S.; Frizzera, D.; Zanni, V.; Galbraith, D.A.; Caprio, E.; Grozinger, C.M.; et al. Haemolymph Removal by Varroa Mite Destabilizes the Dynamical Interaction between Immune Effectors and Virus in Bees, as Predicted by Volterra’s Model. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190331. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet Effects on Honeybee Immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Billiet, A.; Meeus, I.; Van Nieuwerburgh, F.; Deforce, D.; Wäckers, F.; Smagghe, G. Impact of sugar syrup and pollen diet on the bacterial diversity in the gut of indoor-reared bumblebees (Bombus terrestris). Apidologie 2016, 47, 548–560. [Google Scholar] [CrossRef]
- Maes, P.W.; Rodrigues, P.A.P.; Oliver, R.; Mott, B.M.; Anderson, K.E. Diet-related gut bacterial dysbiosis correlates with impaired development, increased mortality and Nosema disease in the honeybee (Apis mellifera). Mol. Ecol. 2016, 25, 5439–5450. [Google Scholar] [CrossRef] [PubMed]
- Haag, K.L.; Caesar, L.; da Silveira Regueira-Neto, M.; de Sousa, D.R.; Montenegro Marcelino, V.; de Queiroz Balbino, V.; Torres Carvalho, A. Temporal Changes in Gut Microbiota Composition and Pollen Diet Associated with Colony Weakness of a Stingless Bee. Microb. Ecol. 2022, 85, 1514–1526. [Google Scholar] [CrossRef]
- Steele, M.I.; Motta, E.V.S.; Gattu, T.; Martinez, D.; Moran, N.A. The Gut Microbiota Protects Bees from Invasion by a Bacterial Pathogen. Microbiol. Spectr. 2021, 9, e00394-21. [Google Scholar] [CrossRef]
- Horak, R.D.; Leonard, S.P.; Moran, N.A. Symbionts Shape Host Innate Immunity in Honeybees: Symbionts Shape Honey Bee Immunity. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201184. [Google Scholar] [CrossRef]
- Raymann, K.; Moran, N.A. The role of the gut microbiome in health and disease of adult honey bee workers. Curr. Opin. Insect Sci. 2018, 26, 97–104. [Google Scholar] [CrossRef]
- Deboutte, W.; Beller, L.; Yinda, C.K.; Maes, P.; de Graaf, D.C.; Matthijnssens, J. Honey-bee–associated prokaryotic viral communities reveal wide viral diversity and a profound metabolic coding potential. Proc. Natl. Acad. Sci. USA 2020, 117, 10511–10519. [Google Scholar] [CrossRef]
- Dosch, C.; Manigk, A.; Streicher, T.; Tehel, A.; Paxton, R.J.; Tragust, S. The Gut Microbiota Can Provide Viral Tolerance in the Honey Bee. Microorganisms 2021, 9, 871. [Google Scholar] [CrossRef]
- Kim, C.; Kim, J.M.; Choi, H.; Choi, Y.S.; Jin, B.R.; Lee, K.S.; Choi, K. Analysis of the gut microbiome of susceptible and resistant honeybees (Apis cerana) against sacbrood virus disease. J. Appl. Entomol. 2022, 146, 1078–1086. [Google Scholar] [CrossRef]
- Yun, B.R.; Truong, A.T.; Choi, Y.S.; Lee, M.Y.; Kim, B.Y.; Seo, M.; Yoon, S.S.; Yoo, M.S.; Van Quyen, D.; Cho, Y.S. Comparison of the gut microbiome of sacbrood virus-resistant and -susceptible Apis cerana from South Korea. Sci. Rep. 2022, 12, 10010. [Google Scholar] [CrossRef] [PubMed]
- Raymann, K.; Shaffer, Z.; Moran, N.A. Antibiotic exposure perturbs the gut microbiota and elevates mortality in honeybees. PLoS Biol. 2017, 15, e2001861. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yang, S.; Zhao, H.; Luo, J.; Yang, W.; Hou, C. Antibiotics-induced changes in intestinal bacteria result in the sensitivity of honey bee to virus. Environ. Pollut. 2022, 314, 120278. [Google Scholar] [CrossRef] [PubMed]
- Bazin, T.; Chiu, L.; Pradeu, T. Host-Microbiota Co-Immunity: An Intimate Relationship That Goes Beyond Protection. Philos. Theory Pract. Biol. 2022, 14. [Google Scholar] [CrossRef]
- Chiu, L.; Bazin, T.; Truchetet, M.E.; Schaeverbeke, T.; Delhaes, L.; Pradeu, T. Protective Microbiota: From Localized to Long-Reaching Co-Immunity. Front. Immunol. 2017, 8, 1678. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Wood, G.R.; Fannon, J.M.; Moore, J.D.; Bull, J.C.; Chandler, D.; Mead, A.; Burroughs, N.; Evans, D.J. A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees (Apis mellifera) Prevails after Varroa destructor-Mediated, or In Vitro, Transmission. PLoS Pathog. 2014, 10, e1004230. [Google Scholar] [CrossRef]
- Dalmon, A.; Desbiez, C.; Coulon, M.; Thomasson, M.; Le Conte, Y.; Alaux, C.; Vallon, J.; Moury, B. Evidence for Positive Selection and Recombination Hotspots in Deformed Wing Virus (DWV). Sci. Rep. 2017, 7, srep41045. [Google Scholar] [CrossRef]
- Levin, S.; Sela, N.; Erez, T.; Nestel, D.; Pettis, J.; Neumann, P.; Chejanovsky, N. New Viruses from the Ectoparasite Mite Varroa destructor Infesting Apis mellifera and Apis cerana. Viruses 2019, 11, 94. [Google Scholar] [CrossRef]
- Posada-Florez, F.; Ryabov, E.V.; Heerman, M.C.; Chen, Y.; Evans, J.D.; Sonenshine, D.E.; Cook, S.C. Varroa destructor Mites Vector and Transmit Pathogenic Honey Bee Viruses Acquired from an Artificial Diet. PLoS ONE 2020, 15, e0242688. [Google Scholar] [CrossRef]
- Martin, S.J.; Highfield, A.C.; Brettell, L.; Villalobos, E.M.; Budge, G.E.; Powell, M.; Nikaido, S.; Schroeder, D.C. Global Honey Bee Viral Landscape Altered by a Parasitic Mite. Science 2012, 336, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.M.; Davis, S.L.; Rasgon, J.L.; Grozinger, C.M. Simulated Vector Transmission Differentially Influences Dynamics of Two Viral Variants of Deformed Wing Virus in Honey Bees (Apis mellifera). J. Gen. Virol. 2021, 102, 001687. [Google Scholar] [CrossRef] [PubMed]
- Piou, V.; Schurr, F.; Dubois, E.; Vétillard, A. Transmission of Deformed Wing Virus between Varroa destructor Foundresses, Mite Offspring and Infested Honey Bees. Parasites Vectors 2022, 15, 333. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, E.V.; Childers, A.K.; Lopez, D.; Grubbs, K.; Posada-Florez, F.; Weaver, D.; Girten, W.; van Engelsdorp, D.; Chen, Y.; Evans, J.D. Dynamic Evolution in the Key Honey Bee Pathogen Deformed Wing Virus: Novel Insights into Virulence and Competition Using Reverse Genetics. PLoS Biol. 2019, 17, e3000502. [Google Scholar] [CrossRef]
- Ongus, J.R.; Peters, D.; Bonmatin, J.M.; Bengsch, E.; Vlak, J.M.; van Oers, M.M. Complete Sequence of a Picorna-like Virus of the Genus Iflavirus Replicating in the Mite Varroa destructor. J. Gen. Virol. 2004, 85, 3747–3755. [Google Scholar] [CrossRef]
- Gisder, S.; Genersch, E. Direct Evidence for Infection of Varroa destructor Mites with the Bee-Pathogenic Deformed Wing Virus Variant B, but Not Variant A, via Fluorescence In Situ Hybridization Analysis. J. Virol. 2021, 95, e01786-20. [Google Scholar] [CrossRef]
- Moore, J.; Jironkin, A.; Chandler, D.; Burroughs, N.; Evans, D.J.; Ryabov, E.V. Recombinants between Deformed Wing Virus and Varroa destructor Virus-1 May Prevail in Varroa destructor-Infested Honeybee Colonies. J. Gen. Virol. 2011, 92, 156–161. [Google Scholar] [CrossRef]
- Di Prisco, G.; Pennacchio, F.; Caprio, E.; Boncristiani, H.F.; Evans, J.D.; Chen, Y. Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. J. Gen. Virol. 2011, 92, 151–155. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Fannon, J.M.; Moore, J.D.; Wood, G.R.; Evans, D.J. The Iflaviruses Sacbrood Virus and Deformed Wing Virus Evoke Different Transcriptional Responses in the Honeybee Which May Facilitate Their Horizontal or Vertical Transmission. PeerJ 2016, 4, e1591. [Google Scholar] [CrossRef]
- Shan, L.; Liuhao, W.; Jun, G.; Yujie, T.; Yanping, C.; Jie, W.; Jilian, L. Chinese Sacbrood Virus Infection in Asian Honey Bees (Apis cerana cerana) and Host Immune Responses to the Virus Infection. J. Invertebr. Pathol. 2017, 150, 63–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Xu, Z.; Han, R.; Chen, J. Differential Gene Transcription in Honeybee (Apis cerana) Larvae Challenged by Chinese Sacbrood Virus (CSBV). Sociobiology 2013, 60, 413–420. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Z.; Zhuang, M.; Wang, L.; Li, K.; Yao, J.; Yang, H.; Huang, J.; Hao, Y.; Ying, F.; et al. Transcriptome Profiling Reveals a Novel Mechanism of Antiviral Immunity Upon Sacbrood Virus Infection in Honey Bee Larvae (Apis cerana). Front. Microbiol. 2021, 12, 615893. [Google Scholar] [CrossRef] [PubMed]
- Quintana, S.; Brasesco, C.; Negri, P.; Marin, M.; Pagnuco, I.; Szawarski, N.; Reynaldi, F.J.; Larsen, A.; Eguaras, M.; Maggi, M. Up-Regulated Pathways in Response to Deformed Wing Virus Infection in Apis mellifera (Hymenoptera: Apidae). Rev. Soc. Entomol. Argent. 2019, 78, 1–11. [Google Scholar] [CrossRef]
- Yang, X.; Cox-Foster, D.L. Impact of an Ectoparasite on the Immunity and Pathology of an Invertebrate: Evidence for Host Immunosuppression and Viral Amplification. Proc. Natl. Acad. Sci. USA 2005, 102, 7470–7475. [Google Scholar] [CrossRef]
- Di Prisco, G.; Annoscia, D.; Margiotta, M.; Ferrara, R.; Varricchio, P.; Zanni, V.; Caprio, E.; Nazzi, F.; Pennacchio, F. A Mutualistic Symbiosis between a Parasitic Mite and a Pathogenic Virus Undermines Honey Bee Immunity and Health. Proc. Natl. Acad. Sci. USA 2016, 113, 3203–3208. [Google Scholar] [CrossRef]
- Barroso-Arévalo, S.; Vicente-Rubiano, M.; Puerta, F.; Molero, F.; Sánchez-Vizcaíno, J.M. Immune Related Genes as Markers for Monitoring Health Status of Honey Bee Colonies. BMC Vet. Res. 2019, 15, 72. [Google Scholar] [CrossRef]
- Mookhploy, W.; Krongdang, S.; Chantawannakul, P. Effects of Deformed Wing Virus Infection on Expressions of Immune-and Apoptosis-Related Genes in Western Honeybees (Apis mellifera). Insects 2021, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic Parasite-Pathogen Interactions Mediated by Host Immunity Can Drive the Collapse of Honeybee Colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Heerman, M.; Peng, W.; Evans, J.D.; Rose, R.; Degrandi-Hoffman, G.; Simone-Finstrom, M.; Li, J.; Li, Z.; Cook, S.C.; et al. The Dynamics of Deformed Wing Virus Concentration and Host Defensive Gene Expression after Varroa Mite Parasitism in Honey Bees, Apis mellifera. Insects 2019, 10, 16. [Google Scholar] [CrossRef]
- Chen, Y.P.; Pettis, J.S.; Corona, M.; Chen, W.P.; Li, C.J.; Spivak, M.; Visscher, P.K.; DeGrandi-Hoffman, G.; Boncristiani, H.; Zhao, Y.; et al. Israeli Acute Paralysis Virus: Epidemiology, Pathogenesis and Implications for Honey Bee Health. PLoS Pathog. 2014, 10, e1004261. [Google Scholar] [CrossRef]
- Chejanovsky, N.; Ophir, R.; Schwager, M.S.; Slabezki, Y.; Grossman, S.; Cox-Foster, D. Characterization of Viral SiRNA Populations in Honey Bee Colony Collapse Disorder. Virology 2014, 454–455, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, D.A.; Yang, X.; Niño, E.L.; Yi, S.; Grozinger, C. Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees (Apis mellifera). PLoS Pathog. 2015, 11, e1004713. [Google Scholar] [CrossRef] [PubMed]
- Azzami, K.; Ritter, W.; Tautz, J.; Beier, H. Infection of Honey Bees with Acute Bee Paralysis Virus Does Not Trigger Humoral or Cellular Immune Responses. Arch. Virol. 2012, 157, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.; Takahashi, T.; Bendena, W.G.; Tobe, S.S.; Hui, J.H.L. Comparative Genomic and Phylogenetic Analysis of Vitellogenin and Other Large Lipid Transfer Proteins in Metazoans. FEBS Lett. 2010, 584, 1273–1278. [Google Scholar] [CrossRef]
- Hagedorn, H.H.; Kunkel, J.G. Vitellogenin and Vitellin in Insects. Annu. Rev. Entomol. 1979, 24, 475–505. [Google Scholar] [CrossRef]
- Amdam, G.V.; Norberg, K.; Hagen, A.; Omholt, S.W. Social Exploitation of Vitellogenin. Proc. Natl. Acad. Sci. USA 2003, 100, 1799–1802. [Google Scholar] [CrossRef]
- Amdam, G.V.; Omholt, S.W. The Hive Bee to Forager Transition in Honeybee Colonies: The Double Repressor Hypothesis. J. Theor. Biol. 2003, 223, 451–464. [Google Scholar] [CrossRef]
- Seehuus, S.C.; Norberg, K.; Krekling, T.; Fondrk, K.; Amdam, G.V. Immunogold Localization of Vitellogenin in the Ovaries, Hypopharyngeal Glands and Head Fat Bodies of Honeybee Workers, Apis mellifera. J. Insect Sci. 2007, 7, 52. [Google Scholar] [CrossRef]
- Fluri, P.; Lüscher, M.; Wille, H.; Gerig, L. Changes in Weight of the Pharyngeal Gland and Haemolymph Titres of Juvenile Hormone, Protein and Vitellogenin in Worker Honey Bees. J. Insect Physiol. 1982, 28, 61–68. [Google Scholar] [CrossRef]
- Pinto, L.Z.; Bitondi, M.M.G.; Simões, Z.L.P. Inhibition of Vitellogenin Synthesis in Apis Mellifera Workers by a Juvenile Hormone Analogue, Pyriproxyfen. J. Insect Physiol. 2000, 46, 153–160. [Google Scholar] [CrossRef]
- Piulachs, M.D.; Guidugli, K.R.; Barchuk, A.R.; Cruz, J.; Simões, Z.L.P.; Bellés, X. The Vitellogenin of the Honey Bee, Apis mellifera: Structural Analysis of the CDNA and Expression Studies. Insect Biochem. Mol. Biol. 2003, 33, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Simões, Z.L.P.; Hagen, A.; Norberg, K.; Schrøder, K.; Mikkelsen, Ø.; Kirkwood, T.B.L.; Omholt, S.W. Hormonal Control of the Yolk Precursor Vitellogenin Regulates Immune Function and Longevity in Honeybees. Exp. Gerontol. 2004, 39, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Norberg, K.; Page, R.E.; Erber, J.; Scheiner, R. Downregulation of Vitellogenin Gene Activity Increases the Gustatory Responsiveness of Honey Bee Workers (Apis mellifera). Behav. Brain Res. 2006, 169, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Aase, A.L.T.O.; Seehuus, S.C.; Kim Fondrk, M.; Norberg, K.; Hartfelder, K. Social Reversal of Immunosenescence in Honey Bee Workers. Exp. Gerontol. 2005, 40, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Seehuus, S.C.; Norberg, K.; Gimsa, U.; Krekling, T.; Amdam, G.V. Reproductive Protein Protects Functionally Sterile Honey Bee Workers from Oxidative Stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Bordier, C.; Suchail, S.; Pioz, M.; Devaud, J.M.; Collet, C.; Charreton, M.; Le Conte, Y.; Alaux, C. Stress Response in Honeybees Is Associated with Changes in Task-Related Physiology and Energetic Metabolism. J. Insect Physiol. 2017, 98, 47–54. [Google Scholar] [CrossRef]
- Prado, A.; Brunet, J.L.; Peruzzi, M.; Bonnet, M.; Bordier, C.; Crauser, D.; Le Conte, Y.; Alaux, C. Warmer Winters Are Associated with Lower Levels of the Cryoprotectant Glycerol, a Slower Decrease in Vitellogenin Expression and Reduced Virus Infections in Winter Honeybees. J. Insect Physiol. 2022, 136, 104348. [Google Scholar] [CrossRef] [PubMed]
- Dalmon, A.; Peruzzi, M.; Le Conte, Y.; Alaux, C.; Pioz, M. Temperature-Driven Changes in Viral Loads in the Honey Bee Apis mellifera. J. Invertebr. Pathol. 2019, 160, 87–94. [Google Scholar] [CrossRef]
- Lin, Y.W.; Chen, C.H.; Hsu, C.Y. Middle-Aged Worker Bees Express Higher Innate Immunity than Young Worker Bees in the Abdomen without the Digestive Tract of Worker Bees Reared in an Incubator. Insects 2022, 13, 209. [Google Scholar] [CrossRef]
- Bull, J.C.; Ryabov, E.V.; Prince, G.; Mead, A.; Zhang, C.; Baxter, L.A.; Pell, J.K.; Osborne, J.L.; Chandler, D. A Strong Immune Response in Young Adult Honeybees Masks Their Increased Susceptibility to Infection Compared to Older Bees. PLoS Pathog. 2012, 8, e1003083. [Google Scholar] [CrossRef]
- Steinmann, N.; Corona, M.; Neumann, P.; Dainat, B. Overwintering Is Associated with Reduced Expression of Immune Genes and Higher Susceptibility to Virus Infection in Honey Bees. PLoS ONE 2015, 10, e0129956. [Google Scholar] [CrossRef] [PubMed]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Virus and DsRNA-Triggered Transcriptional Responses Reveal Key Components of Honey Bee Antiviral Defense. Sci. Rep. 2017, 7, 6448. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M. Dicing and Slicing: The Core Machinery of the RNA Interference Pathway. FEBS Lett. 2005, 579, 5822–5829. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Miesen, P.; van Rij, R.P. Antiviral RNAi in insects and mammals: Parallels and differences. Viruses 2019, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, S.A.; Koonin, E. V Origins and Evolution of Eukaryotic RNA Interference the MiRNA and SiRNA Machinery. Trends Ecol. Evol. 2008, 23, 578–587. [Google Scholar] [CrossRef]
- Torri, A.; Jaeger, J.; Pradeu, T.; Saleh, M.C. The Origin of RNA Interference: Adaptive or Neutral Evolution? PLoS Biol. 2022, 20, e3001715. [Google Scholar] [CrossRef]
- Zamore, P.D.; Haley, B. Ribo-Gnome: The Big World of Small RNAs. Science 2005, 309, 1519–1524. [Google Scholar] [CrossRef]
- Neumeier, J.; Meister, G. SiRNA Specificity: RNAi Mechanisms and Strategies to Reduce Off-Target Effects. Front. Plant Sci. 2021, 11, 526455. [Google Scholar] [CrossRef]
- Deddouche, S.; Matt, N.; Budd, A.; Mueller, S.; Kemp, C.; Galiana-Arnoux, D.; Dostert, C.; Antoniewski, C.; Hoffmann, J.A.; Imler, J.L. The DExD/H-Box Helicase Dicer-2 Mediates the Induction of Antiviral Activity in Drosophila. Nat. Immunol. 2008, 9, 1425–1432. [Google Scholar] [CrossRef]
- Paradkar, P.N.; Trinidad, L.; Voysey, R.; Duchemin, J.B.; Walker, P.J. Secreted Vago Restricts West Nile Virus Infection in Culex Mosquito Cells by Activating the Jak-STAT Pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 18915–18920. [Google Scholar] [CrossRef]
- Bang, I.S. INVI TED R EVI EW JAK/STAT Signaling in Insect Innate Immunity. Entomol. Res. 2019, 49, 339–353. [Google Scholar] [CrossRef]
- Merkling, S.H.; van Rij, R.P. Beyond RNAi: Antiviral defense strategies in Drosophila and mosquito. J. Insect Physiol. 2013, 59, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Schlüns, H.; Crozier, R.H. Relish Regulates Expression of Antimicrobial Peptide Genes in the Honeybee, Apis mellifera, Shown by RNA Interference. Insect Mol. Biol. 2007, 16, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune Pathways and Defence Mechanisms in Honey Bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef]
- Nishide, Y.; Kageyama, D.; Yokoi, K.; Jouraku, A.; Tanaka, H.; Futahashi, R.; Fukatsu, T. Functional Crosstalk across IMD and Toll Pathways: Insight into the Evolution of Incomplete Immune Cascades. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182207. [Google Scholar] [CrossRef]
- Aronstein, K.A.; Murray, K.D.; Saldivar, E. Transcriptional Responses in Honey Bee Larvae Infected with Chalkbrood Fungus. BMC Genom. 2010, 11, 391. [Google Scholar] [CrossRef]
- Lourenço, A.P.; Florecki, M.M.; Simões, Z.L.P.; Evans, J.D. Silencing of Apis mellifera Dorsal Genes Reveals Their Role in Expression of the Antimicrobial Peptide Defensin-1. Insect Mol. Biol. 2018, 27, 577–589. [Google Scholar] [CrossRef]
- Kingsolver, M.B.; Huang, Z.; Hardy, R.W. Insect Antiviral Innate Immunity: Pathways, Effectors, and Connections. J. Mol. Biol. 2013, 425, 4921–4936. [Google Scholar] [CrossRef]
- Feng, M.; Fei, S.; Xia, J.; Labropoulou, V.; Swevers, L.; Sun, J. Antimicrobial Peptides as Potential Antiviral Factors in Insect Antiviral Immune Response. Front. Immunol. 2020, 11, 2030. [Google Scholar] [CrossRef]
- Millanta, F.; Sagona, S.; Mazzei, M.; Forzan, M.; Poli, A.; Felicioli, A. Phenoloxidase Activity and Haemolymph Cytology in Honeybees Challenged with a Virus Suspension (Deformed Wings Virus DWV) or Phosphate Buffered Suspension (PBS). Cienc. Rural 2019, 49, 1–9. [Google Scholar] [CrossRef]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A Key Component of the Insect Immune System. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- Laughton, A.M.; Boots, M.; Siva-Jothy, M.T. The Ontogeny of Immunity in the Honey Bee, Apis mellifera L. Following an Immune Challenge. J. Insect Physiol. 2011, 57, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Zufelato, M.S.; Lourenço, A.P.; Simões, Z.L.P.; Jorge, J.A.; Bitondi, M.M.G. Phenoloxidase Activity in Apis mellifera Honey Bee Pupae, and Ecdysteroid-Dependent Expression of the Prophenoloxidase MRNA. Insect Biochem. Mol. Biol. 2004, 34, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Elias-Neto, M.; Nascimento, A.L.O.; Bonetti, A.M.; Nascimento, F.S.; Mateus, S.; Garófalo, C.A.; Bitondi, M.M.G. Heterochrony of Cuticular Differentiation in Eusocial Corbiculate Bees. Apidologie 2014, 45, 397–408. [Google Scholar] [CrossRef]
- Washburn, J.O.; Kirkpatrick, B.A.; Volkman, L.E. Insect Protection against Viruses. Nature 1996, 383, 767. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Kryukova, N.A.; Glupov, V.V.; Ratcliffe, N.A. Encapsulation and Nodulation in Insects. Invertebr. Surviv. J. 2016, 13, 229–246. [Google Scholar]
- Schmid, M.R.; Brockmann, A.; Pirk, C.W.W.; Stanley, D.W.; Tautz, J. Adult Honeybees (Apis mellifera L.) Abandon Hemocytic, but Not Phenoloxidase-Based Immunity. J. Insect Physiol. 2008, 54, 439–444. [Google Scholar] [CrossRef]
- Hystad, E.M.; Salmela, H.; Amdam, G.V.; Münch, D. Hemocyte-Mediated Phagocytosis Differs between Honey Bee (Apis mellifera) Worker Castes. PLoS ONE 2017, 12, e0184108. [Google Scholar] [CrossRef]
- Wilson-Rich, N.; Dres, S.T.; Starks, P.T. The Ontogeny of Immunity: Development of Innate Immune Strength in the Honey Bee (Apis mellifera). J. Insect Physiol. 2008, 54, 1392–1399. [Google Scholar] [CrossRef]
- Schmid-Hempel, P. Parasites and Their Social Hosts. Apidologie 1995, 26, 255–271. [Google Scholar] [CrossRef]
- Desai, S.D.; Currie, R.W. Genetic Diversity within Honey Bee Colonies Affects Pathogen Load and Relative Virus Levels in Honey Bees, Apis mellifera L. Behav. Ecol. Sociobiol. 2015, 69, 1527–1541. [Google Scholar] [CrossRef]
- Evans, J.D.; Spivak, M. Socialized Medicine: Individual and Communal Disease Barriers in Honey Bees. J. Invertebr. Pathol. 2010, 103, S62–S72. [Google Scholar] [CrossRef] [PubMed]
- Castella, G.; Chapuisat, M.; Moret, Y.; Christe, P. The Presence of Conifer Resin Decreases the Use of the Immune System in Wood Ants. Ecol. Entomol. 2008, 33, 408–412. [Google Scholar] [CrossRef]
- Wilson-Rich, N.; Spivak, M.; Fefferman, N.H.; Starks, P.T. Genetic, Individual, and Group Facilitation of Disease Resistance in Insect Societies. Annu. Rev. Entomol. 2009, 54, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Harpur, B.A.; Chernyshova, A.; Soltani, A.; Tsvetkov, N.; Mahjoorighasrodashti, M.; Xu, Z.; Zayed, A. No Genetic Tradeoffs between Hygienic Behaviour and Individual Innate Immunity in the Honey Bee, Apis mellifera. PLoS ONE 2014, 9, e104214. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sato, J.A.; Chline, N.; Martin, S.J.; Hughes, W.O.H.; Ratnieks, F.L.W. Multi-Level Selection for Hygienic Behaviour in Honeybees. Heredity 2009, 102, 609–615. [Google Scholar] [CrossRef]
- Oxley, P.R.; Spivak, M.; Oldroyd, B.P. Six Quantitative Trait Loci Influence Task Thresholds for Hygienic Behaviour in Honeybees (Apis mellifera). Mol. Ecol. 2010, 19, 1452–1461. [Google Scholar] [CrossRef]
- Le Conte, Y.; Meixner, M.D.; Brandt, A.; Carreck, N.L.; Costa, C.; Mondet, F.; Büchler, R. Geographical Distribution and Selection of European Honey Bees Resistant to Varroa destructor. Insects 2020, 11, 873. [Google Scholar] [CrossRef]
- Pusceddu, M.; Cini, A.; Alberti, S.; Salaris, E. Honey Bees Increase Social Distancing When Facing the Ectoparasite Varroa destructor. Sci. Adv. 2021, 7, eabj1398. [Google Scholar] [CrossRef]
- Conroy, T.E.; Holman, L. Social Immunity in the Honey Bee: Do Immune-Challenged Workers Enter Enforced or Self-Imposed Exile? Behav. Ecol. Sociobiol. 2022, 76, 32. [Google Scholar] [CrossRef]
- Starks, P.; Blackie, C.; Seeley, T. Fever in Honeybee Colonies. Naturwissenschaften 2000, 87, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Goblirsch, M.; Warner, J.F.; Sommerfeldt, B.A.; Spivak, M. Social Fever or General Immune Response? Revisiting an Example of Social Immunity in Honey Bees. Insects 2020, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Bordier, C.; Dechatre, H.; Suchail, S.; Peruzzi, M.; Soubeyrand, S.; Pioz, M.; Pélissier, M.; Crauser, D.; Le Conte, Y.; Alaux, C. Colony Adaptive Response to Simulated Heat Waves and Consequences at the Individual Level in Honeybees (Apis mellifera). Sci. Rep. 2017, 7, 3760. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Gaifullina, L.R.; Saltykova, E.S.; Poskryakov, A.V.; Nikolaenko, A.G. Defensins in the Honeybee Antiinfectious Protection. J. Evol. Biochem. Physiol. 2013, 49, 1–9. [Google Scholar] [CrossRef]
- Harwood, G.; Salmela, H.; Freitak, D.; Amdam, G. Social Immunity in Honey Bees: Royal Jelly as a Vehicle in Transferring Bacterial Pathogen Fragments between Nestmates. J. Exp. Biol. 2021, 224, jeb231076. [Google Scholar] [CrossRef] [PubMed]
- Borba, R.S.; Klyczek, K.K.; Mogen, K.L.; Spivak, M. Seasonal Benefits of a Natural Propolis Envelope to Honey Bee Immunity and Colony Health. J. Exp. Biol. 2015, 218, 3689–3699. [Google Scholar] [CrossRef] [PubMed]
- Drescher, N.; Klein, A.M.; Neumann, P.; Yañez, O.; Leonhardt, S.D. Inside Honeybee Hives: Impact of Natural Propolis on the Ectoparasitic Mite Varroa destructor and Viruses. Insects 2017, 8, 15. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.D.; Spivak, M. Increased Resin Collection after Parasite Challenge: A Case of Self-Medication in Honey Bees? PLoS ONE 2012, 7, 17–21. [Google Scholar] [CrossRef]
- Ripari, N.; Sartori, A.A.; Honorio, M.D.S.; Conte, F.L.; Tasca, K.I.; Santiago, K.B.; Sforcin, J.M. Propolis Antiviral and Immunomodulatory Activity: A Review and Perspectives for COVID-19 Treatment. J. Pharm. Pharmacol. 2021, 73, 281–299. [Google Scholar] [CrossRef]
- Contreras-Garduño, J.; Lanz-Mendoza, H.; Franco, B.; Nava, A.; Pedraza-Reyes, M.; Canales-Lazcano, J. Insect Immune Priming: Ecology and Experimental Evidences. Ecol. Entomol. 2016, 41, 351–366. [Google Scholar] [CrossRef]
- Harwood, G.; Amdam, G.; Freitak, D. The Role of Vitellogenin in the Transfer of Immune Elicitors from Gut to Hypopharyngeal Glands in Honey Bees (Apis mellifera). J. Insect Physiol. 2019, 112, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Sadd, B.M.; Kleinlogel, Y.; Schmid-Hempel, R.; Schmid-Hempel, P. Trans-Generational Immune Priming in a Social Insect. Biol. Lett. 2005, 1, 386–388. [Google Scholar] [CrossRef] [PubMed]
- DeGrandi-Hoffman, G.; Chen, Y.; Huang, E.; Huang, M.H. The Effect of Diet on Protein Concentration, Hypopharyngeal Gland Development and Virus Load in Worker Honey Bees (Apis mellifera L.). J. Insect Physiol. 2010, 56, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, T.; Krejcova, G.; Bajgar, A.; Nedbalova, P.; Strasser, P. Molecular Regulations of Metabolism during Immune Response in Insects. Insect Biochem. Mol. Biol. 2019, 109, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Walton, A.; Toth, A.L.; Dolezal, A.G. Developmental Environment Shapes Honeybee Worker Response to Virus Infection. Sci. Rep. 2021, 11, 13961. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed]
- Branchiccela, B.; Castelli, L.; Corona, M.; Díaz-Cetti, S.; Invernizzi, C.; Martínez de la Escalera, G.; Mendoza, Y.; Santos, E.; Silva, C.; Zunino, P.; et al. Impact of Nutritional Stress on the Honeybee Colony Health. Sci. Rep. 2019, 9, 10156. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.P.; Powell, J.E.; Perutka, J.; Geng, P.; Heckmann, L.C.; Horak, R.D.; Davies, B.W.; Ellington, A.D.; Barrick, J.E.; Moran, N.A. Engineered Symbionts Activate Honey Bee Immunity and Limit Pathogens. Science 2020, 576, 573–576. [Google Scholar] [CrossRef]
- Kuster, R.D.; Boncristiani, H.F.; Rueppell, O. Immunogene and Viral Transcript Dynamics during Parasitic Varroa destructor Mite Infection of Developing Honey Bee (Apis mellifera) Pupae. J. Exp. Biol. 2014, 217, 1710–1718. [Google Scholar] [CrossRef]
- Navajas, M.; Migeon, A.; Alaux, C.; Martin-Magniette, M.L.; Robinson, G.E.; Evans, J.D.; Cros-Arteil, S.; Crauser, D.; Le Conte, Y. Differential Gene Expression of the Honey Bee Apis mellifera Associated with Varroa destructor Infection. BMC Genom. 2008, 9, 301. [Google Scholar] [CrossRef]
- Castelli, L.; García, M.L.G.; Dalmon, A.; Arredondo, D.; Antúnez, K.; Invernizzi, C.; Reynaldi, F.J.; Le Conte, Y.; Beaurepaire, A. Intra-Colonial Viral Infections in Western Honey Bees (Apis mellifera). Microorganisms 2021, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- DaPalma, T.; Doonan, B.P.; Trager, N.M.; Kasman, L.M. A Systematic Approach to Virus-Virus Interactions. Virus Res. 2010, 149, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maori, E.; Lavi, S.; Mozes-Koch, R.; Gantman, Y.; Peretz, Y.; Edelbaum, O.; Tanne, E.; Sela, I. Isolation and Characterization of Israeli Acute Paralysis Virus, a Dicistrovirus Affecting Honeybees in Israel: Evidence for Diversity Due to Intra- and Inter-Species Recombination. J. Gen. Virol. 2007, 88, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, G.J.; Brettell, L.E.; Martin, S.J.; Dixon, D.; Jones, I.M.; Schroeder, D.C. Superinfection Exclusion and the Long-Term Survival of Honey Bees in Varroa-Infested Colonies. ISME J. 2016, 10, 1182–1191. [Google Scholar] [CrossRef]
- Gusachenko, O.N.; Woodford, L.; Balbirnie-Cumming, K.; Evans, D.J. First Come, First Served: Superinfection Exclusion in Deformed Wing Virus Is Dependent upon Sequence Identity and Not the Order of Virus Acquisition. ISME J. 2021, 15, 3704–3713. [Google Scholar] [CrossRef]
- Nickbakhsh, S.; Mair, C.; Matthews, L.; Reeve, R.; Johnson, P.C.D.; Thorburn, F.; Von Wissmann, B.; Reynolds, A.; McMenamin, J.; Gunson, R.N.; et al. Virus-Virus Interactions Impact the Population Dynamics of Influenza and the Common Cold. Proc. Natl. Acad. Sci. USA 2019, 116, 27142–27150. [Google Scholar] [CrossRef]
- Biancotto, A.; Iglehart, S.J.; Lisco, A.; Vanpouille, C.; Grivel, J.C.; Lurain, N.S.; Reichelderfer, P.S.; Margolis, L.B. Upregulation of Human Cytomegalovirus by HIV Type 1 in Human Lymphoid Tissue Ex Vivo. AIDS Res. Hum. Retrovir. 2008, 24, 453–462. [Google Scholar] [CrossRef]
- Cox-Foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Quan, P.; Briese, T.; Hornig, M.; Geiser, D.M.; et al. A Metagenomic Survey of Microbes in Honey Bee Colony Collapse Disorder. Science 2007, 318, 283–288. [Google Scholar] [CrossRef]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive Markers of Honey Bee Colony Collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Wilfert, L.; Martin, S.J.; Jones, I.M.; Schroeder, D.C. Diversity in a honey bee pathogen: First report of a third master variant of the Deformed Wing Virus quasispecies. ISME J. 2016, 10, 1264–1273. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Brettell, L.E.; Chejanovsky, N.; Childers, A.K.; Dalmon, A.; Deboutte, W.; de Graaf, D.C.; Doublet, V.; Gebremedhn, H.; Genersch, E.; et al. Cold case: The disappearance of Egypt bee virus, a fourth distinct master strain of deformed wing virus linked to honeybee mortality in 1970’s Egypt. Virol. J. 2022, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Norton, A.M.; Remnant, E.J.; Buchmann, G.; Beekman, M. Accumulation and Competition Amongst Deformed Wing Virus Genotypes in Naïve Australian Honeybees Provides Insight into the Increasing Global Prevalence of Genotype B. Front. Microbiol. 2020, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.P.; Natsopoulou, M.E.; Doublet, V.; Fürst, M.; Weging, S.; Brown, M.J.F.; Gogol-Döring, A.; Paxton, R.J. Elevated Virulence of an Emerging Viral Genotype as a Driver of Honeybee Loss. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160811. [Google Scholar] [CrossRef] [PubMed]
- Natsopoulou, M.E.; McMahon, D.P.; Doublet, V.; Frey, E.; Rosenkranz, P.; Paxton, R.J. The Virulent, Emerging Genotype B of Deformed Wing Virus Is Closely Linked to Overwinter Honeybee Worker Loss. Sci. Rep. 2017, 7, 5242. [Google Scholar] [CrossRef] [PubMed]
- Al Naggar, Y.; Paxton, R.J. The novel insecticides flupyradifurone and sulfoxaflor do not act synergistically with viral pathogens in reducing honey bee (Apis mellifera) survival but sulfoxaflor modulates host immunocompetence. Microb. Biotechnol. 2021, 14, 227–240. [Google Scholar] [CrossRef]
- Tehel, A.; Vu, Q.; Bigot, D.; Gogol-Döring, A.; Koch, P.; Jenkins, C.; Doublet, V.; Theodorou, P.; Paxton, R. The Two Prevalent Genotypes of an Emerging Infectious Disease, Deformed Wing Virus, Cause Equally Low Pupal Mortality and Equally High Wing Deformities in Host Honey Bees. Viruses 2019, 11, 114. [Google Scholar] [CrossRef]
- Zioni, N.; Soroker, V.; Chejanovsky, N. Replication of Varroa destructor Virus 1 (VDV-1) and a Varroa destructor Virus 1-Deformed Wing Virus Recombinant (VDV-1-DWV) in the Head of the Honey Bee. Virology 2011, 417, 106–112. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Childers, A.K.; Chen, Y.; Madella, S.; Nessa, A.; VanEngelsdorp, D.; Evans, J.D. Recent Spread of Varroa destructor Virus-1, a Honey Bee Pathogen, in the United States. Sci. Rep. 2017, 7, 17447. [Google Scholar] [CrossRef]
- Woodford, L.; Evans, D.J. Deformed Wing Virus: Using Reverse Genetics to Tackle Unanswered Questions about the Most Important Viral Pathogen of Honey Bees. FEMS Microbiol. Rev. 2021, 45, fuaa070. [Google Scholar] [CrossRef]
- Amiri, E.; Herman, J.J.; Strand, M.K.; Tarpy, D.R.; Rueppell, O. Egg Transcriptome Profile Responds to Maternal Virus Infection in Honey Bees, Apis mellifera. Infect. Genet. Evol. 2020, 85, 104558. [Google Scholar] [CrossRef]
- Dubois, E.; Dardouri, M.; Schurr, F.; Cougoule, N.; Sircoulomb, F.; Thiéry, R. Outcomes of Honeybee Pupae Inoculated with Deformed Wing Virus Genotypes A and B. Apidologie 2020, 51, 18–34. [Google Scholar] [CrossRef]
- Mondet, F.; de Miranda, J.R.; Kretzschmar, A.; Le Conte, Y.; Mercer, A.R. On the Front Line: Quantitative Virus Dynamics in Honeybee (Apis mellifera L.) Colonies along a New Expansion Front of the Parasite Varroa destructor. PLoS Pathog. 2014, 10, e1004323. [Google Scholar] [CrossRef] [PubMed]
- BAILEY, L.; BALL, B.V.; PERRY, J.N. The Prevalence of Viruses of Honey Bees in Britain. Ann. Appl. Biol. 1981, 97, 109–118. [Google Scholar] [CrossRef]
- Carrillo-Tripp, J.; Dolezal, A.G.; Goblirsch, M.J.; Miller, W.A.; Toth, A.L.; Bonning, B.C. In Vivo and in Vitro Infection Dynamics of Honey Bee Viruses. Sci. Rep. 2016, 6, 22265. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Berry, B.; Tassetto, M.; Kunitomi, M.; Acevedo, A.; Deng, C.; Krutchinsky, A.; Gross, J.; Antoniewski, C.; Andino, R. Cricket Paralysis Virus Antagonizes Argonaute 2 to Modulate Antiviral Defense in Drosophila. Nat. Struct. Mol. Biol. 2010, 17, 547–554. [Google Scholar] [CrossRef]
- Bailey, L.; Milne, R.G. The Multiplication Regions and Interaction of Acute and Chronic Bee-Paralysis Viruses in Adult Honey Bees. J. Gen. Virol. 1969, 4, 9–14. [Google Scholar] [CrossRef]
- Olivier, V.; Massou, I.; Celle, O.; Blanchard, P.; Schurr, F.; Ribière, M.; Gauthier, M. In Situ Hybridization Assays for Localization of the Chronic Bee Paralysis Virus in the Honey Bee (Apis mellifera) Brain. J. Virol. Methods 2008, 153, 232–237. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, S.; Zhao, H.; Diao, Q.; Hou, C. IAPV-Induced Paralytic Symptoms Associated with Tachypnea via Impaired Tracheal System Function. Int. J. Mol. Sci. 2021, 22, 10078. [Google Scholar] [CrossRef]
- Konig, C.; Schmid-Hempel, P. Foraging Activity and Immunocompetence in Workers of the Bumble Bee, Bombus terrestris L. Proc. R. Soc. B Biol. Sci. 1995, 260, 225–227. [Google Scholar] [CrossRef]
- Moret, Y.; Schmid-Hempel, P. Survival for Immunity: The Price of Immune System Activation for Bumblebee Workers. Science 2000, 290, 1166–1168. [Google Scholar] [CrossRef]
- Mobley, M.W.; Gegear, R.J. Immune-Cognitive System Connectivity Reduces Bumblebee Foraging Success in Complex Multisensory Floral Environments. Sci. Rep. 2018, 8, 5953. [Google Scholar] [CrossRef]
- Riessberger-Gallé, U.; Hernández López, J.; Schuehly, W.; Crockett, S.; Krainer, S.; Crailsheim, K. Immune Responses of Honeybees and Their Fitness Costs as Compared to Bumblebees. Apidologie 2015, 46, 238–249. [Google Scholar] [CrossRef]
- Vilcinskas, A. Evolutionary Plasticity of Insect Immunity. J. Insect Physiol. 2013, 59, 123–129. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Paxton, R.J. Mode of transmission determines the virulence of black queen cell virus in adult honey bees, posing a future threat to bees and apiculture. Viruses 2020, 12, 535. [Google Scholar] [CrossRef] [PubMed]
- Le Clec’h, W.; Dittmer, J.; Raimond, M.; Bouchon, D.; Sicard, M. Phenotypic shift in Wolbachia virulence towards its native host across serial horizontal passages. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171076. [Google Scholar] [CrossRef] [PubMed]
- Lark, K.K.; Un, Y.C.; Hwan, S.C.; Jung, S.L.; Bin Lee, W.; Kim, J.; Jeong, K.; Shim, J.; Kim-Ha, J.; Kim, Y.J. Down-Regulation of NF-ΚB Target Genes by the AP-1 and STAT Complex during the Innate Immune Response in Drosophila. PLoS Biol. 2007, 5, 2064–2076. [Google Scholar] [CrossRef]
- De Smet, L.; Ravoet, J.; Wenseleers, T.; de Graaf, D.C. Expression of Key Components of the RNAi Machinery Are Suppressed in Apis mellifera That Suffer a High Virus Infection. Entomol. Sci. 2017, 20, 76–85. [Google Scholar] [CrossRef]
- Adamo, S.A. Stress Responses Sculpt the Insect Immune System, Optimizing Defense in an Ever-Changing World. Dev. Comp. Immunol. 2017, 66, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Adamo, S.A. The Stress Response and Immune System Share, Borrow, and Reconfigure Their Physiological Network Elements: Evidence from the Insects. Horm. Behav. 2017, 88, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ravoet, J.; De Smet, L.; Wenseleers, T.; de Graaf, D.C. Vertical Transmission of Honey Bee Viruses in a Belgian Queen Breeding Program. BMC Vet. Res. 2015, 11, 61. [Google Scholar] [CrossRef]
- Shen, M.; Cui, L.; Ostiguy, N.; Cox-Foster, D. Intricate Transmission Routes and Interactions between Picorna-like Viruses (Kashmir Bee Virus and Sacbrood Virus) with the Honeybee Host and the Parasitic Varroa Mite. J. Gen. Virol. 2005, 86, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Yan, X.; Han, R. Prevention of Chinese Sacbrood Virus Infection in Apis cerana Using RNA Interference. Curr. Microbiol. 2010, 61, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, E.V.; Christmon, K.; Heerman, M.C.; Posada-Florez, F.; Harrison, R.L.; Chen, Y.; Evans, J.D. Development of a Honey Bee RNA Virus Vector Based on the Genome of a Deformed Wing Virus. Viruses 2020, 12, 374. [Google Scholar] [CrossRef]
- Jin, L.; Mehmood, S.; Zhang, G.; Song, Y.; Su, S.; Huang, S.; Huang, H.; Zhang, Y.; Geng, H.; Huang, W.F. Visualizing Sacbrood Virus of Honey Bees via Transformation and Coupling with Enhanced Green Fluorescent Protein. Viruses 2020, 12, 224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durand, A.M.; Bonjour-Dalmon, A.; Dubois, E. Viral Co-Infections and Antiviral Immunity in Honey Bees. Viruses 2023, 15, 1217. https://doi.org/10.3390/v15051217
Durand AM, Bonjour-Dalmon A, Dubois E. Viral Co-Infections and Antiviral Immunity in Honey Bees. Viruses. 2023; 15(5):1217. https://doi.org/10.3390/v15051217
Chicago/Turabian StyleDurand, Alice Mélusine, Anne Bonjour-Dalmon, and Eric Dubois. 2023. "Viral Co-Infections and Antiviral Immunity in Honey Bees" Viruses 15, no. 5: 1217. https://doi.org/10.3390/v15051217
APA StyleDurand, A. M., Bonjour-Dalmon, A., & Dubois, E. (2023). Viral Co-Infections and Antiviral Immunity in Honey Bees. Viruses, 15(5), 1217. https://doi.org/10.3390/v15051217






