Abstract
Culex mosquitoes are the primary vectors of the Japanese encephalitis virus (JEV). Since its discovery in 1935, Japanese encephalitis (JE), caused by JEV, has posed a significant threat to human health. Despite the widespread implementation of several JEV vaccines, the transmission chain of JEV in the natural ecosystem has not changed, and the vector of transmission cannot be eradicated. Therefore, JEV is still the focus of attention for flaviviruses. At present, there is no clinically specific drug for JE treatment. JEV infection is a complex interaction between the virus and the host cell, which is the focus of drug design and development. An overview of antivirals that target JEV elements and host factors is presented in this review. In addition, drugs that balance antiviral effects and host protection by regulating innate immunity, inflammation, apoptosis, or necrosis are reviewed to treat JE effectively.
1. Introduction
Japanese encephalitis virus (JEV) is a single-stranded plus-stranded RNA virus belonging to the genus Flavivirus of the family Flaviridae [1]. Based on the nucleotide sequence of the envelope (E) gene, JEV can be divided into five genotypes: GI, GII, GIII, GIV, and GV [2]. GI type is the predominant endemic strain throughout Asia and the Indo-Pacific region [3]. As an arthropod-borne virus, JEV is transmitted by the bites of Culex species mosquitoes, particularly Culex tritaeniorhynchus [4]. Domestic pigs and water birds have been recognized as the two most important JEV-amplifying hosts, which have high enough levels of blood viremia to enable transmission to mosquitoes [5]. Humans and horses are considered to be dead-end hosts [6]. The prevalence of JEV shows specific distribution characteristics on the geographical scale, seasonal scale, climate scale, and population scale [7]. In temperate and subtropical regions, populations in agricultural areas are the primary populations exposed to JEV with the onset of the rainy season, among which, unvaccinated populations and children are at high risk of JEV infection.
The main endemic areas of JEV include Eastern, Southern, and Southeastern Asia, the Western Pacific coast, central and eastern Australia, etc. [8]. According to data released by the World Health Organization in 2011 [9], there are approximately ~68,000 cases of JEV infection each year, including at least 13,000 deaths. Most JE cases are asymptomatic or mild and cause fever and headache; a case fatality rate of ~30% can be observed in those with encephalitis, and permanent neurological or psychiatric sequelae can occur in approximately 30–50% of cases [10]. Several vaccines have been applied in order to prevent the effects of JEV infection, including the attenuated vaccine SAl4-14-2, which has been applied in many countries, inactivated mouse brain-derived vaccines, inactivated Vero cell culture vaccines, and attenuated chimeric vaccines [11]. As such, the incidence of JEV has decreased significantly globally, but vaccination rates remain low in less developed areas. Combined with climate change and the zoonotic cycle of JEV, it is still circulating in some areas. In 2022, 30 confirmed JE cases and 6 deaths were reported in Australia [12]. Due to the convenience and cost-effectiveness of drug therapy, the development of anti-JEV-specific drugs remains a necessary option. This article focuses mainly on a review of the recent developments in research and the mechanisms of antiviral drugs for JEV.
2. Structural and Nonstructural Components of JEV
JEV’s genomic RNA size is about 11 kb, with a methylated cap structure at the 5′ terminus and no poly (A) at the 3′ terminus. A single open-reading-frame (ORF) is found between the 5′- and 3′- untranslated regions (UTRs); these encode three structural proteins, including capsid(C), precursor membrane (PrM), and envelope, and seven nonstructural (NS) proteins, including NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5 [13]. Structural and nonstructural proteins are essential targets for antiviral drug design.
2.1. Structural Proteins
The JEV C protein, with a molecular weight of 11 kDa, can pack viral RNA and is the core component of the nucleocapsid. Each monomer molecule of the C protein dimer has four α-helix structures. C protein dimerization is essential for its association with viral RNA [14,15].
The E proteins form the outer shell and are anchored to the lipid bilayer envelope through their transmembrane helices and the M protein. The E protein plays a role in receptor binding and membrane fusion and belongs to the classII fusion glycoprotein and contains five domains, among which DI, DII, and DIII are the ectodomains of the virus. The DI domain connects the DII and DIII domains and participates in E protein conformational changes and stability. The DII domain contains fusion peptides and has the function of viral membrane fusion. The fusion peptide of one E monomer is buried between DI and DIII of the adjacent monomer within a dimer. DIII, containing linear antigenic epitopes, is regarded as an antigen [16]. The prM protein might function as a chaperone for the folding and assembly of the E protein [17]. In the trans-Golgi network, furin or furin-like protease cleave the prM protein to produce M protein. The outer surface of mature JEV virions comprises 180 copies of the E and M proteins. Three E-M-M-E heterodimers lying parallel to each other form a raft, and 30 such rafts cover the viral surface [18].
2.2. Nonstructural Proteins
JEV nonstructural proteins are essential in viral genome replication, protein translation, and viral particle assembly, and they regulate innate immunity. NS1 is a glycoprotein that plays a role in the replication process of viruses [19]. NS2A is a small hydrophobic transmembrane protein with no enzymatic activity that plays a role in JEV replication and assembly [20]. NS2B is also an integral membrane protein as a cofactor of NS3 and binds to NS3 to perform the NS2B/NS3 protease function [21]. NS3 is a multifunctional enzyme with three domains. The N-terminal of NS3 contains the protease domain, which exhibits serine protease activity. With the cofactor NS2B, NS3 is required to process polyproteins into individual NS proteins. The C-terminal of NS3 has a RNA helicase/NTPase domain and RNA triphosphatase, which are required for unwinding the double-stranded RNA during viral RNA synthesis; this is the first step required for RNA capping by NS5 [22,23]. Due to its crucial enzyme function, NS3 is a key target for the development of anti-JEV drugs [24]. NS4A and NS4B are transmembrane proteins that play a role in viral replication and the regulation of the host immune response [25,26].
NS5 is the most highly conserved and largest nonstructural protein. Its N-terminal contains an S-adenosyl methyltransferase (SAM) (MTase) domain structure and its C-terminal contains an RNA-dependent RNA polymerase (RdRp) domain. MTase has seven β folds, surrounded by four α helices, that can cap viral mRNA. RdRp has RNA-dependent RNA polymerase activity and plays a significant role in viral RNA replication [27,28,29]. In addition to its above function as an enzyme, NS5 can also antagonize the interferon (IFN)-mediated innate immune response through its interaction with hSTAT2 [30]. Due to its highly conserved nature in flaviviruses and its essential role in promoting viral infection, NS5 is a preferred target for anti-JEV drug design [24].
3. Lifecycle and Pathogenesis of JEV Infection
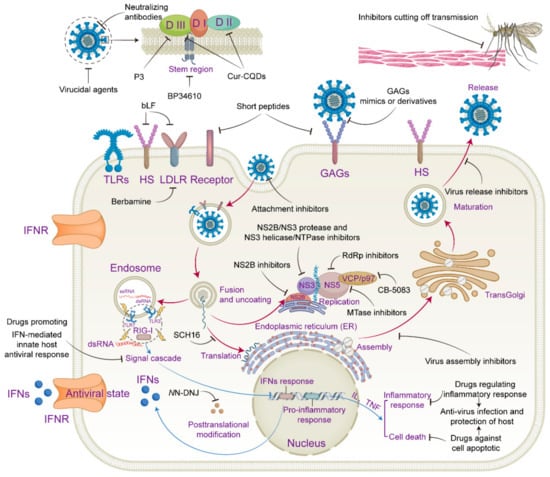
The lifecycle of JEV can be divided into entry, RNA replication, viral protein translation, and viral particle assembly and release. An investigation into which stage of the virus lifecycle antiviral drugs act on will pave the way for revealing the specific target of drug action. The replication cycle of JEV is similar to that of other flaviviruses [18,31,32]. Firstly, the E protein binds to functional receptors and adhesion receptors to initiate the endosome-dependent endocytosis pathway. As the virus undergoes membrane fusion with the endosome, the viral genomic RNA is released into the host cytoplasm, initiating the replication journey of JEV. After the synthesis of the viral RNA and proteins, the newly synthesized viral RNA and structural proteins (C, prM, and E) are transferred to the lumen of the endoplasmic reticulum (ER) and assembled into immature infection-defective JEV virus particles. These immature virions are further transported to the trans-Golgi network (TGN), where prM cleavage occurs at specific sites under the action of the cellular serine protease furin and mature infectious virus particles are finally formed. Finally, cellular exocytosis releases mature JEV virions from the host cell.
JEV is a neurotropic flavivirus; however, its precise route of brain penetration remains unclear. Microglia activation, the innate immune response, the inflammatory response, and neuronal cell death are recognized as essential hallmarks of JE during JEV infection of the central nervous system (CNS). Microglia are considered an essential starting point of inflammatory response when the CNS is triggered by JEV infection [33]. The activation of microglia following JEV infection can release a variety of pro-inflammatory cytokines such as interleukin (IL) and tumor necrosis factor (TNF), triggering a cascade of the inflammatory response, which can prime cell death pathways such as pyroptosis, apoptosis, and necroptosis [34]. The initial line of defense against JEV infection is the host’s innate immune system. Once JEV enters the cell, the viral RNA can be recognized by pattern recognition receptors (PRRs) [35,36], such as Toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs). Then, the succeeding signal cascade causes the production of IFNs and pro-inflammatory cytokines such as IL-1 and IL-18, and the released IFNs attach to their receptor, activating a signaling cascade to commence an antiviral state. Although the innate immune response and inflammatory response stimulated by JEV infection are the host cell’s natural defense line against virus infection, an excessive or prolonged inflammatory response can cause harm to the body. To accomplish the dual effect of anti-JEV and host protection, it is crucial to develop drugs that modify the innate immune response and inflammatory response in an optimal manner.
5. Outlook
Currently, more and more drugs have been reported to be able to inhibit JEV infection. Nonetheless, there are still no clinically approved anti-JE drugs to date. Apart from minocycline, a second-generation antibiotic that has been demonstrated to have a neuroprotective effect on JE in several animal model experiments, no meaningful results have been summarized from the clinical trials of the minocycline treatment of JE due to the low number of JE infection cases [126]. Nonetheless, it remains of concern to continually explore the clinical benefit of minocycline against JEV infection.
In the Flaviviridae family, NS proteins are essential viral elements for the replication of viruses and are crucial targets and hot spots for antiviral drug design. Although no drugs characteristically target NS proteins for the clinical treatment of JEV infection, several inhibitors targeting HCV NSs have been widely used in clinical settings with beneficial outcomes [127]. For example, the NS3/4A protease inhibitor pibrentasvir, NS5A inhibitor glecaprevir, NS5B polymerase inhibitor sofosbuvir, NS5A inhibitor velpatasvir and NS3/4A protease inhibitor voxilaprevir, all of which have shown promising effects in the clinical treatment of anti-HCV infection, may subsequently provide insights that can be used in the research of novel anti-JEV drugs.
Additionally, SCH16, an N-methylisatin-β-thiosemicarbazone derivative, showed promising and intense anti-JEV infection effects at concentrations below the nanomolar level, and significant protection against JEV infection in JEV-infected mice. Furthermore, another study demonstrated that SCH16, combined with ribavirin and mycophenolic acid at the cellular level, had a synergistic effect against JEV infection [106]. Due to this, it is necessary to investigate further the anti-JEV infection mechanism of SCH16 and its potential clinical applications.
6. Final Conclusions
JEV is a mosquito-transmitted flavivirus, and current research focuses on targets for JEV antivirals, mainly the virus itself and factors related to the host. The development of antiviral drugs should be based on a thorough understanding of the lifecycle of JEV. It is, therefore, important to carefully select drug targets to ensure effective antiviral activity, broad-spectrum properties, and minimal toxicity to the host. This review has highlighted several approaches to the design of anti-JEV infection drugs, as follows:
- (1)
- Computer drug design is a convenient and rapid means of drug development and can save costs and time in drug screening. It can identify the amino acid residues in the target protein that interacts with the drug when the precise structure of the viral target protein is known. However, this is still at the theoretical design and analysis stage, and the actual situation needs to be validated by cellular and animal assays. Combined with the results of computer theoretical analyses, the targeted mutation of specific amino acids in target proteins may be an effective means to explore the exact molecular information regarding the interaction between drugs and their targets.
- (2)
- High-throughput drug screening (HTS) at the cellular level is currently an increasingly used tool for antiviral drug development. Several compound libraries contain hundreds of thousands of compounds, and with automated cell manipulation techniques and convenient viral labeling and detection tools, candidate drugs can be identified efficiently and rapidly. In those compound libraries, natural compounds are deserving of attention since they show few side effects and are also easily accessible from natural sources, such as berbamine, rosmarinic acid, arctigenin, and indirubin, all of which could effectively inhibit JEV infection in animal models.
- (3)
- The synthesis of derivatives based on the modification of effective leading compounds. For example, the excellent anti-JEV infection effect shown in vitro and in vivo by the anilidoquinoline analogue PP2 suggests that modifications based on clinically used drugs with potential antiviral effects are an effective means of developing novel anti-JEV drugs.
- (4)
- Drug repurposing, which has become a popular and convenient drug discovery method, intends to uncover new therapeutic properties of old drugs. Minocycline, temoporfin, niclosamide, nitazoxanide, berbamine and fenofibrate are all in clinical use for the treatment of different diseases, but they have also been shown to have potential effects against JEV infection in vivo. Together with these drugs’ safety in humans, they are promising for the clinical treatment of JE.
- (5)
- In-depth analysis of the functions and sequences of the E proteins and NSs proteins of flaviviruses in order to locate the conserved sequences that affect the functions of these viral proteins, as well as the recombinant expression or chemical synthesis of antiviral short peptide inhibitors based on these sequences, is a theoretically feasible and convenient method for antiviral drug development. However, due to the complex structure of E or NSs, these short peptides of viral origin may not completely inhibit the function of E proteins or NSs, and their immunogenicity is of concern.
Besides the methods mentioned above, the development of drugs that specifically cut off the transmission route through mosquito bites is not only a novel idea for JEV antiviral research, but is also a practical and feasible approach. Retnla, which encodes resistin-like molecule-α (RELMα), is an antimicrobial protein on host skin; flaviviruses, such as DENV2 and ZIKV, can suppress the expression of RELMα, which leads to the robust growth of acetophenone-producing bacteria on host skin, resulting in increased acetophenone levels on the skin; this is a volatile compound that is produced by skin-resident bacteria and is attractive to mosquitoes. Vitamin A derivatives such as isotretinoin have been shown to induce RELMa expression, thereby ultimately reducing acetophenone levels on the skin and thus reducing mosquito bites [128]. Therefore, the dietary administration of vitamin A derivatives can contribute to the prevention of JEV infection, especially in high-JE-endemic regions.
Knowledge of JEV has increased dramatically via in-depth studies of its virology, host factors, and innate immune and inflammatory response, which give a boost to the continuous development of candidate drugs in JE treatment. Therefore, further efforts are required in order to research and develop JEV antiviral drugs.
Author Contributions
Y.Z. and S.C. wrote the paper; Q.L. drew the figure; Z.Q. contributed to the proofreading of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (31770181).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations

| Acronym | Full Name |
|---|---|
| JEV | Japanese encephalitis virus |
| DENV | Dengue virus |
| ZIKV | Zika virus |
| YFV | Yellow fever virus |
| SINV | Sindbis virus |
| WNV | West Nile virus |
| HCV | Hepatitis C virus |
| TBEV | Tick-borne encephalitis virus |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| JE | Japanese encephalitis |
| COVID-19 | Coronavirus disease 2019 |
| YF | Yellow fever |
| GI | Genotypes I |
| GII | Genotypes II |
| GIII | Genotypes III |
| GIV | Genotypes IV |
| GV | Genotypes V |
| ORF | Open-reading-frame |
| UTRs | Untranslated regions |
| C | Capsid protein |
| PrM | Precursor membrane protein |
| M | Membrane protein |
| E | Envelope protein |
| FP | Fusion peptide |
| DI | DomainI |
| DII | DomainII |
| DIII | DomainIII |
| NS | Nonstructural protein |
| SAM | S-adenosyl methyltransferase |
| MTase | Methyltransferase |
| RdRp | RNA-dependent RNA polymerase |
| ssRNA | Single-stranded RNA |
| IFN | Interferon |
| TNF | Tumor necrosis factor |
| PRRs | Pattern recognition receptors |
| TLRs | Toll-like receptors |
| RIG-I | Retinoic acid-inducible gene I |
| RLRs | Retinoic acid-inducible gene I (RIG-I)-like receptor |
| IL | Interleukin |
| ROS | Reactive oxygen species |
| Bcl2 | B-cell lymphoma 2 protein |
| GAS | Gamma-activated sequence |
| ISRE | IFN-stimulated response element |
| CNS | Central nervous system |
| BBB | Blood-brain barrier |
| ER | Endoplasmic reticulum |
| TGN | Trans-Golgi network |
| POMs | Polyoxometalates |
| GRFT | Griffithsin |
| CQDs | Carbon quantum dots |
| CUR | Curcumin |
| NN-DNJ | N-nonyl-deoxynojirimycin |
| B200 | A hydroalcoholic belladonna formulation |
| TPB | 3-chloro-N-[({4-[4-(2-thienylcarbonyl)-1-piperazinyl]phenyl}amino)carbonothioyl]-1-benzothiophene-2-carboxamide |
| PP2 | 2-(2-Methyl-quinoline-4ylamino)-N-(2-chlorophenyl)-acetamide |
| mAbs | Monoclonal antibodies |
| CbAE | Chromobacterium antiviral effector |
| bLF | Bovine lactoferrin |
| PAP | Plant-derived antiviral protein |
| AMPs | Antimicrobial peptides |
| RA | Rosmarinic acid |
| UPS | Ubiquitin-proteasome system |
| PF4 | Platelet factor 4 |
| HDAC6 | Histone deacetylase 6 |
| Hsp90 | Heat shock protein 90 |
| RELMα | Retnla encodes resistin-like molecule-α |
| MMP | Matrix metalloproteinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| GAGs | Glycosaminoglycans |
| HS | Heparan sulfate |
| LDLR | Low-density lipoprotein receptor |
| TRPMLs | Ca2+ permeable non-selective cation channels in endosomes and Lysosomes |
| PPARα | Peroxisome proliferator-activated receptor-α |
| EVs | Extracellular vesicles |
| VCP | Valosin-containing protein |
| CCV | Clathrin-coated vesicle |
| CAM | Chorioallantoic membrane |
| PTSA | Protein thermal shift assay |
| HTS | High-throughput drug screening |
References
- McMinn, P.C. The molecular basis of virulence of the encephalitogenic flaviviruses. J. Gen. Virol. 1997, 78 Pt 11, 2711–2722. [Google Scholar] [CrossRef]
- Solomon, T.; Ni, H.; Beasley, D.W.; Ekkelenkamp, M.; Cardosa, M.J.; Barrett, A.D. Origin and evolution of Japanese encephalitis virus in southeast Asia. J. Virol. 2003, 77, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Liu, K.; Anwar, M.N.; Wahaab, A.; Safdar, A.; Di, D.; Boruah, P.; Xu, J.; Wang, X.; Li, B.; et al. The emerged genotype I of Japanese encephalitis virus shows an infectivity similar to genotype III in Culex pipiens mosquitoes from China. PLoS Negl. Trop. Dis. 2019, 13, e0007716. [Google Scholar] [CrossRef] [PubMed]
- Su, C.L.; Yang, C.F.; Teng, H.J.; Lu, L.C.; Lin, C.; Tsai, K.H.; Chen, Y.Y.; Chen, L.Y.; Chang, S.F.; Shu, P.Y. Molecular epidemiology of Japanese encephalitis virus in mosquitoes in Taiwan during 2005–2012. PLoS Negl. Trop. Dis. 2014, 8, e3122. [Google Scholar] [CrossRef] [PubMed]
- Buescher, E.L.; Scherer, W.F.; Rosenberg, M.Z.; Gresser, I.; Hardy, J.L.; Bullock, H.R. Ecologic studies of Japanese encephalitis virus in Japan. II. Mosquito infection. Am. J. Trop. Med. Hyg. 1959, 8, 651–664. [Google Scholar] [CrossRef]
- Weaver, S.C.; Barrett, A.D. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2004, 2, 789–801. [Google Scholar] [CrossRef]
- Joe, S.; Salam, A.A.A.; Neogi, U.; Mudgal, P.P. Antiviral drug research for Japanese encephalitis: An updated review. Pharmacol. Rep. 2022, 74, 273–296. [Google Scholar] [CrossRef]
- Pearce, J.C.; Learoyd, T.P.; Langendorf, B.J.; Logan, J.G. Japanese encephalitis: The vectors, ecology and potential for expansion. J. Travel Med. 2018, 25 (Suppl. S1), S16–S26. [Google Scholar] [CrossRef]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Horton, D.L.; Johnson, N.; Li, L.; Barrett, A.D.T.; Smith, D.J.; Galbraith, S.E.; Solomon, T.; Fooks, A.R. Flavivirus-induced antibody cross-reactivity. J. Gen. Virol. 2011, 92 Pt 12, 2821–2829. [Google Scholar] [CrossRef]
- Hegde, N.R.; Gore, M.M. Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum. Vaccines Immunother. 2017, 13, 1320–1337. [Google Scholar] [CrossRef]
- Yakob, L.; Hu, W.; Frentiu, F.D.; Gyawali, N.; Hugo, L.E.; Johnson, B.; Lau, C.; Furuya-Kanamori, L.; Magalhaes, R.S.; Devine, G. Japanese Encephalitis Emergence in Australia: The Potential Population at Risk. In Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America; Oxford University Press: Oxford, UK, 2022. [Google Scholar]
- Erlanger, T.E.; Weiss, S.; Keiser, J.; Utzinger, J.; Wiedenmayer, K. Past, present, and future of Japanese encephalitis. Emerg. Infect. Dis. 2009, 15, 1–7. [Google Scholar] [CrossRef]
- Jones, C.T.; Ma, L.; Burgner, J.W.; Groesch, T.D.; Post, C.B.; Kuhn, R.J. Flavivirus capsid is a dimeric alpha-helical protein. J. Virol. 2003, 77, 7143–7149. [Google Scholar] [CrossRef]
- Tan, T.Y.; Fibriansah, G.; Kostyuchenko, V.A.; Ng, T.S.; Lim, X.X.; Zhang, S.; Lim, X.N.; Wang, J.; Shi, J.; Morais, M.C.; et al. Capsid protein structure in Zika virus reveals the flavivirus assembly process. Nat. Commun. 2020, 11, 895. [Google Scholar] [CrossRef]
- Hu, T.; Wu, Z.; Wu, S.; Chen, S.; Cheng, A. The key amino acids of E protein involved in early flavivirus infection: Viral entry. Virol. J. 2021, 18, 136. [Google Scholar] [CrossRef]
- Lorenz, I.C.; Allison, S.L.; Heinz, F.X.; Helenius, A. Folding and dimerization of tick-borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J. Virol. 2002, 76, 5480–5491. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.H.; Zhu, L.; Nian, Q.G.; Yuan, S.; Gao, Q.; Hu, Z.; Ye, Q.; Li, X.F.; Xie, D.Y.; et al. Near-atomic structure of Japanese encephalitis virus reveals critical determinants of virulence and stability. Nat. Commun. 2017, 8, 14. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Rice, C.M. trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J. Virol. 1997, 71, 9608–9617. [Google Scholar] [CrossRef]
- Leung, J.Y.; Pijlman, G.P.; Kondratieva, N.; Hyde, J.; Mackenzie, J.M.; Khromykh, A.A. Role of nonstructural protein NS2A in flavivirus assembly. J. Virol. 2008, 82, 4731–4741. [Google Scholar] [CrossRef] [PubMed]
- Sampath, A.; Padmanabhan, R. Molecular targets for flavivirus drug discovery. Antivir. Res. 2009, 81, 6–15. [Google Scholar] [CrossRef]
- Benarroch, D.; Selisko, B.; Locatelli, G.A.; Maga, G.; Romette, J.L.; Canard, B. The RNA helicase, nucleotide 5′-triphosphatase, and RNA 5′-triphosphatase activities of Dengue virus protein NS3 are Mg2+-dependent and require a functional Walker B motif in the helicase catalytic core. Virology 2004, 328, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Falgout, B.; Pethel, M.; Zhang, Y.M.; Lai, C.J. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J. Virol. 1991, 65, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, K.; Chew, B.L.A.; Ho, J.S.; Luo, D. Flavivirus nonstructural proteins and replication complexes as antiviral drug targets. Curr. Opin. Virol. 2023, 59, 101305. [Google Scholar] [CrossRef] [PubMed]
- Klaitong, P.; Smith, D.R. Roles of Non-Structural Protein 4A in Flavivirus Infection. Viruses 2021, 13, 2077. [Google Scholar] [CrossRef]
- Xie, X.; Zou, J.; Wang, Q.Y.; Shi, P.Y. Targeting dengue virus NS4B protein for drug discovery. Antivir. Res. 2015, 118, 39–45. [Google Scholar] [CrossRef]
- Zhou, Y.; Ray, D.; Zhao, Y.; Dong, H.; Ren, S.; Li, Z.; Guo, Y.; Bernard, K.A.; Shi, P.Y.; Li, H. Structure and function of flavivirus NS5 methyltransferase. J. Virol. 2007, 81, 3891–3903. [Google Scholar] [CrossRef]
- Egloff, M.P.; Benarroch, D.; Selisko, B.; Romette, J.L.; Canard, B. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: Crystal structure and functional characterization. EMBO J. 2002, 21, 2757–2768. [Google Scholar] [CrossRef]
- Qian, X.; Qi, Z. Mosquito-Borne Flaviviruses and Current Therapeutic Advances. Viruses 2022, 14, 1226. [Google Scholar] [CrossRef]
- Wang, B.; Thurmond, S.; Zhou, K.; Sanchez-Aparicio, M.T.; Fang, J.; Lu, J.; Gao, L.; Ren, W.; Cui, Y.; Veit, E.C.; et al. Structural basis for STAT2 suppression by flavivirus NS5. Nat. Struct. Mol. Biol. 2020, 27, 875–885. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Yun, S.I.; Lee, Y.M. Early Events in Japanese Encephalitis Virus Infection: Viral Entry. Pathogens 2018, 7, 68. [Google Scholar] [CrossRef]
- McMillan, R.E.; Wang, E.; Carlin, A.F.; Coufal, N.G. Human microglial models to study host-virus interactions. Exp. Neurol. 2023, 363, 114375. [Google Scholar] [CrossRef]
- Ghoshal, A.; Das, S.; Ghosh, S.; Mishra, M.K.; Sharma, V.; Koli, P.; Sen, E.; Basu, A. Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia 2007, 55, 483–496. [Google Scholar] [CrossRef]
- Stone, A.E.L.; Green, R.; Wilkins, C.; Hemann, E.A.; Gale, M., Jr. RIG-I-like receptors direct inflammatory macrophage polarization against West Nile virus infection. Nat. Commun. 2019, 10, 3649. [Google Scholar] [CrossRef]
- Town, T.; Bai, F.; Wang, T.; Kaplan, A.T.; Qian, F.; Montgomery, R.R.; Anderson, J.F.; Flavell, R.A.; Fikrig, E. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity 2009, 30, 242–253. [Google Scholar] [CrossRef]
- Chang, S.J.; Chang, Y.C.; Lu, K.Z.; Tsou, Y.Y.; Lin, C.W. Antiviral Activity of Isatis indigotica Extract and Its Derived Indirubin against Japanese Encephalitis Virus. Evid.-Based Complement. Altern. Med. 2012, 2012, 925830. [Google Scholar] [CrossRef]
- Johari, J.; Kianmehr, A.; Mustafa, M.R.; Abubakar, S.; Zandi, K. Antiviral activity of baicalein and quercetin against the Japanese encephalitis virus. Int. J. Mol. Sci. 2012, 13, 16785–16795. [Google Scholar] [CrossRef]
- Chang, S.J.; Huang, S.H.; Lin, Y.J.; Tsou, Y.Y.; Lin, C.W. Antiviral activity of Rheum palmatum methanol extract and chrysophanol against Japanese encephalitis virus. Arch. Pharmacal Res. 2014, 37, 1117–1123. [Google Scholar] [CrossRef]
- Qi, Y.; Han, L.; Qi, Y.; Jin, X.; Zhang, B.; Niu, J.; Zhong, J.; Xu, Y. Anti-flavivirus activity of polyoxometalate. Antivir. Res. 2020, 179, 104813. [Google Scholar] [CrossRef]
- Ishag, H.Z.; Li, C.; Huang, L.; Sun, M.X.; Wang, F.; Ni, B.; Malik, T.; Chen, P.Y.; Mao, X. Griffithsin inhibits Japanese encephalitis virus infection in vitro and in vivo. Arch. Virol. 2013, 158, 349–358. [Google Scholar] [CrossRef]
- Yu, X.; Tong, L.; Zhang, L.; Yang, Y.; Xiao, X.; Zhu, Y.; Wang, P.; Cheng, G. Lipases secreted by a gut bacterium inhibit arbovirus transmission in mosquitoes. PLoS Pathog. 2022, 18, e1010552. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Lei, Y.; Yang, P.; Gao, Q.; Wang, N.; Cao, L.; Yuan, S.; Huang, X.; Deng, Y.; Ma, W.; et al. Structural basis for neutralization of Japanese encephalitis virus by two potent therapeutic antibodies. Nat. Microbiol. 2018, 3, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hu, H.S.; Lin, H.M.; Wu, P.S.; Wu, R.H.; Tian, J.N.; Wu, S.H.; Tsou, L.K.; Song, J.S.; Chen, H.W.; et al. A novel flavivirus entry inhibitor, BP34610, discovered through high-throughput screening with dengue reporter viruses. Antivir. Res. 2019, 172, 104636. [Google Scholar] [CrossRef] [PubMed]
- Zu, X.; Liu, Y.; Wang, S.; Jin, R.; Zhou, Z.; Liu, H.; Gong, R.; Xiao, G.; Wang, W. Peptide inhibitor of Japanese encephalitis virus infection targeting envelope protein domain III. Antivir. Res. 2014, 104, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Chen, H.H.; Lin, C.J.; Anand, A.; Lin, H.J.; Lin, H.Y.; Mao, J.Y.; Wang, P.H.; Tseng, Y.J.; Tzou, W.S.; Huang, C.C.; et al. Development of antiviral carbon quantum dots that target the Japanese encephalitis virus envelope protein. J. Biol. Chem. 2022, 298, 101957. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lei, H.Y.; Lin, Y.S.; Yeh, T.M.; Chen, S.H.; Liu, H.S. Heparin inhibits dengue-2 virus infection of five human liver cell lines. Antivir. Res. 2002, 56, 93–96. [Google Scholar] [CrossRef]
- Lee, E.; Pavy, M.; Young, N.; Freeman, C.; Lobigs, M. Antiviral effect of the heparan sulfate mimetic, PI-88, against dengue and encephalitic flaviviruses. Antivir. Res. 2006, 69, 31–38. [Google Scholar] [CrossRef]
- Chen, J.; Yamada, S.; Hama, Y.; Shetty, A.K.; Kobayashi, T.; Oda, H.; Seiki, K.; Kim, E.; Kimura, T.; Takahashi, N.; et al. Unique heparan sulfate from shrimp heads exhibits a strong inhibitory effect on infections by dengue virus and Japanese encephalitis virus. Biochem. Biophys. Res. Commun. 2011, 412, 136–142. [Google Scholar] [CrossRef]
- Kim, E.; Okumura, M.; Sawa, H.; Miyazaki, T.; Fujikura, D.; Yamada, S.; Sugahara, K.; Sasaki, M.; Kimura, T. Paradoxical effects of chondroitin sulfate-E on Japanese encephalitis viral infection. Biochem. Biophys. Res. Commun. 2011, 409, 717–722. [Google Scholar] [CrossRef]
- Samrat, S.K.; Xu, J.; Li, Z.; Zhou, J.; Li, H. Antiviral Agents against Flavivirus Protease: Prospect and Future Direction. Pathogens 2022, 11, 293. [Google Scholar] [CrossRef]
- Seniya, C.; Mishra, H.; Yadav, A.; Sagar, N.; Chaturvedi, B.; Uchadia, K.; Wadhwa, G. Antiviral potential of 4-hydroxypanduratin A, secondary metabolite of Fingerroot, Boesenbergia pandurata (Schult.), towards Japanese Encephalitis virus NS2B/NS3 protease. Bioinformation 2013, 9, 54–60. [Google Scholar] [CrossRef]
- Li, Z.; Brecher, M.; Deng, Y.Q.; Zhang, J.; Sakamuru, S.; Liu, B.; Huang, R.; Koetzner, C.A.; Allen, C.A.; Jones, S.A.; et al. Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction. Cell Res. 2017, 27, 1046–1064. [Google Scholar] [CrossRef]
- Li, Z.; Sakamuru, S.; Huang, R.; Brecher, M.; Koetzner, C.A.; Zhang, J.; Chen, H.; Qin, C.F.; Zhang, Q.Y.; Zhou, J.; et al. Erythrosin B is a potent and broad-spectrum orthosteric inhibitor of the flavivirus NS2B-NS3 protease. Antivir. Res. 2018, 150, 217–225. [Google Scholar] [CrossRef]
- Pambudi, S.; Kawashita, N.; Phanthanawiboon, S.; Omokoko, M.D.; Masrinoul, P.; Yamashita, A.; Limkittikul, K.; Yasunaga, T.; Takagi, T.; Ikuta, K.; et al. A small compound targeting the interaction between nonstructural proteins 2B and 3 inhibits dengue virus replication. Biochem. Biophys. Res. Commun. 2013, 440, 393–398. [Google Scholar] [CrossRef]
- Bhosale, S.; Kumar, A. Screening of phytoconstituents of Andrographis paniculata against various targets of Japanese encephalitis virus: An in-silico and in-vitro target-based approach. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100043. [Google Scholar] [CrossRef]
- Bhimaneni, S.; Kumar, A. Abscisic acid and aloe-emodin against NS2B-NS3A protease of Japanese encephalitis virus. Environ. Sci. Pollut. Res. Int. 2022, 29, 8759–8766. [Google Scholar] [CrossRef]
- Borowski, P.; Heising, M.V.; Miranda, I.B.; Liao, C.L.; Choe, J.; Baier, A. Viral NS3 helicase activity is inhibited by peptides reproducing the Arg-rich conserved motif of the enzyme (motif VI). Biochem. Pharmacol. 2008, 76, 28–38. [Google Scholar] [CrossRef]
- Mastrangelo, E.; Pezzullo, M.; De Burghgraeve, T.; Kaptein, S.; Pastorino, B.; Dallmeier, K.; de Lamballerie, X.; Neyts, J.; Hanson, A.M.; Frick, D.N.; et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug. J. Antimicrob. Chemother. 2012, 67, 1884–1894. [Google Scholar] [CrossRef]
- Kumar, S.; Maurya, V.K.; Kabir, R.; Nayak, D.; Khurana, A.; Manchanda, R.K.; Gadugu, S.; Shanker, K.; Saxena, S.K. Antiviral Activity of Belladonna during Japanese Encephalitis Virus Infection via Inhibition of Microglia Activation and Inflammation Leading to Neuronal Cell Survival. ACS Chem. Neurosci. 2020, 11, 3683–3696. [Google Scholar] [CrossRef]
- Fang, J.; Li, H.; Kong, D.; Cao, S.; Peng, G.; Zhou, R.; Chen, H.; Song, Y. Structure-based discovery of two antiviral inhibitors targeting the NS3 helicase of Japanese encephalitis virus. Sci. Rep. 2016, 6, 34550. [Google Scholar] [CrossRef] [PubMed]
- Hale, G.L. Flaviviruses & the Traveler: Around the World and to Your Stage. Mod. Pathol. 2023, in press. [Google Scholar] [CrossRef]
- Yadav, P.; El-Kafrawy, S.A.; El-Day, M.M.; Alghafari, W.T.; Faizo, A.A.; Jha, S.K.; Dwivedi, V.D.; Azhar, E.I. Discovery of Small Molecules from Echinacea angustifolia Targeting RNA-Dependent RNA Polymerase of Japanese Encephalitis Virus. Life 2022, 12, 952. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, V.D.; Singh, A.; El-Kafraway, S.A.; Alandijany, T.A.; Faizo, A.A.; Bajrai, L.H.; Kamal, M.A.; Azhar, E.I. Mechanistic insights into the Japanese encephalitis virus RNA dependent RNA polymerase protein inhibition by bioflavonoids from Azadirachta indica. Sci. Rep. 2021, 11, 18125. [Google Scholar] [CrossRef]
- Malet, H.; Masse, N.; Selisko, B.; Romette, J.L.; Alvarez, K.; Guillemot, J.C.; Tolou, H.; Yap, T.L.; Vasudevan, S.; Lescar, J.; et al. The flavivirus polymerase as a target for drug discovery. Antivir. Res. 2008, 80, 23–35. [Google Scholar] [CrossRef]
- Yokokawa, F.; Nilar, S.; Noble, C.G.; Lim, S.P.; Rao, R.; Tania, S.; Wang, G.; Lee, G.; Hunziker, J.; Karuna, R.; et al. Discovery of Potent Non-Nucleoside Inhibitors of Dengue Viral RNA-Dependent RNA Polymerase from a Fragment Hit Using Structure-Based Drug Design. J. Med. Chem. 2016, 59, 3935–3952. [Google Scholar] [CrossRef]
- Bernatchez, J.A.; Coste, M.; Beck, S.; Wells, G.A.; Luna, L.A.; Clark, A.E.; Zhu, Z.; Hecht, D.; Rich, J.N.; Sohl, C.D.; et al. Activity of Selected Nucleoside Analogue ProTides against Zika Virus in Human Neural Stem Cells. Viruses 2019, 11, 365. [Google Scholar] [CrossRef]
- Zeuzem, S.; Dusheiko, G.M.; Salupere, R.; Mangia, A.; Flisiak, R.; Hyland, R.H.; Illeperuma, A.; Svarovskaia, E.; Brainard, D.M.; Symonds, W.T.; et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N. Engl. J. Med. 2014, 370, 1993–2001. [Google Scholar] [CrossRef]
- Dragoni, F.; Boccuto, A.; Picarazzi, F.; Giannini, A.; Giammarino, F.; Saladini, F.; Mori, M.; Mastrangelo, E.; Zazzi, M.; Vicenti, I. Evaluation of sofosbuvir activity and resistance profile against West Nile virus in vitro. Antivir. Res. 2020, 175, 104708. [Google Scholar] [CrossRef]
- Amstutz, A.; Speich, B.; Mentre, F.; Rueegg, C.S.; Belhadi, D.; Assoumou, L.; Burdet, C.; Murthy, S.; Dodd, L.E.; Wang, Y.; et al. Effects of remdesivir in patients hospitalised with COVID-19: A systematic review and individual patient data meta-analysis of randomised controlled trials. Lancet Respir. Med. 2023. [CrossRef]
- Konkolova, E.; Dejmek, M.; Hrebabecky, H.; Sala, M.; Boserle, J.; Nencka, R.; Boura, E. Remdesivir triphosphate can efficiently inhibit the RNA-dependent RNA polymerase from various flaviviruses. Antivir. Res. 2020, 182, 104899. [Google Scholar] [CrossRef]
- Knyazhanskaya, E.; Morais, M.C.; Choi, K.H. Flavivirus enzymes and their inhibitors. Enzym. 2021, 49, 265–303. [Google Scholar]
- Taylor, R.; Kotian, P.; Warren, T.; Panchal, R.; Bavari, S.; Julander, J.; Dobo, S.; Rose, A.; El-Kattan, Y.; Taubenheim, B.; et al. BCX4430—A broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J. Infect. Public Health 2016, 9, 220–226. [Google Scholar] [CrossRef]
- Julander, J.G.; Demarest, J.F.; Taylor, R.; Gowen, B.B.; Walling, D.M.; Mathis, A.; Babu, Y.S. An update on the progress of galidesivir (BCX4430), a broad-spectrum antiviral. Antivir. Res. 2021, 195, 105180. [Google Scholar] [CrossRef]
- Pattnaik, A.; Palermo, N.; Sahoo, B.R.; Yuan, Z.; Hu, D.; Annamalai, A.S.; Vu, H.L.X.; Correas, I.; Prathipati, P.K.; Destache, C.J.; et al. Discovery of a non-nucleoside RNA polymerase inhibitor for blocking Zika virus replication through in silico screening. Antivir. Res. 2018, 151, 78–86. [Google Scholar] [CrossRef]
- Lim, S.P.; Noble, C.G.; Seh, C.C.; Soh, T.S.; El Sahili, A.; Chan, G.K.; Lescar, J.; Arora, R.; Benson, T.; Nilar, S.; et al. Potent Allosteric Dengue Virus NS5 Polymerase Inhibitors: Mechanism of Action and Resistance Profiling. PLoS Pathog. 2016, 12, e1005737. [Google Scholar] [CrossRef]
- Hernandez, J.; Hoffer, L.; Coutard, B.; Querat, G.; Roche, P.; Morelli, X.; Decroly, E.; Barral, K. Optimization of a fragment linking hit toward Dengue and Zika virus NS5 methyltransferases inhibitors. Eur. J. Med. Chem. 2019, 161, 323–333. [Google Scholar] [CrossRef]
- Thames, J.E.; Waters, C.D., 3rd; Valle, C.; Bassetto, M.; Aouadi, W.; Martin, B.; Selisko, B.; Falat, A.; Coutard, B.; Brancale, A.; et al. Synthesis and biological evaluation of novel flexible nucleoside analogues that inhibit flavivirus replication in vitro. Bioorg. Med. Chem. 2020, 28, 115713. [Google Scholar] [CrossRef]
- Spizzichino, S.; Mattedi, G.; Lauder, K.; Valle, C.; Aouadi, W.; Canard, B.; Decroly, E.; Kaptein, S.J.F.; Neyts, J.; Graham, C.; et al. Design, Synthesis and Discovery of N,N’-Carbazoyl-aryl-urea Inhibitors of Zika NS5 Methyltransferase and Virus Replication. ChemMedChem 2020, 15, 385–390. [Google Scholar] [CrossRef]
- Brecher, M.; Chen, H.; Li, Z.; Banavali, N.K.; Jones, S.A.; Zhang, J.; Kramer, L.D.; Li, H. Identification and Characterization of Novel Broad-Spectrum Inhibitors of the Flavivirus Methyltransferase. ACS Infect. Dis. 2015, 1, 340–349. [Google Scholar] [CrossRef]
- Fan, J.; Liu, Y.; Xie, X.; Zhang, B.; Yuan, Z. Inhibition of Japanese encephalitis virus infection by flavivirus recombinant E protein domain III. Virol. Sin. 2013, 28, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, L.Y.; Sun, M.X.; Li, P.P.; Huang, L.; Wei, J.C.; Yao, Y.L.; Isahg, H.; Chen, P.Y.; Mao, X. Inhibition of Japanese encephalitis virus entry into the cells by the envelope glycoprotein domain III (EDIII) and the loop3 peptide derived from EDIII. Antivir. Res. 2012, 94, 179–183. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wang, S.; Sun, J.; Wang, P.; Xin, Q.; Zhang, L.; Xiao, G.; Wang, W. Antiviral activity of peptide inhibitors derived from the protein E stem against Japanese encephalitis and Zika viruses. Antivir. Res. 2017, 141, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.S.; Liu, H.; Chuang, C.K.; Lin, C.C.; Chen, W.J. Fitness of Japanese encephalitis virus to Neuro-2a cells is determined by interactions of the viral envelope protein with highly sulfated glycosaminoglycans on the cell surface. J. Med. Virol. 2005, 76, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, H.; Ye, Z.; Xu, Q.; Fu, Q.; Sun, W.; Qi, W.; Yue, J. Berbamine inhibits Japanese encephalitis virus (JEV) infection by compromising TPRMLs-mediated endolysosomal trafficking of low-density lipoprotein receptor (LDLR). Emerg. Microbes Infect. 2021, 10, 1257–1271. [Google Scholar] [CrossRef]
- Chien, Y.J.; Chen, W.J.; Hsu, W.L.; Chiou, S.S. Bovine lactoferrin inhibits Japanese encephalitis virus by binding to heparan sulfate and receptor for low density lipoprotein. Virology 2008, 379, 143–151. [Google Scholar] [CrossRef]
- Sehrawat, S.; Khasa, R.; Deb, A.; Prajapat, S.K.; Mallick, S.; Basu, A.; Surjit, M.; Kalia, M.; Vrati, S. Valosin-Containing Protein/p97 Plays Critical Roles in the Japanese Encephalitis Virus Life Cycle. J. Virol. 2021, 95, e02336-20. [Google Scholar] [CrossRef]
- Sebastian, L.; Desai, A.; Shampur, M.N.; Perumal, Y.; Sriram, D.; Vasanthapuram, R. N-methylisatin-beta-thiosemicarbazone derivative (SCH 16) is an inhibitor of Japanese encephalitis virus infection in vitro and in vivo. Virol. J. 2008, 5, 64. [Google Scholar] [CrossRef]
- Wu, S.F.; Lee, C.J.; Liao, C.L.; Dwek, R.A.; Zitzmann, N.; Lin, Y.L. Antiviral effects of an iminosugar derivative on flavivirus infections. J. Virol. 2002, 76, 3596–3604. [Google Scholar] [CrossRef]
- Cannalire, R.; Tarantino, D.; Piorkowski, G.; Carletti, T.; Massari, S.; Felicetti, T.; Barreca, M.L.; Sabatini, S.; Tabarrini, O.; Marcello, A.; et al. Broad spectrum anti-flavivirus pyridobenzothiazolones leading to less infective virions. Antivir. Res. 2019, 167, 6–12. [Google Scholar] [CrossRef]
- Ratheesh, A.; Jain, M.; Gude, R.P. Antimetastatic Action of Pentoxifylline, a Methyl Xanthine Derivative, Through its Effect on PKC Mediated Integrin Transport in B16F10 Melanoma Cells. World J. Oncol. 2010, 1, 194–203. [Google Scholar]
- Sebastian, L.; Desai, A.; Madhusudana, S.N.; Ravi, V. Pentoxifylline inhibits replication of Japanese encephalitis virus: A comparative study with ribavirin. Int. J. Antimicrob. Agents 2009, 33, 168–173. [Google Scholar] [CrossRef]
- Yang, C.F.; Gopula, B.; Liang, J.J.; Li, J.K.; Chen, S.Y.; Lee, Y.L.; Chen, C.S.; Lin, Y.L. Novel AR-12 derivatives, P12-23 and P12-34, inhibit flavivirus replication by blocking host de novo pyrimidine biosynthesis. Emerg. Microbes Infect. 2018, 7, 187. [Google Scholar] [CrossRef]
- Fan, W.; Qian, S.; Qian, P.; Li, X. Antiviral activity of luteolin against Japanese encephalitis virus. Virus Res. 2016, 220, 112–116. [Google Scholar] [CrossRef]
- Liang, J.J.; Wei, J.C.; Lee, Y.L.; Hsu, S.H.; Lin, J.J.; Lin, Y.L. Surfactant-modified nanoclay exhibits an antiviral activity with high potency and broad spectrum. J. Virol. 2014, 88, 4218–4228. [Google Scholar] [CrossRef]
- Navyashree, V.; Kant, K.; Kumar, A. Natural chemical entities from Arisaema genus might be a promising break-through against Japanese encephalitis virus infection: A molecular docking and dynamics approach. J. Biomol. Struct. Dyn. 2021, 39, 1404–1416. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, H.M.; Koch, V.; Schmitz, H.; Liao, C.L.; Bretner, M.; Bhadti, V.S.; Fattom, A.I.; Naso, R.B.; Hosmane, R.S.; et al. Ring-expanded (“fat”) nucleoside and nucleotide analogues exhibit potent in vitro activity against flaviviridae NTPases/helicases, including those of the West Nile virus, hepatitis C virus, and Japanese encephalitis virus. J. Med. Chem. 2003, 46, 4149–4164. [Google Scholar] [CrossRef]
- Zandi, K.; Bassit, L.; Amblard, F.; Cox, B.D.; Hassandarvish, P.; Moghaddam, E.; Yueh, A.; Libanio Rodrigues, G.O.; Passos, I.; Costa, V.V.; et al. Nucleoside Analogs with Selective Antiviral Activity against Dengue Fever and Japanese Encephalitis Viruses. Antimicrob. Agents Chemother. 2019, 63, e00397-19. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Chaudhuri, R.; Dash, J.J.; Saha, M.; Choudhury, L.; Roy, S. Pre-treatment with Scopolamine Naturally Suppresses Japanese Encephalitis Viral Load in Embryonated Chick Through Regulation of Multiple Signaling Pathways. Appl. Biochem. Biotechnol. 2021, 193, 1654–1674. [Google Scholar] [CrossRef]
- Lu, C.Y.; Chang, Y.C.; Hua, C.H.; Chuang, C.; Huang, S.H.; Kung, S.H.; Hour, M.J.; Lin, C.W. Tubacin, an HDAC6 Selective Inhibitor, Reduces the Replication of the Japanese Encephalitis Virus via the Decrease of Viral RNA Synthesis. Int. J. Mol. Sci. 2017, 18, 954. [Google Scholar] [CrossRef]
- Ishag, H.Z.; Li, C.; Huang, L.; Sun, M.X.; Ni, B.; Guo, C.X.; Mao, X. Inhibition of Japanese encephalitis virus infection in vitro and in vivo by pokeweed antiviral protein. Virus Res. 2013, 171, 89–96. [Google Scholar] [CrossRef]
- Fang, J.; Sun, L.; Peng, G.; Xu, J.; Zhou, R.; Cao, S.; Chen, H.; Song, Y. Identification of three antiviral inhibitors against Japanese encephalitis virus from library of pharmacologically active compounds 1280. PLoS ONE 2013, 8, e78425. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Zhang, L.; Zheng, B.; Zhao, Z.; Zhou, D.; Wan, S.; Chen, Z.; Duan, H.; Li, Q.; Liu, X.; et al. Screening of novel synthetic derivatives of dehydroepiandrosterone for antivirals against flaviviruses infections. Virol. Sin. 2022, 37, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.R.; Zhang, H.Q.; Li, X.D.; Deng, C.L.; Wang, Z.; Li, J.Q.; Li, N.; Zhang, Q.Y.; Zhang, H.L.; Zhang, B.; et al. Generation and characterization of Japanese encephalitis virus expressing GFP reporter gene for high throughput drug screening. Antivir. Res. 2020, 182, 104884. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, L.; Desai, A.; Yogeeswari, P.; Sriram, D.; Madhusudana, S.N.; Ravi, V. Combination of N-methylisatin-beta-thiosemicarbazone derivative (SCH16) with ribavirin and mycophenolic acid potentiates the antiviral activity of SCH16 against Japanese encephalitis virus in vitro. Lett. Appl. Microbiol. 2012, 55, 234–239. [Google Scholar] [CrossRef]
- Nam, S.; Na, H.G.; Oh, E.H.; Jung, E.; Lee, Y.H.; Jeong, E.J.; Ou, Y.D.; Zhou, B.; Ahn, S.; Shin, J.S.; et al. Discovery and synthesis of 1,2,4-oxadiazole derivatives as novel inhibitors of Zika, dengue, Japanese encephalitis, and classical swine fever virus infections. Arch. Pharmacal Res. 2022, 45, 280–293. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Z.; Du, J.; Hu, Y.; Liu, L.; Yang, F.; Jin, Q. Anti-Japanese-encephalitis-viral effects of kaempferol and daidzin and their RNA-binding characteristics. PLoS ONE 2012, 7, e30259. [Google Scholar] [CrossRef]
- Lv, B.M.; Tong, X.Y.; Quan, Y.; Liu, M.Y.; Zhang, Q.Y.; Song, Y.F.; Zhang, H.Y. Drug Repurposing for Japanese Encephalitis Virus Infection by Systems Biology Methods. Molecules 2018, 23, 3346. [Google Scholar] [CrossRef]
- Lin, C.W.; Wu, C.F.; Hsiao, N.W.; Chang, C.Y.; Li, S.W.; Wan, L.; Lin, Y.J.; Lin, W.Y. Aloe-emodin is an interferon-inducing agent with antiviral activity against Japanese encephalitis virus and enterovirus 71. Int. J. Antimicrob. Agents 2008, 32, 355–359. [Google Scholar] [CrossRef]
- Ojha, A.; Bhasym, A.; Mukherjee, S.; Annarapu, G.K.; Bhakuni, T.; Akbar, I.; Seth, T.; Vikram, N.K.; Vrati, S.; Basu, A.; et al. Platelet factor 4 promotes rapid replication and propagation of Dengue and Japanese encephalitis viruses. eBioMedicine 2019, 39, 332–347. [Google Scholar] [CrossRef]
- Mishra, M.K.; Basu, A. Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J. Neurochem. 2008, 105, 1582–1595. [Google Scholar] [CrossRef]
- Mishra, M.K.; Dutta, K.; Saheb, S.K.; Basu, A. Understanding the molecular mechanism of blood-brain barrier damage in an experimental model of Japanese encephalitis: Correlation with minocycline administration as a therapeutic agent. Neurochem. Int. 2009, 55, 717–723. [Google Scholar] [CrossRef]
- Swarup, V.; Ghosh, J.; Ghosh, S.; Saxena, A.; Basu, A. Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob. Agents Chemother. 2007, 51, 3367–3670. [Google Scholar] [CrossRef]
- Huang, H.N.; Rajanbabu, V.; Pan, C.Y.; Chan, Y.L.; Hui, C.F.; Chen, J.Y.; Wu, C.J. Modulation of the immune-related gene responses to protect mice against Japanese encephalitis virus using the antimicrobial peptide, tilapia hepcidin 1-5. Biomaterials 2011, 32, 6804–6814. [Google Scholar] [CrossRef]
- Sehgal, N.; Kumawat, K.L.; Basu, A.; Ravindranath, V. Fenofibrate reduces mortality and precludes neurological deficits in survivors in murine model of Japanese encephalitis viral infection. PLoS ONE 2012, 7, e35427. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, M.; Liu, C.; Tang, Y.; Xiao, K.; Dai, J.; Gao, Z.; Siew, L.; Cao, G.; Wu, X.; et al. Memantine can relieve the neuronal impairment caused by neurotropic virus infection. J. Med. Virol. 2019, 91, 935–940. [Google Scholar] [CrossRef]
- Ghosh, J.; Swarup, V.; Saxena, A.; Das, S.; Hazra, A.; Paira, P.; Banerjee, S.; Mondal, N.B.; Basu, A. Therapeutic effect of a novel anilidoquinoline derivative, 2-(2-methyl-quinoline-4ylamino)-N-(2-chlorophenyl)-acetamide, in Japanese encephalitis: Correlation with in vitro neuroprotection. Int. J. Antimicrob. Agents 2008, 32, 349–354. [Google Scholar] [CrossRef]
- Swarup, V.; Ghosh, J.; Mishra, M.K.; Basu, A. Novel strategy for treatment of Japanese encephalitis using arctigenin, a plant lignan. J. Antimicrob. Chemother. 2008, 61, 679–688. [Google Scholar] [CrossRef]
- Gupta, A.; Gawandi, S.; Yadav, I.; Mohan, H.; Desai, V.G.; Kumar, S. Analysis of fluoro based pyrazole analogues as a potential therapeutics candidate against Japanese encephalitis virus infection. Virus Res. 2022, 323, 198955. [Google Scholar] [CrossRef]
- Dutta, K.; Ghosh, D.; Basu, A. Curcumin protects neuronal cells from Japanese encephalitis virus-mediated cell death and also inhibits infective viral particle formation by dysregulation of ubiquitin-proteasome system. J. Neuroimmune Pharmacol. 2009, 4, 328–337. [Google Scholar] [CrossRef]
- Li, Q.; Sun, B.; Zhuo, Y.; Jiang, Z.; Li, R.; Lin, C.; Jin, Y.; Gao, Y.; Wang, D. Interferon and interferon-stimulated genes in HBV treatment. Front. Immunol. 2022, 13, 1034968. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.R.; Besson, V.C.; Palmier, B.; Garcia, Y.; Plotkine, M.; Marchand-Leroux, C. Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR alpha agonist, in traumatic brain injury. J. Neurotrauma 2007, 24, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Deplanque, D.; Gele, P.; Petrault, O.; Six, I.; Furman, C.; Bouly, M.; Nion, S.; Dupuis, B.; Leys, D.; Fruchart, J.C.; et al. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J. Neurosci. 2003, 23, 6264–6271. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.; Ginty, M.; Kemp, K.; Scolding, N.; Wilkins, A. Peroxisome proliferator-activated receptor-alpha agonists protect cortical neurons from inflammatory mediators and improve peroxisomal function. Eur. J. Neurosci. 2011, 33, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Basu, A.; Sinha, S.; Das, M.; Tripathi, P.; Jain, A.; Kumar, C.; Atam, V.; Khan, S.; Singh, A.S. Role of oral Minocycline in acute encephalitis syndrome in India—A randomized controlled trial. BMC Infect. Dis. 2016, 16, 67. [Google Scholar]
- Vermehren, J.; Park, J.S.; Jacobson, I.M.; Zeuzem, S. Challenges and perspectives of direct antivirals for the treatment of hepatitis C virus infection. J. Hepatol. 2018, 69, 1178–1187. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Liu, Z.; Peng, Y.; Peng, W.; Tong, L.; Wang, J.; Liu, Q.; Wang, P.; Cheng, G. A volatile from the skin microbiota of flavivirus-infected hosts promotes mosquito attractiveness. Cell 2022, 185, 2510–2522. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).