SARS-CoV-2 Reinfection and Severity of the Disease: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Search Strategy
2.2. Eligibility Criteria
2.3. Exclusion Criteria
2.4. Data Collection Process
2.5. Assessment for Quality of Studies
2.6. Statistics
3. Results
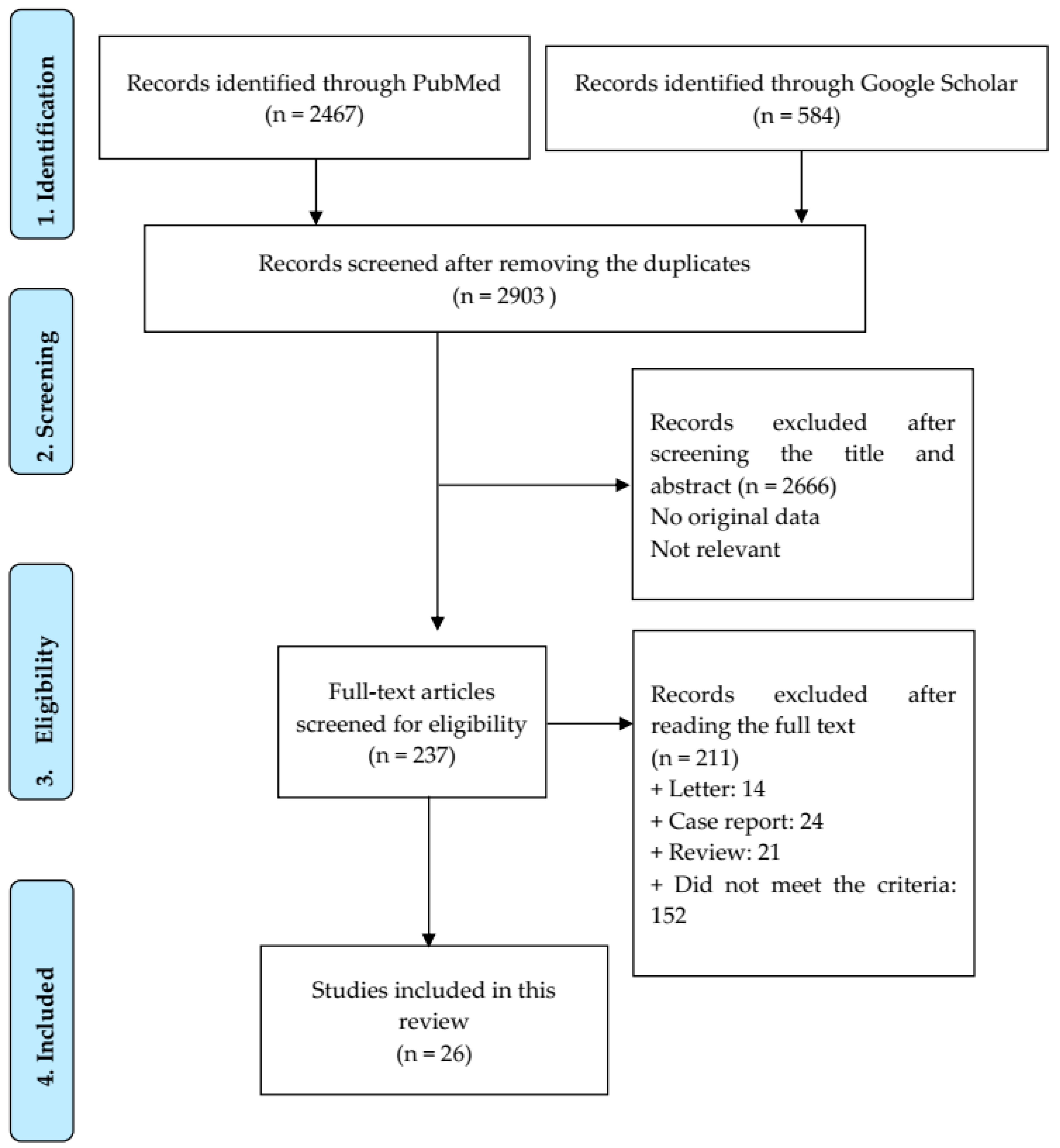
3.1. Study Selection and Quality Assessment
3.2. Characteristics of Selected Studies
3.3. SARS-CoV-2 Variants
3.4. Prevalence of Reinfection and Characteristics of SARS-CoV-2 Reinfected Patients
3.4.1. Prevalence of Reinfection and Effect of SARS-CoV-2 Variants and Region
3.4.2. Demographics
Age
Gender
3.4.3. Comorbidities
3.4.4. Vaccination Status
3.5. Length of Time between Two Episodes
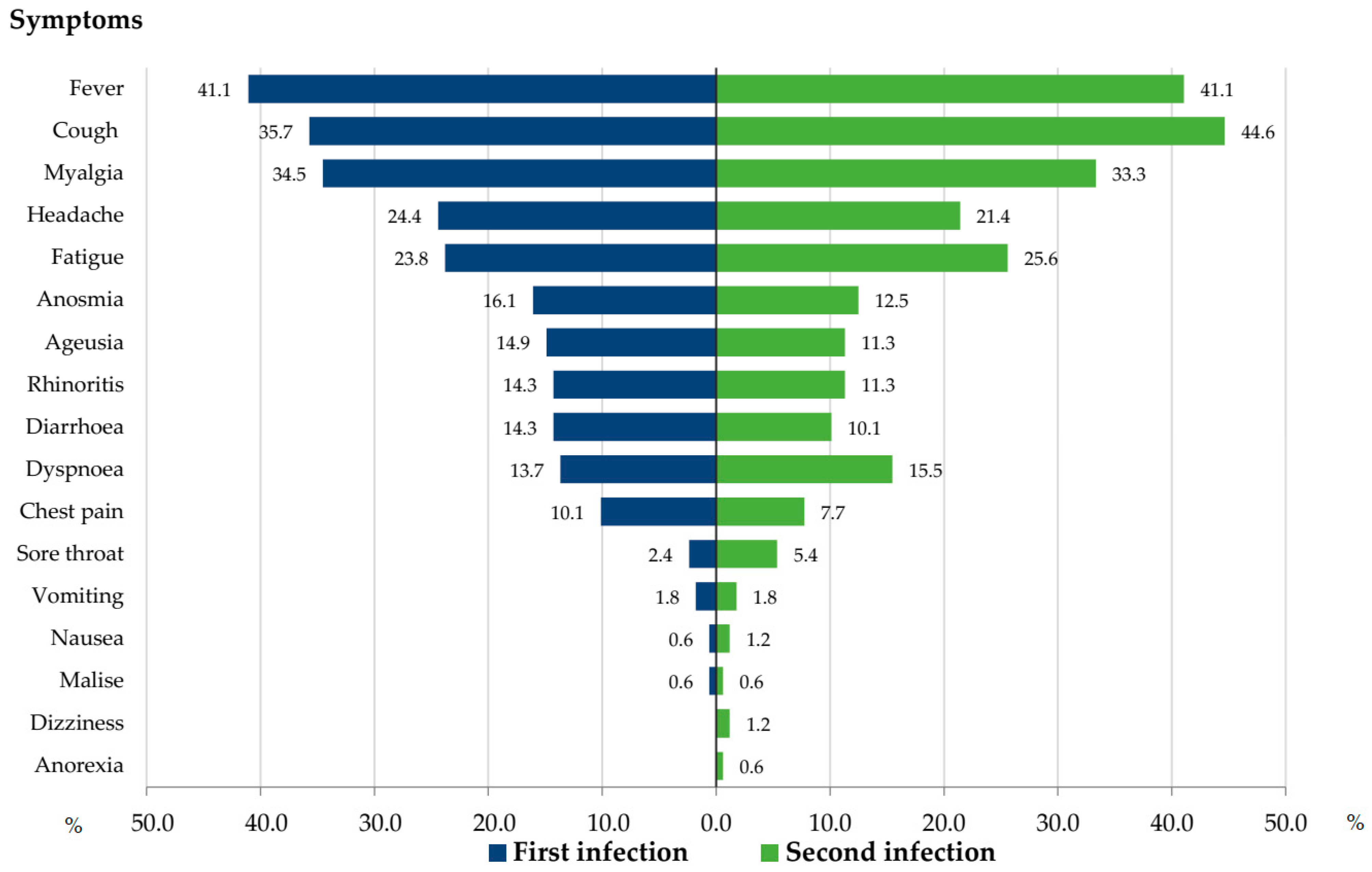
3.6. Clinical Symptoms
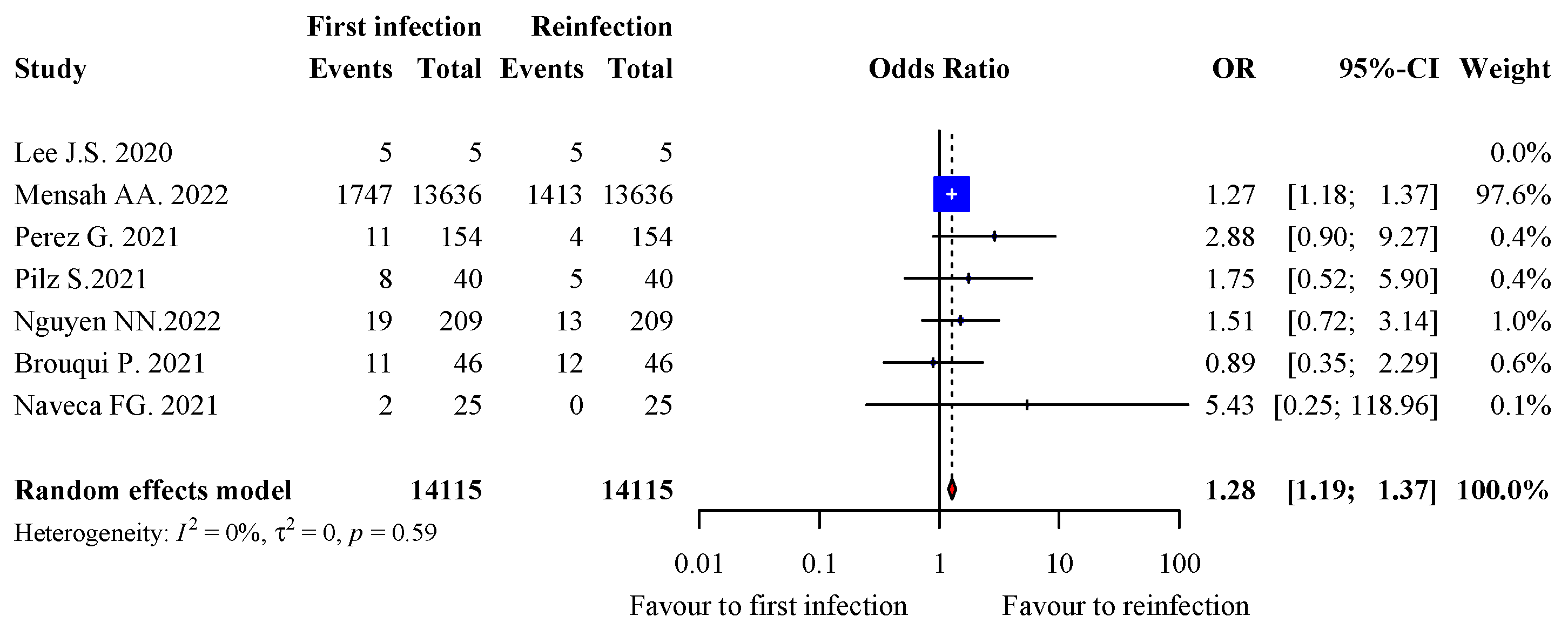
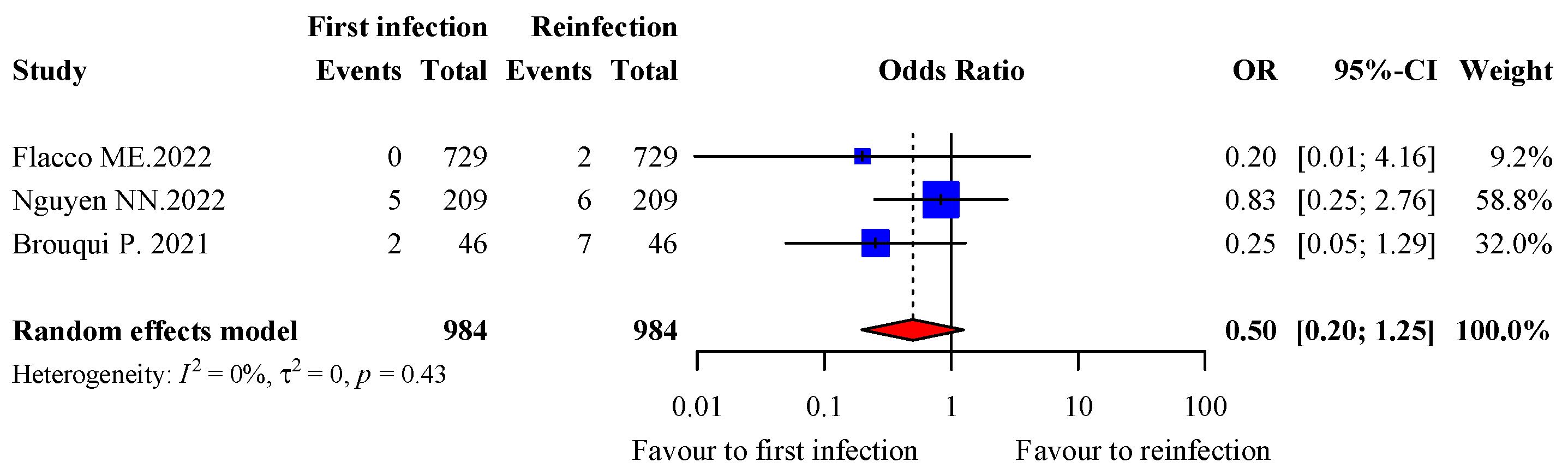
3.7. Severity of Infection
3.8. Risk Factors for Reinfection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Word Health Organization (WHO). Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 25 June 2022).
- To, K.K.; Hung, I.F.; Ip, J.D.; Chu, A.W.; Chan, W.-M.; Tam, A.R.; Fong, C.H.-Y.; Yuan, S.; Tsoi, H.-W.; Ng, A.C.-K.; et al. Coronavirus Disease 2019 (COVID-19) Reinfection by a Phylogenetically Distinct Severe Acute Respiratory Syndrome Coronavirus 2 Strain Confirmed by Whole Genome Sequencing. Clin. Infect. Dis. 2021, 73, e2946–e2951. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, S.S.; Taherpour, N.; Bayat, S.; Ghajari, H.; Mohseni, P.; Nazari, S.S.H. Epidemiologic characteristics of cases with reinfection, recurrence, and hospital readmission due to COVID-19: A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.N.; Houhamdi, L.; Hoang, V.T.; Stoupan, D.; Fournier, P.-E.; Raoult, D.; Colson, P.; Gautret, P. High rate of reinfection with the SARS-CoV-2 Omicron variant. J. Infect. 2022, 23, S0163–S4453. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19) 2021 Case Definition. Available online: https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2021/ (accessed on 4 April 2023).
- European Centre for Disease Prevention and Control. Reinfection with SARS-CoV-2: Implementation of a Surveillance Case Definition within the EU/EEA. Available online: https://www.ecdc.europa.eu/en/publications-data/reinfection-sars-cov-2-implementation-surveillance-case-definition-within-eueea (accessed on 4 April 2023).
- Nevejan, L.; Cuypers, L.; Laenen, L.; Van Loo, L.; Vermeulen, F.; Wollants, E.; Van Hecke, I.; Desmet, S.; Lagrou, K.; Maes, P.; et al. Early SARS-CoV-2 reinfections within 60 days highlight the need to consider antigenic variations together with duration of immunity in defining retesting policies. Emerg Infect. Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control. Common Investigation Protocol for Investigating Suspected SARS-CoV-2 Reinfection (2020). Available online: https://www.cdc.gov/coronavirus/2019-ncov/php/reinfection.html (accessed on 19 April 2022).
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 4 April 2023).
- Tool to Assess Risk of Bias in Case Control Studies. 2017. Available online: https://www.evidencepartners.com/wp-content/uploads/2021/03/Tool-to-Assess-Risk-of-Bias-in-Cohort-Studies-DistillerSR.pdf.%20Accessed%20July%2021%202022 (accessed on 4 April 2023).
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://handbook-5-1.cochrane.org/chapter_16/16_9_3_studies_with_no_events.htm (accessed on 4 April 2023).
- Gupta, V.; Bhoyar, R.C.; Jain, A.; Srivastava, S.; Upadhayay, R.; Imran, M.; Jolly, B.; Divakar, M.K.; Sharma, D.; Sehgal, P.; et al. Asymptomatic reinfection in 2 healthcare workers from India with genetically distinct severe acute respiratory syndrome coronavirus 2. Clin. Infect. Dis. 2021, 73, e2823–e2825. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.Y.; Kim, T.S.; Hong, K.H.; Ryoo, N.-H.; Lee, J.; Park, J.H.; Cho, S.I.; Kim, M.J.; Kim, Y.; et al. Evidence of Severe Acute Respiratory Syndrome Coronavirus 2 Reinfection after Recovery from Mild Coronavirus Disease 2019. Clin. Infect. Dis. 2021, 73, e3002–e3008. [Google Scholar] [CrossRef]
- Salehi-Vaziri, M.; Jalali, T.; Farahmand, B.; Fotouhi, F.; Pouriayevali, M.H.; Sadat Larijani, M.; Afzali, N.; Ramezani, A. Clinical characteristics of SARS-CoV-2 by re-infection vs. reactivation: A case series from Iran. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1713–1719. [Google Scholar] [CrossRef]
- Amorim, M.R.; Souza, W.M.; Barros, A.C.G.; Toledo-Teixeira, D.A.; dos-Santos, K.B.; Simeoni, C.L.; Parise, P.L.; Vieira, A.; Forato, J.; Claro, I.M.; et al. Respiratory viral shedding in healthcare workers reinfected with SARS-CoV-2, Brazil, 2020. Emerg. Infect. Dis. 2021, 27, 1737–1740. [Google Scholar] [CrossRef]
- Yu, A.L.F.; Liphaus, B.L.; Ferreira, P.M.; Tanamachi, A.T.; Masuda, E.T.; Trevisan, C.M.; Lucas, P.C.d.C.; Bugno, A.; Carvalhanas, T.R.M.P. Sars-CoV-2 reinfection: Report of two cases in Southeast Brazil. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, 2–5. [Google Scholar] [CrossRef]
- Naveca, F.G.; Nascimento, V.A.; Nascimento, F.; Ogrzewalska, M.; Pauvolid-Corrêa, A.; Araujo, M.F.; Arantes, I.; Batista, É.L.R.; Magalhães, A.L.Á.; Vinhal, F.; et al. A case series of SARS-CoV-2 reinfections caused by the variant of concern Gamma in Brazil. MedRxiv 2021. [Google Scholar] [CrossRef]
- Sanyang, B.; Kanteh, A.; Usuf, E.; Nadjm, B.; Jarju, S.; Bah, A.; Bojang, A.; Grey-Johnson, M.; Jones, J.C.; Gai, A.; et al. COVID-19 reinfections in The Gambia by phylogenetically distinct SARS-CoV-2 variants—First two confirmed events in west Africa. Lancet Glob. Heal. 2021, 9, e905–e907. [Google Scholar] [CrossRef] [PubMed]
- Sonié, P.; Manuel-Silva, J.; Rafael, A.; Amorim-Alves, L. Reinfection with SARS-CoV-2: An inconvenient truth? J. Fam. Med. Prim. Care. 2022, 11, 366–369. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Bertollini, R.; National Study Group for COVID-19 Epidemiology. Severity of SARS-CoV-2 Reinfections as Compared with Primary Infections. N. Engl. J. Med. 2021, 385, 2487–2489. [Google Scholar] [CrossRef]
- Ali, A.M.; Ali, K.M.; Fatah, M.H.; Tawfeeq, H.M.; Rostam, H.M. SARS-CoV-2 reinfection in patients negative for immunoglobulin G following recovery from COVID-19. New Microbes New Infect. 2021, 43, 100926. [Google Scholar] [CrossRef] [PubMed]
- Jeffery-Smith, A.; Rowland, T.A.J.; Patel, M.; Whitaker, H.; Iyanger, N.; Williams, S.V.; Giddings, R.; Thompson, L.; Zavala, M.; Aiano, F.; et al. Reinfection with new variants of SARS-CoV-2 after natural infection: A prospective observational cohort in 13 care homes in England. Lancet Heal. Longev. 2021, 2, e811–e819. [Google Scholar] [CrossRef]
- Mensah, A.A.; Lacy, J.; Stowe, J.; Seghezzo, G.; Sachdeva, R.; Simmons, R.; Bukasa, A.; O’Boyle, S.; Andrews, N.; Ramsay, M.; et al. Disease severity during SARS-COV-2 reinfection: A nationwide study. J. Infect. 2022, 84, 542–550. [Google Scholar] [CrossRef]
- Rivelli, A.; Fitzpatrick, V.; Blair, C.; Copeland, K.; Richards, J. Incidence of COVID-19 reinfection among Midwestern healthcare employees. PLoS ONE 2022, 17, e0262164. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Houhamdi, L.; Hoang, V.T.; Delerce, J.; Delorme, L.; Colson, P.; Brouqui, P.; Fournier, P.-E.; Raoult, D.; Gautret, P. SARS-CoV-2 reinfection and COVID-19 severity. Emerg. Microbes Infect. 2022, 11, 894–901. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Hasan, M.R.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Benslimane, F.M.; Al-Kanaani, Z.; Al-Kuwari, E.; et al. Protection of SARS-CoV-2 natural infection against reinfection with Omicron BA.4 or BA.5. MedRxiv 2022, 386, 1288–1290. [Google Scholar] [CrossRef]
- Brouqui, P.; Colson, P.; Melenotte, C.; Houhamdi, L.; Bedotto, M.; Devaux, C.; Gautret, P.; Million, M.; Parola, P.; Stoupan, D.; et al. COVID-19 re-infection. Eur. J. Clin. Investig. 2021, 51, eci.13537. [Google Scholar] [CrossRef]
- Fonager, J.; Bennedbæk, M.; Bager, P.; Wohlfahrt, J.; Ellegaard, K.M.; Ingham, A.C.; Edslev, S.M.; Stegger, M.; Sieber, R.N.; Lassauniere, R.; et al. Molecular epidemiology of the SARS-CoV-2 variant Omicron BA.2 sub-lineage in Denmark, 29 November 2021 to 2 January 2022. Euro Surveill. 2022, 27, 2200181. [Google Scholar] [CrossRef] [PubMed]
- Good, M.K.; Czarnik, M.; Harmon, K.G.; Aukerman, D.; O’Neal, C.S.; Day, C.; Goerl, K.; Sifre, K.; Fink, S.; Riggs, M.A. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infections and Reinfections Among Fully Vaccinated and Unvaccinated University Athletes-15 States, January-November 2021. Clin. Infect. Dis. 2022, 75, S236–S242. [Google Scholar] [CrossRef]
- Mack, C.D.; Tai, C.; Sikka, R.; Grad, Y.H.; Maragakis, L.L.; Grubaugh, N.D.; Anderson, D.J.; Ho, D.; Merson, M.; Samant, R.M.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Reinfection: A Case Series From a 12-Month Longitudinal Occupational Cohort. Clin. Infect. Dis. 2022, 74, 1682–1685. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.R.; Oliveira, M.S.; Tavares, B.M.; Luna-Muschi, A.; Lazari, C.d.S.; Montal, A.C.; de Faria, E.; Maia, F.L.; Barboza, A.d.S.; Leme, M.D.; et al. Reinfection rate in a cohort of healthcare workers over 2 years of the COVID-19 pandemic. Sci. Rep. 2023, 13, 712. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, K.D.; Hollister, J.; Porter, C.J.; Khan, S.M.; Feldstein, L.R.; Naleway, A.L.; Gaglani, M.; Caban-Martinez, A.J.; Tyner, H.L.; Lowe, A.A.; et al. Risk Factors for Reinfection with SARS-CoV-2 Omicron Variant among Previously Infected Frontline Workers. Emerg. Infect. Dis. 2023, 29, 599–604. [Google Scholar] [CrossRef]
- Flacco, M.E.; Soldato, G.; Acuti Martellucci, C.; Di Martino, G.; Carota, R.; Caponetti, A.; Manzoli, L. Risk of SARS-CoV-2 Reinfection 18 Months After Primary Infection: Population-Level Observational Study. Front Public Health 2022, 10, 884121. [Google Scholar] [CrossRef]
- Perez, G.; Banon, T.; Gazit, S.; Moshe, S.B.; Wortsman, J.; Grupel, D.; Peretz, A.; Tov, A.B.; Chodick, G.; Mizrahi-Reuveni, M.; et al. A 1 to 1000 SARS-CoV-2 reinfection proportion in members of a large healthcare provider in Israel: A preliminary report. MedRxiv 2021. [Google Scholar] [CrossRef]
- Pilz, S.; Chakeri, A.; Ioannidis, J.P.; Richter, L.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Allerberger, F. SARS-CoV-2 re-infection risk in Austria. Eur. J. Clin. Investig. 2021, 51, e13520. [Google Scholar] [CrossRef]
- Malhotra, S.; Mani, K.; Lodha, R.; Bakhshi, S.; Mathur, V.P.; Gupta, P.; Kedia, S.; Sankar, J.; Kumar, P.; Kumar, A.; et al. SARS-CoV-2 Reinfection Rate and Estimated Effectiveness of the Inactivated Whole Virion Vaccine BBV152 Against Reinfection Among Health Care Workers in New Delhi, India. JAMA Netw. Open 2022, 5, e2142210. [Google Scholar] [CrossRef]
- Al-Otaiby, M.; Krissaane, I.; Seraihi, A.A.; Alshenaifi, J.; Qahtani, M.H.; Aljeri, T.; Zaatari, E.; Hassanain, M.; Algwizani, A.; Albarrag, A.; et al. SARS-CoV-2 Reinfection Rate and Outcomes in Saudi Arabia: A National Retrospective Study. Int. J. Infect. Dis. 2022, 122, 758–766. [Google Scholar] [CrossRef]
- Hoang, T. Characteristics of COVID-19 recurrence: A systematic review and meta-analysis. Ann. Glob. Health 2021, 87, 28. [Google Scholar] [CrossRef]
- Dao, T.L.; Hoang, V.T.; Gautret, P. Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: A narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wang, W.; Ma, J.; Wu, S.; Sun, F. Reinfection rates among patients previously infected by SARS-CoV-2: Systematic review and meta-analysis. Chin. Med. J. Engl. 2022, 135, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.A.; Qamar, M.A.; Gilani, J.A.; Irfan, O.; Waqar, U.; Sajid, M.I.; Mahmood, S.F. The mystery of COVID-19 reinfections: A global systematic review and meta-analysis. Ann. Med. Surg. 2021, 72, 103130. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Rahman, M.M.; Miah, M.; Begum, M.N.; Sarmin, M.; Mahfuz, M.; Hossain, M.E.; Rahman, M.Z.; Chisti, M.J.; Ahmed, T.; et al. COVID-19 reinfections among naturally infected and vaccinated individuals. Sci. Rep. 2022, 12, 1438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.N.; Nguyen, Y.N.; Hoang, V.T.; Million, M.; Gautret, P. SARS-CoV-2 Reinfection and Severity of the Disease: A Systematic Review and Meta-Analysis. Viruses 2023, 15, 967. https://doi.org/10.3390/v15040967
Nguyen NN, Nguyen YN, Hoang VT, Million M, Gautret P. SARS-CoV-2 Reinfection and Severity of the Disease: A Systematic Review and Meta-Analysis. Viruses. 2023; 15(4):967. https://doi.org/10.3390/v15040967
Chicago/Turabian StyleNguyen, Nhu Ngoc, Y Ngoc Nguyen, Van Thuan Hoang, Matthieu Million, and Philippe Gautret. 2023. "SARS-CoV-2 Reinfection and Severity of the Disease: A Systematic Review and Meta-Analysis" Viruses 15, no. 4: 967. https://doi.org/10.3390/v15040967
APA StyleNguyen, N. N., Nguyen, Y. N., Hoang, V. T., Million, M., & Gautret, P. (2023). SARS-CoV-2 Reinfection and Severity of the Disease: A Systematic Review and Meta-Analysis. Viruses, 15(4), 967. https://doi.org/10.3390/v15040967






