Persistence of SARS-CoV-2 Antigens in the Nasal Mucosa of Eight Patients with Inflammatory Rhinopathy for over 80 Days following Mild COVID-19 Diagnosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design
2.3. Ear, Nose and Throat (ENT) Physical Examination
2.4. Immunofluorescence
2.5. Data Analysis
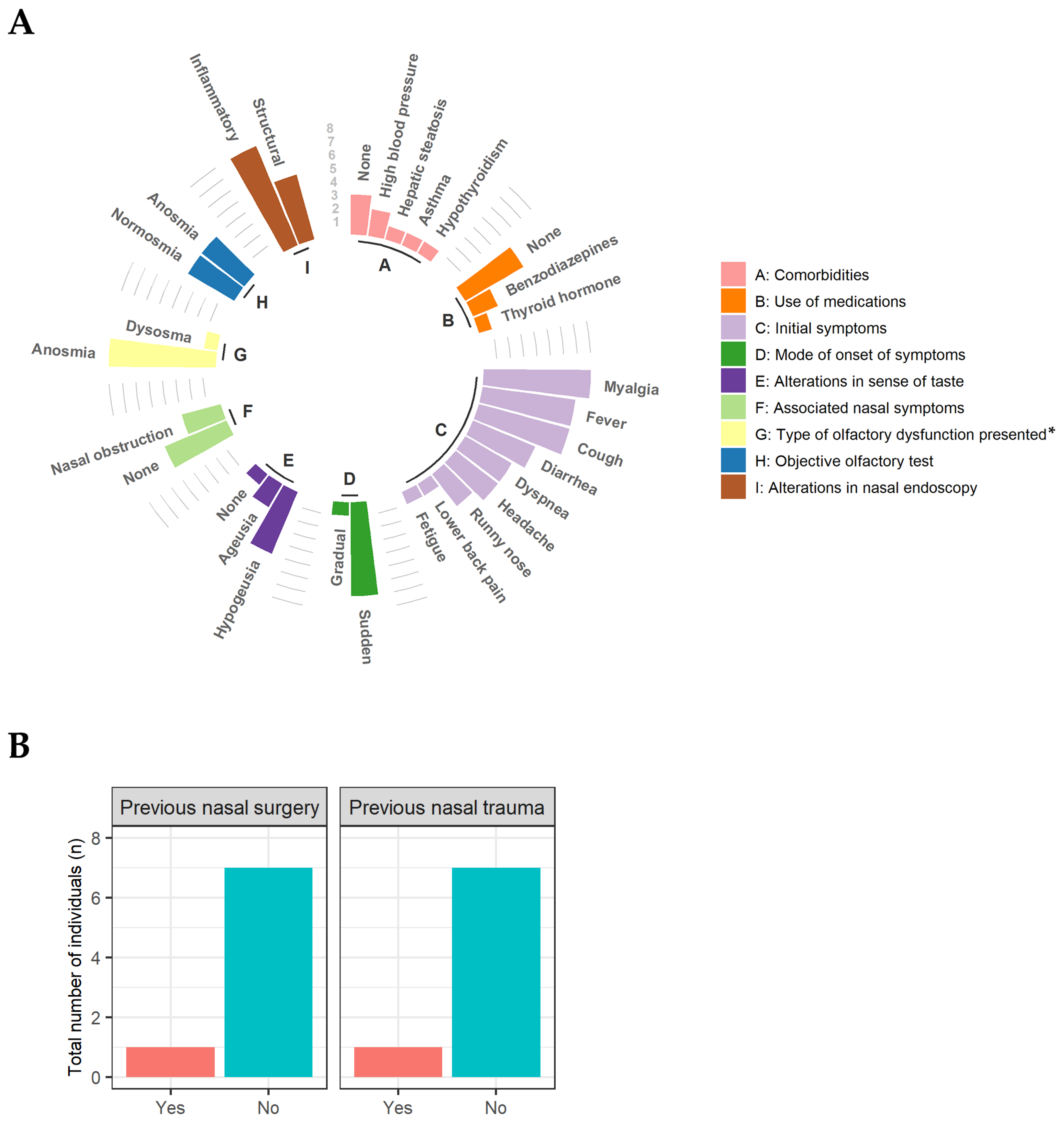
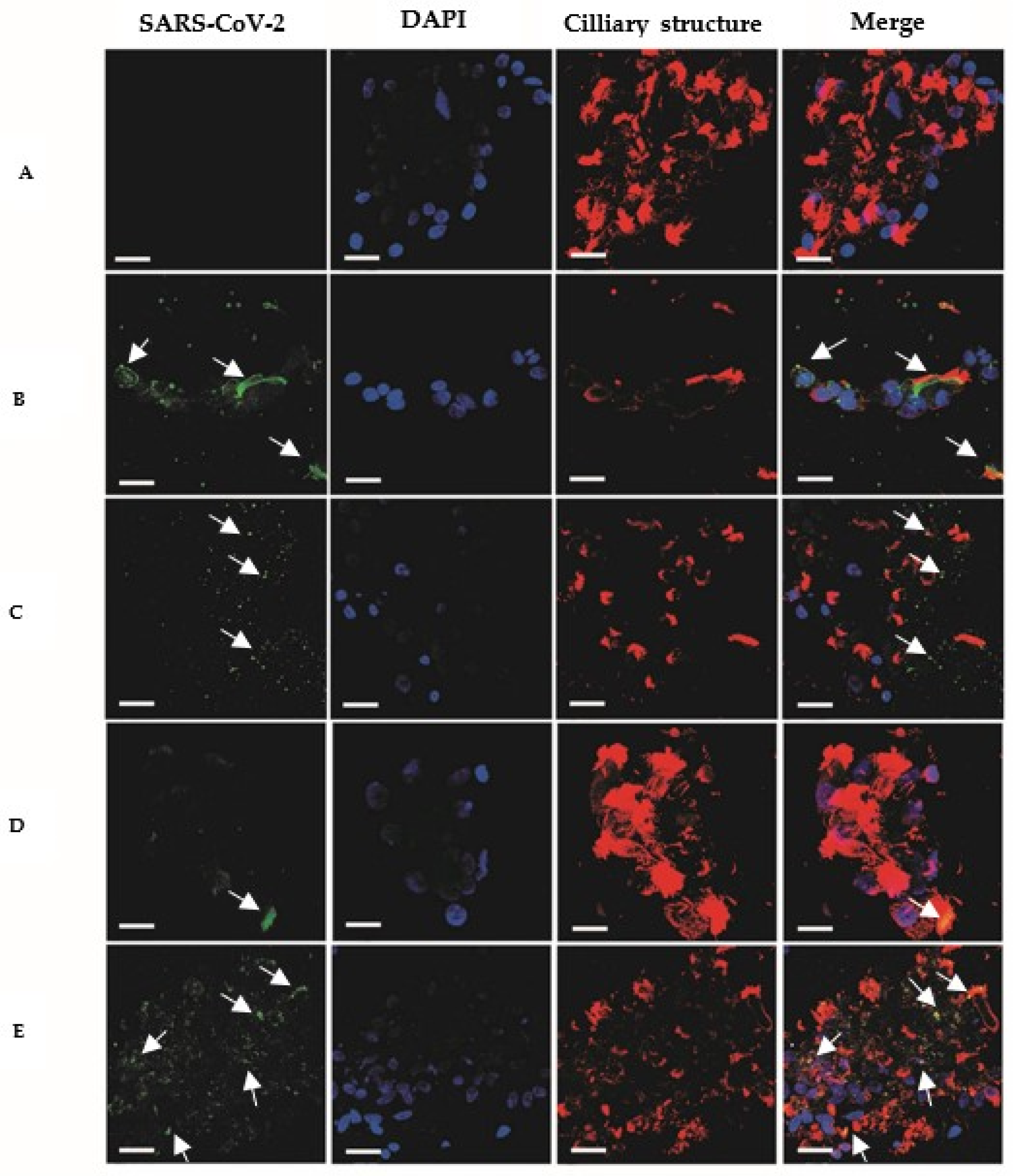
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 Entry Factors Are Highly Expressed in Nasal Epithelial Cells Together with Innate Immune Genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-NCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed on 13 February 2023).
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Director—General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 13 February 2022).
- Lee, I.T.; Nakayama, T.; Wu, C.-T.; Goltsev, Y.; Jiang, S.; Gall, P.A.; Liao, C.-K.; Shih, L.-C.; Schürch, C.M.; McIlwain, D.R.; et al. ACE2 Localizes to the Respiratory Cilia and Is Not Increased by ACE Inhibitors or ARBs. Nat. Commun. 2020, 11, 5453. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, M.; Ou, G. COVID-19, Cilia, and Smell. FEBS J. 2020, 287, 3672–3676. [Google Scholar] [CrossRef]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef]
- Yang, H.; Rao, Z. Structural Biology of SARS-CoV-2 and Implications for Therapeutic Development. Nat. Rev. Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural Basis of Receptor Recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Korompoki, E.; Fotiou, D.; Ntanasis-Stathopoulos, I.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Organ-Specific Manifestations of COVID-19 Infection. Clin. Exp. Med. 2020, 20, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Zapor, M. Persistent Detection and Infectious Potential of SARS-CoV-2 Virus in Clinical Specimens from COVID-19 Patients. Viruses 2020, 12, 1384. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Wang, W.; Liu, Z.; Liang, C.; Wang, W.; Ye, F.; Huang, B.; Zhao, L.; Wang, H.; Zhou, W.; et al. Morphogenesis and Cytopathic Effect of SARS-CoV-2 Infection in Human Airway Epithelial Cells. Nat. Commun. 2020, 11, 3910. [Google Scholar] [CrossRef] [PubMed]
- Umeki, S.; Manabe, T. Structure, Function and Pathophysiology of Mucociliary Transport System. Nihon Rinsho 1992, 50, 892–899. [Google Scholar] [PubMed]
- De Melo, G.D.; Lazarini, F.; Levallois, S.; Hautefort, C.; Michel, V.; Larrous, F.; Verillaud, B.; Aparicio, C.; Wagner, S.; Gheusi, G.; et al. COVID-19–Related Anosmia Is Associated with Viral Persistence and Inflammation in Human Olfactory Epithelium and Brain Infection in Hamsters. Sci. Transl. Med. 2021, 13, eabf8396. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 6 October 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 13 February 2023).
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618–100638. [Google Scholar] [CrossRef]
- Kopańska, M.; Barnaś, E.; Błajda, J.; Kuduk, B.; Łagowska, A.; Banaś-Ząbczyk, A. Effects of SARS-CoV-2 Inflammation on Selected Organ Systems of the Human Body. Int. J. Mol. Sci. 2022, 23, 4178. [Google Scholar] [CrossRef]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the Long Term Effects of COVID-19: Summary of NICE, SIGN, and RCGP Rapid Guideline. BMJ 2021, 372, n136. [Google Scholar] [CrossRef]
- Garg, M.; Maralakunte, M.; Garg, S.; Dhooria, S.; Sehgal, I.; Bhalla, A.S.; Vijayvergiya, R.; Grover, S.; Bhatia, V.; Jagia, P.; et al. The Conundrum of ‘Long-COVID-19ʹ: A Narrative Review. Int. J. Gen. Med. 2021, 14, 2491–2506. [Google Scholar] [CrossRef]
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Boone, C.E.; DeConde, A.S. Association of Chemosensory Dysfunction and COVID-19 in Patients Presenting with Influenza-like Symptoms. Int. Forum Allergy Rhinol. 2020, 10, 806–813. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Borsetto, D.; Fabbris, C.; Spinato, G.; Frezza, D.; Menegaldo, A.; Mularoni, F.; Gaudioso, P.; Cazzador, D.; Marciani, S.; et al. Evolution of Altered Sense of Smell or Taste in Patients with Mildly Symptomatic COVID-19. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Jafar, A.; Lasso, A.; Shorr, R.; Hutton, B.; Kilty, S. Olfactory Recovery Following Infection with COVID-19: A Systematic Review. PLoS ONE 2021, 16, e0259321. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.M.; Murphy, C. Rapid Clinical Evaluation of Anosmia: The Alcohol Sniff Test. Arch. Otolaryngol. Head Neck Surg. 1997, 123, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Jackman, A.H.; Doty, R.L. Utility of a Three-Item Smell Identification Test in Detecting Olfactory Dysfunction. Laryngoscope 2005, 115, 2209–2212. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; de Siati, D.R.; Horoi, M.; le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; el Afia, F.; Distinguin, L.; et al. Olfactory and Gustatory Dysfunctions as a Clinical Presentation of Mild-to-Moderate Forms of the Coronavirus Disease (COVID-19): A Multicenter European Study. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Spinato, G.; Fabbris, C.; Polesel, J.; Cazzador, D.; Borsetto, D.; Hopkins, C.; Boscolo-Rizzo, P. Alterations in Smell or Taste in Mildly Symptomatic Outpatients with SARS-CoV-2 Infection. JAMA 2020, 323, 2089–2090. [Google Scholar] [CrossRef]
- Parma, V.; Ohla, K.; Veldhuizen, M.G.; Niv, M.Y.; Kelly, C.E.; Bakke, A.J.; Cooper, K.W.; Bouysset, C.; Pirastu, N.; Dibattista, M.; et al. More than Smell—COVID-19 Is Associated with Severe Impairment of Smell, Taste, and Chemesthesis. Chem. Senses 2020, 45, 609–622. [Google Scholar] [CrossRef]
- Klopfenstein, T.; Kadiane-Oussou, N.J.; Toko, L.; Royer, P.-Y.; Lepiller, Q.; Gendrin, V.; Zayet, S. Features of Anosmia in COVID-19. Med. Mal. Infect. 2020, 50, 436–439. [Google Scholar] [CrossRef]
- Marin, C.; Hummel, T.; Liu, Z.; Mullol, J. Chronic Rhinosinusitis and COVID-19. J. Allergy Clin. Immunol. Pract. 2022, 10, 1423–1432. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological Assessment of Hospitalized Patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Carmo, A.; Pereira-Vaz, J.; Mota, V.; Mendes, A.; Morais, C.; Silva, A.C.; Camilo, E.; Pinto, C.S.; Cunha, E.; Pereira, J.; et al. Clearance and Persistence of SARS-CoV-2 RNA in Patients with COVID-19. J. Med. Virol. 2020, 92, 2227–2231. [Google Scholar] [CrossRef] [PubMed]
- Buqaileh, R.; Saternos, H.; Ley, S.; Aranda, A.; Forero, K.; AbouAlaiwi, W.A. Can Cilia Provide an Entry Gateway for SARS-CoV-2 to Human Ciliated Cells? Physiol. Genom. 2021, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Hagemann, J.; Döge, J.; Freudelsperger, L.; Cuevas, M.; Klimek, F.; Hummel, T. Olfactory and Gustatory Disorders in COVID-19. Allergo J. Int. 2022, 31, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Von Bartheld, C.S.; Hagen, M.M.; Butowt, R. Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-Analysis Reveals Significant Ethnic Differences. ACS Chem. Neurosci. 2020, 11, 2944–2961. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cao, M.; Zheng, P.; Shen, W. Residual Olfactory Dysfunction in Coronavirus Disease 2019 Patients after Long Term Recovery. J. Clin. Neurosci. 2021, 93, 31–35. [Google Scholar] [CrossRef]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV Viral Load Dynamics, Duration of Viral Shedding, and Infectiousness: A Systematic Review and Meta-Analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and Clinical Application of a Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef]
- Benvari, S.; Mahmoudi, S.; Mohammadi, M. Gastrointestinal Viral Shedding in Children with SARS-CoV-2: A Systematic Review and Meta-Analysis. World J. Pediatr. 2022, 18, 582–588. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.-Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 Infection and Persistence in the Human Body and Brain at Autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- Fernández-Castañeda, A.; Lu, P.; Geraghty, A.C.; Song, E.; Lee, M.-H.; Wood, J.; O’Dea, M.R.; Dutton, S.; Shamardani, K.; Nwangwu, K.; et al. Mild Respiratory COVID Can Cause Multi-Lineage Neural Cell and Myelin Dysregulation. Cell 2022, 185, 2452–2468.e16. [Google Scholar] [CrossRef]
- Hua-Huy, T.; Lorut, C.; Aubourg, F.; Morbieu, C.; Marey, J.; Texereau, J.; Fajac, I.; Mouthon, L.; Roche, N.; Dinh-Xuan, A.T. Persistent Nasal Inflammation 5 Months after Acute Anosmia in Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 1319–1322. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa dos Santos, J.; Ximenes Rabelo, M.; Mattana Sebben, L.; de Souza Carneiro, M.V.; Bosco Lopes Botelho, J.; Cardoso Neto, J.; Nogueira Barbosa, A.; Monteiro de Carvalho, D.; Pontes, G.S. Persistence of SARS-CoV-2 Antigens in the Nasal Mucosa of Eight Patients with Inflammatory Rhinopathy for over 80 Days following Mild COVID-19 Diagnosis. Viruses 2023, 15, 899. https://doi.org/10.3390/v15040899
Costa dos Santos J, Ximenes Rabelo M, Mattana Sebben L, de Souza Carneiro MV, Bosco Lopes Botelho J, Cardoso Neto J, Nogueira Barbosa A, Monteiro de Carvalho D, Pontes GS. Persistence of SARS-CoV-2 Antigens in the Nasal Mucosa of Eight Patients with Inflammatory Rhinopathy for over 80 Days following Mild COVID-19 Diagnosis. Viruses. 2023; 15(4):899. https://doi.org/10.3390/v15040899
Chicago/Turabian StyleCosta dos Santos, Juliana, Marjory Ximenes Rabelo, Luana Mattana Sebben, Matheus Vinicius de Souza Carneiro, João Bosco Lopes Botelho, José Cardoso Neto, Anderson Nogueira Barbosa, Diego Monteiro de Carvalho, and Gemilson Soares Pontes. 2023. "Persistence of SARS-CoV-2 Antigens in the Nasal Mucosa of Eight Patients with Inflammatory Rhinopathy for over 80 Days following Mild COVID-19 Diagnosis" Viruses 15, no. 4: 899. https://doi.org/10.3390/v15040899
APA StyleCosta dos Santos, J., Ximenes Rabelo, M., Mattana Sebben, L., de Souza Carneiro, M. V., Bosco Lopes Botelho, J., Cardoso Neto, J., Nogueira Barbosa, A., Monteiro de Carvalho, D., & Pontes, G. S. (2023). Persistence of SARS-CoV-2 Antigens in the Nasal Mucosa of Eight Patients with Inflammatory Rhinopathy for over 80 Days following Mild COVID-19 Diagnosis. Viruses, 15(4), 899. https://doi.org/10.3390/v15040899






