Prevention of the Vertical Transmission of HIV; A Recap of the Journey so Far
Abstract
1. Introduction
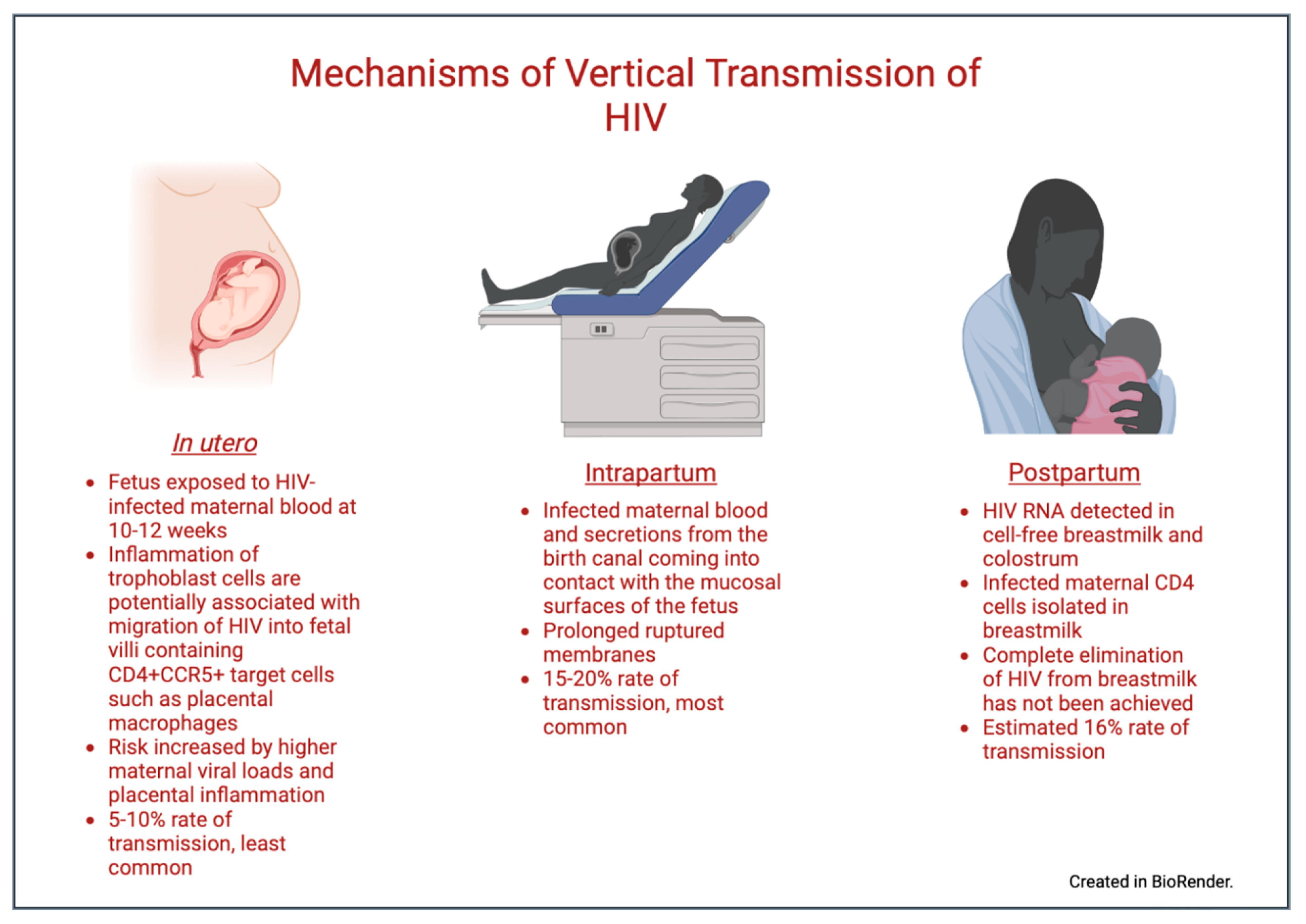
2. Pathophysiology of Vertical Transmission
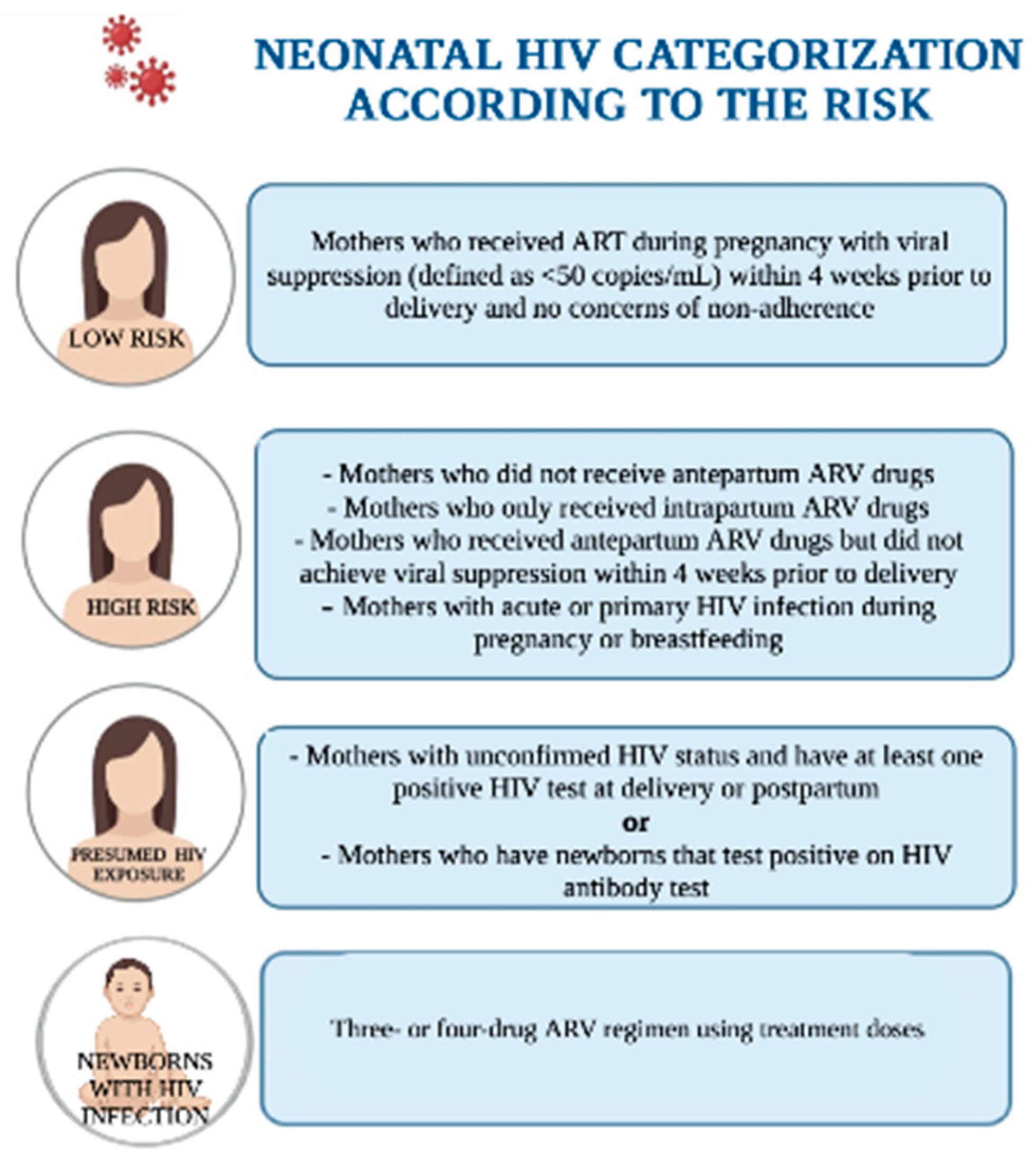
3. Neonatal HIV Infection
4. HIV Testing
4.1. Pre-Pregnancy
4.2. During Pregnancy
4.3. In Labor
4.4. Postpartum
5. Pre-Exposure Prophylaxis (PrEP)
6. Antiretroviral Therapy (ART)
6.1. Pre-Pregnancy
6.2. During Pregnancy and Peripartum
7. Mode of Delivery
8. Other Considerations in the Care of Pregnant PLHIV
9. Postpartum Interventions
9.1. Antiretroviral Medication for the Birthing Parent
9.2. Antiretroviral Medication for Infants
10. Infant Feeding
11. Routine Postnatal Care
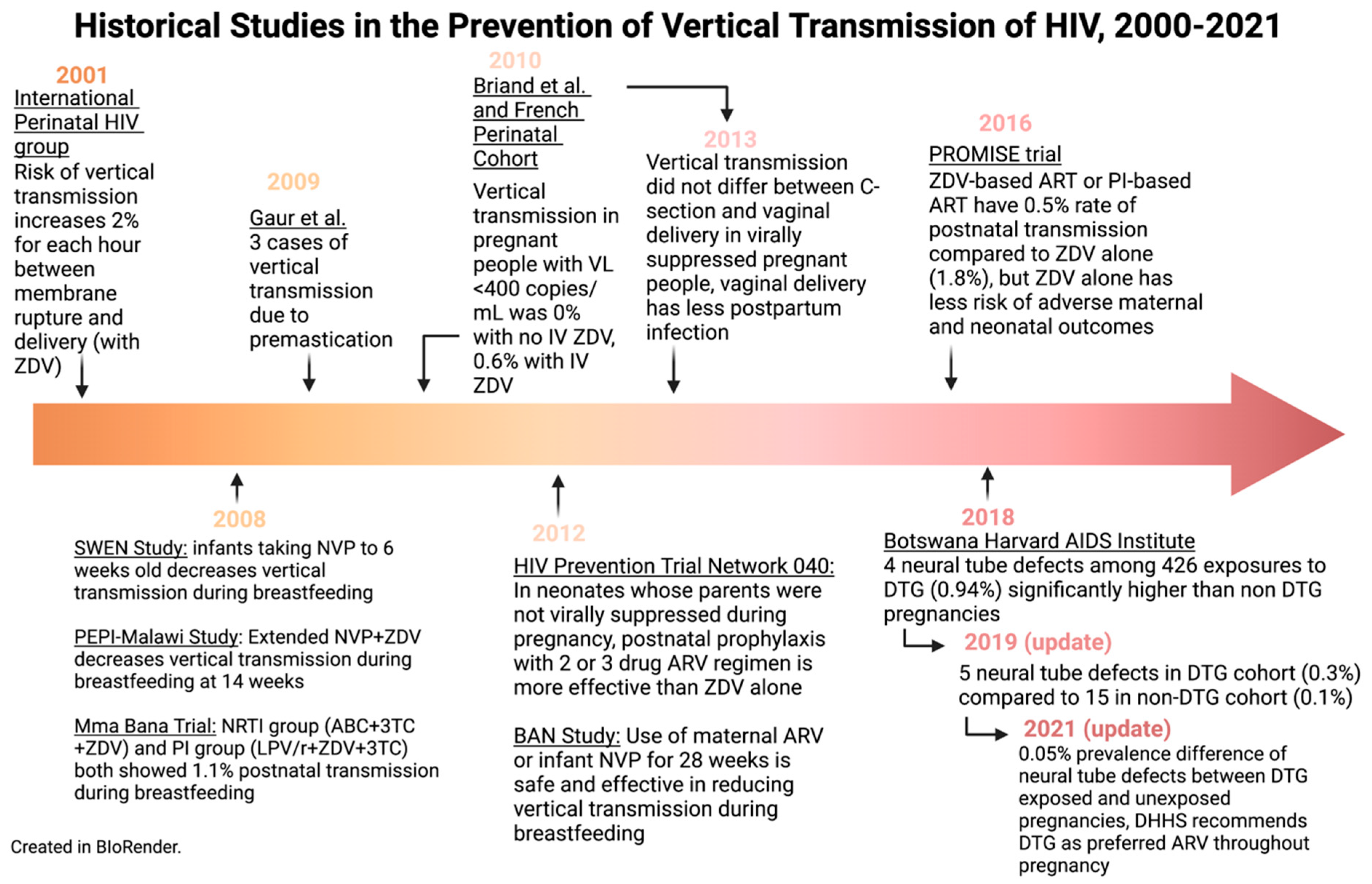
12. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nesheim, S.R.; FitzHarris, L.F.; Gray, K.M.; Lampe, M.A. Epidemiology of Perinatal HIV Transmission in the United States in the Era of Its Elimination. Pediatr. Infect. Dis. J. 2019, 38, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Elgalib, A.; Al-Hinai, F.; Al-Abri, J.; Shah, S.; Al-Habsi, Z.; Al-Fouri, M.; Lau, R.; Al-Kindi, H.; Al-Wahaibi, A.; Al-Maani, A.; et al. Elimination of mother-to-child transmission of HIV in Oman: A success story from the Middle East. East Mediterr. Health J. 2021, 27, 381–389. [Google Scholar] [CrossRef]
- The Working Group on Mother-To-Child Transmission of HIV. Rates of mother-to-child transmission of HIV-1 in Africa, America, and Europe: Results from 13 perinatal studies. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1995, 8, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Guay, L.A.; Musoke, P.; Fleming, T.; Bagenda, D.; Allen, M.; Nakabiito, C.; Sherman, J.; Bakaki, P.; Ducar, C.; Deseyve, M.; et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999, 354, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://data.unicef.org/topic/hivaids/emtct/#status (accessed on 24 January 2023).
- Kourtis, A.P.; Bulterys, M. Mother-to-child transmission of HIV: Pathogenesis, mechanisms and pathways. Clin. Perinatol. 2010, 37, 721–737. [Google Scholar] [CrossRef]
- Amin, O.; Powers, J.; Bricker, K.M.; Chahroudi, A. Understanding Viral and Immune Interplay during Vertical Transmission of HIV: Implications for Cure. Front. Immunol. 2021, 12, 757400. [Google Scholar] [CrossRef]
- Menu, E.; Mbopi-Keou, F.X.; Lagaye, S.; Pissard, S.; Mauclère, P.; Scarlatti, G.; Martin, J.; Goossens, M.; Chaouat, G.; Barré-Sinoussi, F. Selection of maternal human immunodeficiency virus type 1 variants in human placenta European Network for In Utero Transmission of HIV-1. J. Infect. Dis. 1999, 179, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zachar, V.; Zacharova, V.; Fink, T.; Thomas, R.A.; King, B.R.; Ebbesen, P.; Jones, T.B.; Goustin, A.S. Genetic analysis reveals ongoing HIV type 1 evolution in infected human placental trophoblast. AIDS Res. Hum. Retrovir. 1999, 15, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.L.; Boggavarapu, S.; Johnson, E.S.; Lal, A.A.; Agrawal, P.; Bhaumik, S.K.; Murali-Krishna, K.; Chakraborty, R. Human Cytomegalovirus Enhances Placental Susceptibility and Replication of Human Immunodeficiency Virus Type 1 (HIV-1), Which May Facilitate in Utero HIV-1 Transmission. J. Infect. Dis. 2018, 218, 1464–1473. [Google Scholar] [CrossRef]
- Johnson, E.L.; Howard, C.L.; Thurman, J.; Pontiff, K.; Johnson, E.S.; Chakraborty, R. Cytomegalovirus upregulates expression of CCR5 in central memory cord blood mononuclear cells, which may facilitate in utero HIV type 1 transmission. J. Infect. Dis. 2015, 211, 187–196. [Google Scholar] [CrossRef]
- Girsch, J.H.; Plazas, M.C.M.; Olivier, A.; Farah, M.; Littlefield, D.; Behl, S.; Punia, S.R.; Hemsath, J.R.; Norgan, A.; Enninga, E.A.L.; et al. Host-Viral Interactions at the Maternal-Fetal Interface. What We Know and What We Need to Know. Front. Virol. 2022, 2, 833106. Available online: https://www.frontiersin.org/articles/10.3389/fviro.2022.833106/full (accessed on 4 October 2022). [CrossRef] [PubMed]
- Lehman, D.A.; Farquhar, C. Biological mechanisms of vertical human immunodeficiency virus (HIV-1) transmission. Rev. Med. Virol. 2007, 17, 381–403. [Google Scholar] [CrossRef] [PubMed]
- Tobin, N.H.; Aldrovandi, G.M. Immunology of pediatric HIV infection. Immunol. Rev. 2013, 254, 143–169. [Google Scholar] [CrossRef] [PubMed]
- Landesman, S.H.; Kalish, L.A.; Burns, D.N.; Minkoff, H.; Fox, H.E.; Zorrilla, C.; Garcia, P.; Fowler, M.G.; Mofenson, L.; Tuomala, R. Obstetrical factors and the transmission of human immunodeficiency virus type 1 from mother to child. The Women and Infants Transmission Study. N. Engl. J. Med. 1996, 334, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- International Perinatal HIV Group. Duration of ruptured membranes and vertical transmission of HIV-1: A meta-analysis from 15 prospective cohort studies. AIDS 2001, 15, 357–368. [Google Scholar] [CrossRef]
- International Perinatal HIV Group; Andiman, W.; Bryson, Y.; de Martino, M.; Fowler, M.; Harris, D.; Hutto, C.; Korber, B.; Kovacs, A.; Landesman, S.; et al. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1--a meta-analysis of 15 prospective cohort studies. N. Engl. J. Med. 1999, 340, 977–987. [Google Scholar] [CrossRef]
- Goga, A.E.; Van de Perre, P.; Ngandu, N.; Nagot, N.; Abrams, E.J.; Moodley, D.; King, R.; Molès, J.P.; Chirinda, W.; Scarlatti, G.; et al. Eliminating HIV transmission through breast milk from women taking antiretroviral drugs. BMJ 2021, 374, n1697. [Google Scholar] [CrossRef]
- Van de Perre, P.; Simonon, A.; Msellati, P.; Hitimana, D.G.; Vaira, D.; Bazubagira, A.; Van Goethem, C.; Stevens, A.M.; Karita, E.; Sondag-Thull, D. Postnatal transmission of human immunodeficiency virus type 1 from mother to infant. A prospective cohort study in Kigali, Rwanda. N. Engl. J. Med. 1991, 325, 593–598. [Google Scholar] [CrossRef]
- Van de Perre, P.; Lepage, P.; Homsy, J.; Dabis, F. Mother-to-infant transmission of human immunodeficiency virus by breast milk: Presumed innocent or presumed guilty? Clin. Infect. Dis. 1992, 15, 502–507. [Google Scholar] [CrossRef]
- Nduati, R.; John, G.; Mbori-Ngacha, D.; Richardson, B.; Overbaugh, J.; Mwatha, A.; Ndinya-Achola, J.; Bwayo, J.; Onyango, F.E.; Hughes, J.; et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: A randomized clinical trial. JAMA 2000, 283, 1167–1174. [Google Scholar] [CrossRef]
- Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Transmission in the United States. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/Perinatal_GL.pdf (accessed on 10 May 2022).
- Gilleece, D.Y.; Tariq, D.S.; Bamford, D.A.; Bhagani, D.S.; Byrne, D.L.; Clarke, D.E.; Clayden, M.P.; Lyall, D.H.; Metcalfe, D.R.; Palfreeman, D.A.; et al. British HIV Association guidelines for the management of HIV in pregnancy and postpartum 2018. HIV Med. 2019, 20 (Suppl. 3), s2–s85. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, A. Acquired Immunodeficiency Syndrome in Infants. Am. J. Dis Child. 1983, 137, 825–827. [Google Scholar] [CrossRef]
- Oleske, J.; Minnefor, A.; Cooper, R.; Thomas, K.; dela Cruz, A.; Ahdieh, H.; Guerrero, I.; Joshi, V.V.; Desposito, F. Immune Deficiency Syndrome in Children. JAMA 1983, 249, 2345–2349. [Google Scholar] [CrossRef]
- Scott, G.B.; Hutto, C.; Makuch, R.W.; Mastrucci, M.T.; O’Connor, T.; Mitchell, C.D.; Trapido, E.J.; Parks, W.P. Survival in children with perinatally acquired human immunodeficiency virus type 1 infection. N. Engl. J. Med. 1989, 321, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.E.; Graham, S.M.; Kumwenda, N.I.; Broadhead, R.L.; Hoover, D.R.; Markakis, D.; van Der Hoeven, L.; Liomba, G.N.; Chiphangwi, J.D.; Miotti, P.G. Morbidity among human immunodeficiency virus-1-infected and -uninfected African children. Pediatrics 2000, 106, E77. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.merckmanuals.com/professional/pediatrics/human-immunodeficiency-virus-hiv-infection-in-infants-and-children/human-immunodeficiency-virus-hiv-infection-in-infants-and-children (accessed on 21 January 2023).
- Abbas, M.; Bakhtyar, A.; Bazzi, R. Neonatal HIV. [Updated 2022 Sep 20]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- World Health Organization. Global HIV, Hepatitis and Sexually Transmitted Infections Programmes, Guidelines Review Committee. “Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach”. Available online: https://www.who.int/publications/i/item/9789240031593 (accessed on 4 October 2022).
- Salters, K.; Loutfy, M.; de Pokomandy, A.; Money, D.; Pick, N.; Wang, L.; Jabbari, S.; Carter, A.; Webster, K.; Conway, T.; et al. Pregnancy incidence and intention after HIV diagnosis among women living with HIV in Canada. PLoS ONE 2017, 12, e0180524. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28727731 (accessed on 4 October 2022). [CrossRef]
- Aebi-Popp, K.; Mercanti, V.; Voide, C.; Nemeth, J.; Cusini, A.; Jakopp, B.; Bruno, A.; Calmy, A.; de Tejada, B.M. Neglect of attention to reproductive health in women with HIV infection: Contraceptive use and unintended pregnancies in the Swiss HIV Cohort Study. HIV Med. 2018, 19, 339–346. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29336516 (accessed on 4 October 2022). [CrossRef]
- Sutton, M.Y.; Zhou, W.; Frazier, E.L. Unplanned pregnancies and contraceptive use among HIV-positive women in care. PLoS ONE 2018, 13, e0197216. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29771940 (accessed on 4 October 2022). [CrossRef]
- Dude, A.M.; Miller, E.S.; Garcia, P.M.; Yee, L.M. Unintended pregnancy and viral suppression in pregnant women living with HIV. Am. J. Obstet. Gynecol. MFM 2021, 3, 100300. Available online: https://www.ncbi.nlm.nih.gov/pubmed/33359637 (accessed on 4 October 2022). [CrossRef]
- Sekar, V.J.; Lefebvre, E.; Guzman, S.S.; Felicione, E.; De Pauw, M.; Vangeneugden, T.; Hoetelmans, R.M. Pharmacokinetic interaction between ethinyl estradiol, norethindrone and darunavir with low-dose ritonavir in healthy women. Antivir. Ther. 2008, 13, 563–569. [Google Scholar] [CrossRef]
- Vieira, C.S.; Bahamondes, M.V.; de Souza, R.M.; Brito, M.B.; Tatiana, R.R.P.; Eliana, A.; Luis, B.; Geraldo, D.; Silvana, M.Q.; Carolina, S.; et al. Effect of antiretroviral therapy including lopinavir/ritonavir or efavirenz on etonogestrel-releasing implant pharmacokinetics in HIV-positive women. J. Acquir Immune Defic Syndr. 2014, 66, 378–385. Available online: https://pubmed.ncbi.nlm.nih.gov/24798768 (accessed on 4 October 2022). [CrossRef]
- National AIDS and STI Control Program. “Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV in Kenya.” NASCOP. 2018. Available online: https://www.nascop.or.ke/new-guidelines/ (accessed on 4 October 2022).
- Connor, E.M.; Sperling, R.S.; Gelber, R.; Kiselev, P.; Scott, G.; O’Sullivan, M.J.; VanDyke, R.; Bey, M.; Shearer, W.; Jacobson, R.L.; et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N. Engl. J. Med. 1994, 331, 1173–1180. [Google Scholar] [CrossRef]
- Perinatal Human Immunodeficiency Virus Testing. Provisional Committee on Pediatric AIDS, American Academy of Pediatrics. Pediatrics 1995, 95, 303–307. [Google Scholar]
- Centers for Disease Control and Prevention. U.S. Public Health Service recommendations for human immunodeficiency virus counseling and voluntary testing for pregnant women. MMWR Recomm. Rep. 1995, 44, 1–15. [Google Scholar]
- Human Immunodeficiency Virus Screening. Joint statement of the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Pediatrics 1999, 104 Pt 1, 128. [Google Scholar]
- Valentine, S.S.; Caldwell, J.; Tailor, A. Effect of CDC 2006 revised HIV testing recommendations for adults, adolescents, pregnant women, and newborns on state laws, 2018. Public Health Rep. 2020, 135 (Suppl. 1), 189S–196S. Available online: https://www.ncbi.nlm.nih.gov/pubmed/32735201 (accessed on 4 October 2022). [CrossRef] [PubMed]
- Department of Health, Republic of South Africa. “National Consolidated Guidelines for the Prevention of Mother to Child Transmission and the Management of HIV in Children, Adolescents, and Adults.” South African HIV Clinicians Society. 2015. Available online: https://sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf (accessed on 4 October 2022).
- Olakunde, B.O.; Pharr, J.R.; Adeyinka, D.A. HIV testing among pregnant women with prenatal care in the United States: An analysis of the 2011–2017 National Survey of Family Growth. Int. J. STD AIDS 2020, 31, 680–688. Available online: https://www.ncbi.nlm.nih.gov/pubmed/32538331 (accessed on 4 October 2022). [CrossRef]
- Koumans, E.H.; Harrison, A.; House, L.D.; Burley, K.; Ruffo, N.; Smith, R.; Fitzharris, L.; Johnson, C.H.; Taylor, A.W.; Nesheim, S.R. Characteristics associated with lack of HIV testing during pregnancy and delivery in 36 U.S. states, 2004–2013. Int. J. STD AIDS 2018, 29, 1225–1233. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29969977 (accessed on 4 October 2022). [CrossRef]
- Liao, C.; Golden, W.C.; Anderson, J.R.; Coleman, J.S. Missed opportunities for repeat HIV testing in pregnancy: Implications for elimination of mother-to-child transmission in the United States. AIDS Patient Care STDS 2017, 31, 20–26. Available online: https://www.ncbi.nlm.nih.gov/pubmed/27936863 (accessed on 4 October 2022). [CrossRef] [PubMed]
- Trepka, M.J.; Mukherjee, S.; Beck-Sague, C.; Maddox, L.M.; Fennie, K.P.; Sheehan, D.M.; Prabhakar, M.; Thompson, D.; Lieb, S. Missed opportunities for preventing perinatal transmission of human immunodeficiency virus, Florida, 2007–2014. South. Med. J. 2017, 110, 116–128. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28158882 (accessed on 4 October 2022). [CrossRef] [PubMed]
- Szlachta-McGinn, A.; Aserlind, A.; Duthely, L.; Oldak, S.; Babriwala, R.; Montgomerie, E.; Potter, J. HIV screening during pregnancy in a U.S. HIV epicenter. Infect. Dis. Obstet. Gynecol. 2020, 2020, 8196342. Available online: https://www.ncbi.nlm.nih.gov/pubmed/32454582 (accessed on 4 October 2022). [CrossRef] [PubMed]
- Townsend, C.L.; Byrne, L.; Cortina-Borja, M.; Thorne, C.; de Ruiter, A.; Lyall, H.; Taylor, G.P.; Peckham, C.S.; Tookey, P.A. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011. AIDS 2014, 28, 1049–1057. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24566097 (accessed on 4 October 2022). [CrossRef]
- National AIDS Control Organization, Ministry of Health, Government of India. “National Guidelines for HIV Care and Treatment.” NACO. 2021. Available online: http://naco.gov.in/sites/default/files/National_Guidelines_for_HIV_Care_and_Treatment_2021.pdf (accessed on 4 October 2022).
- Boucoiran, I.; Albert, A.; Tulloch, K.; Wagner, E.C.; Pick, N.; Van Schalkwyk, J.; Harrigan, P.R.; Money, D. Human immunodeficiency virus viral load rebound near delivery in previously suppressed, combination antiretroviral therapy-treated pregnant women. Obstet. Gynecol. 2017, 130, 497–501. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28796673 (accessed on 4 October 2022). [CrossRef]
- World Health Organization; United Nations Children’s Fund. Guideline: Updates on HIV and Infant Feeding: The Duration of Breastfeeding, and Support from Health Services to Improve Feeding Practices among Mothers Living with HIV; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Available online: https://www.health.ny.gov/diseases/aids/general/about/perinatal.htm (accessed on 20 January 2023).
- Centers for Disease Control and Prevention and Association of Public Health Laboratories. Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. 27 June 2014. Available online: http://dx.doi.org/10.15620/cdc.23447 (accessed on 4 October 2022).
- Donovan, M.; Palumbo, P. Diagnosis of HIV: Challenges and strategies for HIV prevention and detection among pregnant women and their infants. Clin. Perinatol. 2010, 37, 751–763, viii. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21078448 (accessed on 4 October 2022). [CrossRef] [PubMed]
- Brubaker, S.G.; Bukusi, E.A.; Odoyo, J.; Achando, J.; Okumu, A.; Cohen, C.R. Pregnancy and HIV transmission among HIV-discordant couples in a clinical trial in Kisumu, Kenya. HIV Med. 2011, 12, 316–321. Available online: https://pubmed.ncbi.nlm.nih.gov/21205129/ (accessed on 4 October 2022). [CrossRef] [PubMed]
- Tang, H.; Wu, Z.; Mao, Y.; Cepeda, J.; Morano, J. Risk factor associated with negative spouse HIV seroconversion among sero-different couples: A nested case-control retrospective survey study in 30 counties in rural China. PLoS ONE 2016, 11, e0164761. Available online: https://pubmed.ncbi.nlm.nih.gov/27741292/ (accessed on 4 October 2022). [CrossRef]
- Groer, M.; El-Badri, N.; Djeu, J.; Harrington, M.; Van Eepoel, J. Suppression of natural killer cell cytotoxicity in postpartum women. Am. J. Reprod. Immunol. 2010, 63, 209–213. Available online: https://pubmed.ncbi.nlm.nih.gov/20055786/ (accessed on 4 October 2022). [CrossRef] [PubMed]
- Hapgood, J.P.; Kaushic, C.; Hel, Z. Hormonal contraception and HIV-1 acquisition: Biological mechanisms. Endocr. Rev. 2018, 39, 36–78. Available online: https://pubmed.ncbi.nlm.nih.gov/29309550/ (accessed on 4 October 2022). [CrossRef] [PubMed]
- Mugo, N.R.; Heffron, R.; Donnell, D.; Wald, A.; Were, E.O.; Rees, H.; Celum, C.; Kiarie, J.N.; Cohen, C.R.; Kayintekore, K.; et al. Increased risk of HIV-1 transmission in pregnancy: A prospective study among African HIV-1-serodiscordant couples. AIDS 2011, 25, 1887–1895. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21785321 (accessed on 4 October 2022). [CrossRef]
- Keating, M.A.; Hamela, G.; Miller, W.C.; Moses, A.; Hoffman, I.F.; Hosseinipour, M.C. High HIV incidence and sexual behavior change among pregnant women in Lilongwe, Malawi: Implications for the risk of HIV acquisition. PLoS ONE 2012, 7, e39109. Available online: https://pubmed.ncbi.nlm.nih.gov/22768063/ (accessed on 4 October 2022). [CrossRef]
- Moodley, D.; Esterhuizen, T.M.; Pather, T.; Chetty, V.; Ngaleka, L. High HIV incidence during pregnancy: Compelling reason for repeat HIV testing. AIDS 2009, 23, 1255–1259. Available online: https://pubmed.ncbi.nlm.nih.gov/19455017/ (accessed on 4 October 2022). [CrossRef]
- Gray, R.H.; Li, X.; Kigozi, G.; Serwadda, D.; Brahmbhatt, H.; Wabwire-Mangen, F.; Nalugoda, F.; Kiddugavu, M.; Sewankambo, N.; Quinn, T.C.; et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: A prospective study. Lancet 2005, 366, 1182–1188. Available online: https://pubmed.ncbi.nlm.nih.gov/16198767/ (accessed on 4 October 2022). [CrossRef] [PubMed]
- Graybill, L.A.; Kasaro, M.; Freeborn, K.; Walker, J.S.; Poole, C.; Powers, K.A.; Mollan, K.R.; Rosenberg, N.E.; Vermund, S.H.; Mutale, W.; et al. Incident HIV among pregnant and breast-feeding women in sub-Saharan Africa: A systematic review and meta-analysis. AIDS 2020, 34, 761–776. Available online: https://pubmed.ncbi.nlm.nih.gov/32167990/ (accessed on 4 October 2022). [CrossRef]
- Humphrey, J.H.; Marinda, E.; Mutasa, K.; Moulton, L.H.; Iliff, P.J.; Ntozini, R.; Chidawanyika, H.; Nathoo, H.J.; Tavengwa, N.; Jenkins, A.; et al. Mother to child transmission of HIV among Zimbabwean women who seroconverted postnatally: Prospective cohort study. BMJ 2010, 341, c6580. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21177735 (accessed on 4 October 2022). [CrossRef]
- Morrison, S.; John-Stewart, G.; Egessa, J.J.; Mubezi, S.; Kusemererwa, S.; Bii, D.K.; Bulya, N.; Mugume, F.; Campbell, J.D.; Wangisi, J.; et al. Rapid antiretroviral therapy initiation for women in an HIV-1 prevention clinical trial experiencing primary HIV-1 infection during pregnancy or breastfeeding. PLoS ONE 2015, 10, e0140773. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26469986 (accessed on 4 October 2022). [CrossRef] [PubMed]
- Okwundu, C.I.; Uthman, O.A.; Okoromah, C.A. Antiretroviral pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals. Cochrane Database Syst. Rev. 2012, 7, CD007189. [Google Scholar] [CrossRef]
- Hanscom, B.; Janes, H.E.; Guarino, P.D.; Huang, Y.; Brown, W.R.; Chen, Y.Q.; Hammer, S.M.; Gilbert, P.B.; Donnel, D.J. Brief report: Preventing HIV-1 infection in women using oral preexposure prophylaxis: A meta-analysis of current evidence. J. Acquir. Immune Defic. Syndr. 2016, 73, 606–608. Available online: https://pubmed.ncbi.nlm.nih.gov/27846073/ (accessed on 4 October 2022). [CrossRef] [PubMed]
- Heffron, R.; Ngure, K.; Velloza, J.; Kiptinness, C.; Quame-Amalgo, J.; Oluch, L.; Thuo, N.; Njoroge, J.; Momanyi, R.; Gakuo, S. Implementation of a comprehensive safer conception intervention for HIV-serodiscordant couples in Kenya: Uptake, use and effectiveness. J. Int. AIDS Soc. 2019, 22, e25261. Available online: https://pubmed.ncbi.nlm.nih.gov/30957420/ (accessed on 4 October 2022). [CrossRef]
- Schwartz, S.R.; Bassett, J.; Mutunga, L.; Yenden, N.; Mudavanhu, M.; Phofa, R.; Sanne, I.; Van Rie, A. HIV incidence, pregnancy, and implementation outcomes from the Sakh’umndeni safer conception project in South Africa: A prospective cohort study. Lancet HIV. 2019, 6, e438–e446. Available online: https://pubmed.ncbi.nlm.nih.gov/31160268/ (accessed on 4 October 2022). [CrossRef]
- Leech, A.A.; Biancarelli, D.; Aaron, E.; Miller, E.S.; Coleman, J.S.; Anderson, P.L.; Nkwihoreze, H.; Condron, B.; Sullivan, M. Hiv pre-exposure prophylaxis for conception among HIV serdiscordant couplsin the United States: A cohort study. AIDS Patient Care STDS. 2020, 34, 295–302. Available online: https://www.ncbi.nlm.nih.gov/pubmed/32639209 (accessed on 4 October 2022). [CrossRef]
- British HIV Association. BHIVA Guidelines for the Treatment of HIV-1 Positive Adults with Antiretroviral Therapy 2015 (2016 interim update). 2016. Available online: www.bhiva.org/HIV-1-treatment-guidelines (accessed on 4 October 2022).
- van der Galien, R.; Ter Heine, R.; Greupink, R.; van Herwaarden, A.E.; Colbers, A.; Burger, D.M. Pharmacokinetics of HIV-integrase inhibitors during pregnancy: Mechanisms, clinical implications and knowledge gaps. Clin. Pharmacokinet. 2018, 58, 309–323. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29915921 (accessed on 4 October 2022). [CrossRef] [PubMed]
- Galli, L.; Puliti, D.; Chiappini, E.; Gabiano, C.; Ferraris, G.; Mignone, F.; Viganò, A.; Giaquinto, C.; Genovese, O.; Anzidei, G.; et al. Is the interruption of antiretroviral treatment during pregnancy an additional major risk factor for mother-to-child transmission of HIV type 1? Clin. Infect. Dis. 2009, 48, 1310–1317. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19309307 (accessed on 4 October 2022). [CrossRef] [PubMed]
- Wiktor, S.Z.; Ekpini, E.; Karon, J.M.; Nkengasong, J.; Maurice, C.; Severin, S.T.; Roels, T.H.; Kouassi, M.K.; Lackritz, E.M.; Coulibaly, I.M.; et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Côte d’Ivoire: A randomised trial. Lancet 1999, 353, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Dabis, F.; Msellati, P.; Meda, N.; elffens-Ekra, C.; You, B.; Manigart, O.; Leroy, V.; Simonon, A.; Cartoux, M.; Combe, P.; et al. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Côte d’Ivoire and Burkina Faso: A double-blind placebo-controlled multicentre trial. Lancet 1999, 353, 786–792. [Google Scholar] [CrossRef]
- Sperling, R.S.; Shapiro, D.E.; Coombs, R.W.; Todd, J.A.; Herman, S.A.; McSherry, G.D.; O′Sullivan, M.J.; Van Dyke, R.B.; Jimenez, E.; Rouzioux, C.; et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. N. Engl. J. Med. 1998, 335, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.B.; Musoke, P.; Fleming, T.; Guay, L.A.; Bagenda, D.; Allen, M.; Nakabiito, C.; Sherman, J.; Bakaki, P.; Owor, M.; et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet 2003, 362, 859–868. [Google Scholar] [CrossRef]
- Institute of Medicine Committee on Reviewing the HPHIVPS. The National Academies Collection: Reports Funded by National Institutes of Health. Review of the HIVNET 012 Perinatal HIV Prevention Study; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Shaffer, N.; Roongpisuthipong, A.; Siriwasin, W.; Chotpitayasunondh, T.; Chearskul, S.; Young, N.L.; Parekh, B.; Mock, P.A.; Bhadrakom, C.; Chinayon, P.; et al. Maternal virus load and perinatal human immunodeficiency virus type 1 subtype E transmission, Thailand. Bangkok Collaborative Perinatal HIV Transmission Study Group. J. Infect. Dis. 1999, 179, 590–599. [Google Scholar] [CrossRef]
- Garcia, P.M.; Kalish, L.A.; Pitt, J.; Minkoff, H.; Quinn, T.C.; Burchett, S.K.; Kornegay, J.; Jackson, B.; Moye, J.; Hanson, C.; et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N. Engl. J. Med. 1999, 341, 394–402. [Google Scholar] [CrossRef]
- de Vincenzi, I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): A randomised controlled trial. Lancet Infect. Dis. 2011, 11, 171–180. [Google Scholar] [CrossRef]
- Shapiro, R.L.; Hughes, M.D.; Ogwu, A.; Kitch, D.; Lockman, S.; Moffat, C.; Makhema, J.; Moyo, S.; Thior, I.; McIntosh, K.; et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N. Engl. J. Med. 2010, 362, 2282–2294. [Google Scholar] [CrossRef]
- Briand, N.; Warszawski, J.; Mandelbrot, L.; Dollfus, C.; Pannier, E.; Cravello, L.; Nguyen, R.; Matheron, I.; Winer, N.; Tubiana, R.; et al. Is intrapartum intravenous zidovudine for prevention of mother-to-child HIV-1 transmission still useful in the combination antiretroviral therapy era? Clin. Infect. Dis. 2013, 57, 903–914. [Google Scholar] [CrossRef]
- Briand, N.; Jasseron, C.; Sibiude, J.; Azria, E.; Pollet, J.; Hammou, Y.; Warszawski, J.; Mandelbrot, L. Cesarean section for HIV-infected women in the combination antiretroviral therapies era, 2000–2010. Am. J. Obstet. Gynecol. 2013, 209, 335.e1–335.e12. [Google Scholar] [CrossRef] [PubMed]
- National AIDS and STIs Control Program, Federal Ministry of Health Nigeria. “National Guidelines for HIV Prevention, Treatment, and Care.” NASCP Federal Ministry of Health. 2020. Available online: https://nascp.gov.ng/resources/view/1 (accessed on 4 October 2022).
- Garcia-Tejedor, A.; Maiques, V.; Perales, A.; López-Aldeguer, J. Influence of highly active antiretroviral treatment (HAART) on risk factors for vertical HIV transmission. , Acta Obstet. Gynecol. Scand. 2009, 88, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Cotter, A.M.; Brookfield, K.F.; Duthely, L.M.; Quintero, V.H.G.; Potter, J.E.; O’Sullivan, M.J. Duration of membrane rupture and risk of perinatal transmission of HIV-1 in the era of combination antiretroviral therapy. Am. J. Obstet. Gynecol. 2012, 207, 482.e1–482.e5. [Google Scholar] [CrossRef] [PubMed]
- Mark, S.; Murphy, K.E.; Read, S.; Bitnun, A.; Yudin, M.H. HIV Mother-to-Child Transmission, Mode of Delivery, and Duration of Rupture of Membranes: Experience in the Current Era. Infect. Dis. Obstet. Gynecol. 2012, 2012, 267969. [Google Scholar] [CrossRef]
- Peters, H.; Byrne, L.; De Ruiter, A.; Francis, K.; Harding, K.; Taylor, G.P.; Tookey, P.A.; Townsend, C.L. Duration of ruptured membranes and mother-to-child HIV transmission: A prospective population-based surveillance study. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 975–981. [Google Scholar] [CrossRef]
- Peters, H.; Francis, K.; Harding, K.; Tookey, P.A.; Thorne, C. Operative vaginal delivery and invasive procedures in pregnancy among women living with HIV. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 210, 295–299. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28092853 (accessed on 4 October 2022). [CrossRef]
- Carias, A.M.; Hope, T.J. Barriers of Mucosal Entry of HIV/SIV. Curr. Immunol. Rev. 2019, 15, 4–13. [Google Scholar] [CrossRef]
- McGoldrick, E.; Stewart, F.; Parker, R.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020, 12, CD004454. [Google Scholar] [CrossRef]
- Burns, E.; Feeley, C.; Hall, P.J.; Vanderlaan, J. Systematic review and meta-analysis to examine intrapartum interventions, and maternal and neonatal outcomes following immersion in water during labour and waterbirth. BMJ Open 2022, 12, e056517. [Google Scholar] [CrossRef]
- McKinney, J.; Jackson, J.; Sangi-Haghpeykar, H.; Hickerson, L.; Hawkins, J.; Peters, Y.; Levison, J. HIV-adapted group prenatal care: Assessing viral suppression and postpartum retention in care. AIDS Patient Care STDS 2021, 35, 39–46. Available online: https://www.ncbi.nlm.nih.gov/pubmed/33571047 (accessed on 4 October 2022). [CrossRef]
- Nora, E.R.; van Lettow, M.; Tweya, H.; Kapito-Tembo, A.; Bourdon, C.M.; Cataldo, F.; Chiwaula, L.; Sampathkumar, V.; Trapence, C.; Kayoyo, V.; et al. Improving PMTCT Uptake and Retention Services through Novel Approaches in Peer-Based Family-Supported Care in the Clinic and Community: A three-arm cluster randomized trial (PURE Malawi). J. Acquir. Immune Defic. Syndr. 2014, 67, S114–S119. [Google Scholar] [CrossRef]
- Soepnel, L.M.; Norris, S.A.; Schrier, V.J.; Browne, J.L.; Rijken, M.J.; Gray, G.; Klipstein-Grobusch, K. The association between HIV, antiretroviral therapy, and gestational diabetes mellitus. AIDS 2017, 31, 113–125. Available online: https://www.ncbi.nlm.nih.gov/pubmed/27677165 (accessed on 4 October 2022). [CrossRef]
- Floridia, M.; Masuelli, G.; Meloni, A.; Cetin, I.; Tamburrini, E.; Cavaliere, A.F.; Dalzero, S.; Sansone, M.; Alberico, S.; Guerra, B.; et al. Amniocentesis and chorionic villus sampling in HIV-infected pregnant women: A multicentre case series. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1218–1223. Available online: https://www.ncbi.nlm.nih.gov/pubmed/27319948 (accessed on 4 October 2022). [CrossRef] [PubMed]
- Maiques, V.; Garcia-Tejedor, A.; Perales, A.; Cordoba, J.; Esteban, R.J. HIV detection in amniotic fluid samples. Amniocentesis can be performed in HIV pregnant women? Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 108, 137–141. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12781400 (accessed on 4 October 2022). [CrossRef]
- Rabe, H.; Diaz-Rossello, J.L.; Duley, L.; Dowswell, T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst. Rev. 2012, 8, CD003248. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22895933 (accessed on 4 October 2022). [CrossRef]
- McDonald, S.J.; Middleton, P.; Dowswell, T.; Morris, P.S. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst. Rev. 2013, 7, CD004074. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23843134 (accessed on 4 October 2022). [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Committee opinion No. 684: Delayed umbilical cord clamping after birth. Obstet. Gynecol. 2017, 129, e5–e10. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28002310 (accessed on 4 October 2022). [CrossRef]
- Navarro, J.; Curran, A.; Burgos, J.; Torrella, A.; Ocaña, I.; Falcó, V.; Crespo, M.; Ribera, E. Acute leg ischaemia in an HIV-infected patient receiving antiretroviral treatment. Antivir. Ther. 2017, 22, 89–90. Available online: https://www.ncbi.nlm.nih.gov/pubmed/27546463 (accessed on 4 October 2022). [CrossRef]
- Shapiro, R.L.; Kitch, D.; Ogwu, A.; Hughes, M.D.; Lockman, S.; Powis, K.; Souda, S.; Moffat, C.; Moyo, S.; McIntosh, K.; et al. HIV transmission and 24-month survival in a randomized trial of HAART to prevent MTCT during pregnancy and breastfeeding in Botswana. AIDS 2013, 27, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, L.; Sinkala, M.; Kankasa, C.; Semrau, K.; Kasonde, P.; Scott, N.; Mwiya, M.; Vwalika, C.; Walter, J.; Tsai, W.Y.; et al. High uptake of exclusive breastfeeding and reduced early post-natal HIV transmission. PLoS ONE 2007, 2, e1363. [Google Scholar] [CrossRef]
- Bedri, A.; Gudetta, B.; Isehak, A.; Kumbi, S.; Lulseged, S.; Mengistu, Y.; Bhore, A.V.; Bhosale, R.; Varadhrajan, V.; Gupte, N.; et al. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: An analysis of three randomised controlled trials. Lancet 2008, 372, 300–313. [Google Scholar] [CrossRef]
- Taha, T.E.; Li, Q.; Hoover, D.R.; Mipando, L.; Nkanaunena, K.; Thigpen, M.C.; Taylor, A.; Kumwenda, J.; Fowler, M.G.; Mofenson, L.M.; et al. Postexposure prophylaxis of breastfeeding HIV-exposed infants with antiretroviral drugs to age 14 weeks: Updated efficacy results of the PEPI-Malawi trial. J. Acquir. Immune Defic. Syndr. 2011, 57, 319–325. [Google Scholar] [CrossRef]
- Nagot, N.; Kankasa, C.; Tumwine, J.K.; Meda, N.; Hofmeyr, G.J.; Vallo, R.; Mwiya, M.; Kwagala, M.; Traore, H.; Sunday, A.; et al. Extended pre-exposure prophylaxis with lopinavir-ritonavir versus lamivudine to prevent HIV-1 transmission through breastfeeding up to 50 weeks in infants in Africa (ANRS 12174): A randomised controlled trial. Lancet 2016, 387, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Chasela, C.S.; Hudgens, M.G.; Jamieson, D.J.; Kayira, D.; Hosseinipour, M.C.; Kourtis, A.P.; Martinson, F.; Tegha, G.; Knight, R.J.; Ahmed, Y.I.; et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N. Engl. J. Med. 2010, 362, 2271–2281. [Google Scholar] [CrossRef]
- The Kesho Bora Study Group; Dioulasso, B.; Faso, B.; Meda, N.; Fao, P.; Ky-Zerbo, O.; Gouem, C.; Somda, P.; Hien, H.; Ouedraogo, P.E.; et al. Maternal HIV-1 disease progression 18-24 months postdelivery according to antiretroviral prophylaxis regimen (triple-antiretroviral prophylaxis during pregnancy and breastfeeding vs zidovudine/single-dose nevirapine prophylaxis): The Kesho Bora randomized controlled trial. Clin. Infect. Dis. 2012, 55, 449–460. [Google Scholar] [CrossRef]
- Fowler, M.G.; Qin, M.; Fiscus, S.A.; Currier, J.S.; Flynn, P.M.; Chipato, T.; McIntyre, J.; Gnanashanmugam, D.; Siberry, G.K.; Coletti, A.S.; et al. Benefits and Risks of Antiretroviral Therapy for Perinatal HIV Prevention. N. Engl. J. Med. 2016, 375, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.A.; Momplaisir, F.M.; Corson, C.; Brady, K.A. Assessing the impact of perinatal HIV case management on outcomes along the HIV care continuum for pregnant and postpartum women living with HIV, Philadelphia 2005–2013. AIDS Behav. 2017, 21, 2670–2681. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28176167 (accessed on 4 October 2022). [CrossRef] [PubMed]
- Wade, N.A.; Birkhead, G.S.; Warren, B.L.; Charbonneau, T.T.; French, P.T.; Wang, L.; Baum, J.B.; Tesoriero, J.M.; Savicki, R. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N. Engl. J. Med. 1998, 339, 1409–1414. [Google Scholar] [CrossRef]
- Nielsen-Saines, K.; Watts, D.H.; Veloso, V.G.; Bryson, Y.J.; Joao, E.C.; Pilotto, J.H.; Gray, G.; Theron, G.; Santos, B.; Fonseca, R.; et al. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N. Engl. J. Med. 2012, 366, 2368–2379. [Google Scholar] [CrossRef]
- Swiss Federal Office of Public Health. Recommendations of the Swiss Federal Commission for Sexual Health (FCHS) for Medical Care of HIV-Infected Women and Their Offspring. 2018. Available online: https://www.bag.admin.ch/dam/bag/en/dokumente/mt/p-und-p/richtlinien-empfehlungen/fcsh-mtct-hiv.pdf.download.pdf/fcsh-mtct-hiv.pdf (accessed on 4 October 2022).
- Flynn, P.M.; Taha, T.E.; Cababasay, M.; Fowler, M.G.; Mofenson, L.M.; Owor, M.; Fiscus, S.; Stranix-Chibanda, L.; Coutsoudis, A.; Gnanashanmugam, D.; et al. Prevention of HIV-1 Transmission Through Breastfeeding: Efficacy and Safety of Maternal Antiretroviral Therapy versus Infant Nevirapine Prophylaxis for Duration of Breastfeeding in HIV-1-Infected Women With High CD4 Cell Count (IMPAACT PROMISE): A Randomized, Open-Label, Clinical Trial. J. Acquir. Immune Defic. Syndr. 2018, 77, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Miotti, P.G.; Taha, T.E.T.; Kumwenda, N.I.; Broadhead, R.; Mtimavalye, L.A.R.; Van der Hoeven, L.; Chiphangwi, J.D.; Liomba, G.; Biggar, R.J. HIV Transmission through Breastfeeding A Study in Malawi. JAMA 1999, 282, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.; Chung, M.; Raman, G.; Chew, P.; Magula, N.; DeVine, D.; Trikalinos, T.; Lau, J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid. Rep. Technol. Assess 2007, 153, 1–186. [Google Scholar]
- Akobeng, A.K.; Ramanan, A.V.; Buchan, I.; Heller, R.F. Effect of breast feeding on risk of coeliac disease: A systematic review and meta-analysis of observational studies. Arch. Dis. Child. 2006, 91, 39–43. [Google Scholar] [CrossRef]
- Barclay, A.R.; Russell, R.K.; Wilson, M.L.; Gilmour, W.H.; Satsangi, J.; Wilson, D.C. Systematic review: The role of breastfeeding in the development of pediatric inflammatory bowel disease. J. Pediatr. 2009, 155, 421–426. [Google Scholar] [CrossRef]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Smith, G.D.; Cook, D.G. Effect of infant feeding on the risk of obesity across the life course: A quantitative review of published evidence. Pediatrics 2005, 115, 1367–1377. [Google Scholar] [CrossRef]
- Rosenbauer, J.; Herzig, P.; Giani, G. Early infant feeding and risk of type 1 diabetes mellitus-a nationwide population-based case-control study in pre-school children. Diabetes Metab. Res. Rev. 2008, 24, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.J.; Evans, S.F.; Straton, J.A.; Priest, S.R.; Hagan, R. Impact of postnatal depression on breastfeeding duration. Birth 2003, 30, 175–180. [Google Scholar] [CrossRef]
- Meek, J.Y.; Noble, L. Section on Breastfeeding; Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Chasela, C.S.; Hudgens, M.G.; King, C.C.; Kourtis, A.P.; Kayira, D.; Hosseinipour, M.C.; Kamwendo, D.D.; Ellington, S.R.; Wiener, J.B.; et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet 2012, 379, 2449–2458. [Google Scholar] [CrossRef]
- Waitt, C.; Olagunju, A.; Nakalema, S.; Kyohaire, I.; Owen, A.; Lamorde, M.; Khoo, S. Plasma and breast milk pharmacokinetics of emtricitabine, tenofovir and lamivudine using dried blood and breast milk spots in nursing African mother-infant pairs. J. Antimicrob. Chemother. 2018, 73, 1013–1019. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29309634 (accessed on 4 October 2022). [CrossRef] [PubMed]
- Mugwanya, K.K.; Hendrix, C.W.; Mugo, N.R.; Marzinke, M.; Katabira, E.T.; Ngure, K.; Semiyaga, N.B.; John-Stewart, G.; Muwonge, T.R.; Muthuri, G.; et al. Pre-exposure prophylaxis use by breastfeeding HIV-uninfected women: A prospective short-term study of antiretroviral excretion in breast milk and infant absorption. PLoS Med 2016, 13, e1002132. Available online: https://www.pubmed.ncbi.nlm.nih.gov/pubmed/27676257 (accessed on 4 October 2022). [CrossRef]
- Palombi, L.; Pirillo, M.F.; Marchei, E.; Jere, H.; Sagno, J.B.; Luhanga, R.; Floridia, M.; Andreotti, M.; Galluzzo, C.M.; Pichini, S.; et al. Concentrations of tenofovir, lamivudine and efavirenz in mothers and children enrolled under the option B-plus approach in Malawi. J. Antimicrob. Chemother. 2016, 71, 1027–1030. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26679247 (accessed on 4 October 2022). [CrossRef] [PubMed]
- Dryden-Peterson, S.; Shapiro, R.L.; Hughes, M.D.; Powis, K.; Ogwu, A.; Moffat, C.; Moyo, S.; Makhema, J.; Essex, M.; Lockman, S. Increased risk of severe infant anemia after exposure to maternal HAART, Botswana. J. Acquir. Immune Defic. Syndr. 2011, 56, 428–436. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21266910 (accessed on 4 October 2022). [CrossRef]
- Fogel, J.; Li, Q.; Taha, T.E.; Hoover, D.R.; Kumwenda, N.I.; Mofenson, L.M.; Kumwenda, J.J.; Fowler, M.G.; Thigpen, M.C.; Eshleman, S.H. Initiation of antiretroviral treatment in women after delivery can induce multiclass drug resistance in breastfeeding HIV-infected infants. Clin. Infect. Dis. 2011, 52, 1069–1076. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21460326 (accessed on 4 October 2022). [CrossRef] [PubMed]
- Kwan, M.L.; Buffler, P.A.; Abrams, B.; Kiley, V.A. Breastfeeding and the risk of childhood leukemia: A meta-analysis. Public Health Rep. 2004, 119, 521–535. [Google Scholar] [CrossRef]
- Etowa, J.; Nare, H.; Kakuru, D.M.; Etowa, E.B. Psychosocial Experiences of HIV-Positive Women of African Descent in the Cultural Context of Infant Feeding: A Three-Country Comparative Analyses. Int. J. Environ. Res. Public Health 2020, 17, 7150. [Google Scholar] [CrossRef]
- Becquet, R.; Ekouevi, D.K.; Menan, H.; Amani-Bosse, C.; Bequet, L.; Viho, I.; Dabis, F.; Timite-Konan, M.; Leroy, V. Early mixed feeding and breastfeeding beyond 6 months increase the risk of postnatal HIV transmission: ANRS 1201/1202 Ditrame Plus, Abidjan, Côte d’Ivoire. Prev. Med. 2008, 47, 27–33. [Google Scholar] [CrossRef]
- Charurat, M.; Datong, P.; Matawal, B.; Ajene, A.; Blattner, W.; Abimiku, A. Timing and determinants of mother-to-child transmission of HIV in Nigeria. Int. J. Gynaecol. Obstet. 2009, 106, 8–13. [Google Scholar] [CrossRef]
- Coovadia, H.M.; Rollins, N.C.; Bland, R.M.; Little, K.; Coutsoudis, A.; Bennish, M.L.; Newell, M.L. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: An intervention cohort study. Lancet 2007, 369, 1107–1116. [Google Scholar] [CrossRef]
- Iliff, P.J.; Piwoz, E.G.; Tavengwa, N.V.; Zunguza, C.D.; Marinda, E.T.; Nathoo, K.J.; Moulton, L.H.; Ward, B.J.; Humphrey, J.H. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS 2005, 19, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Coutsoudis, A.; Pillay, K.; Spooner, E.; Kuhn, L.; Coovadia, H.M. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: A prospective cohort study. South African Vitamin A Study Group. Lancet 1999, 354, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, L.; Aldrovandi, G.M.; Sinkala, M.; Kankasa, C.; Semrau, K.; Mwiya, M.; Kasonde, P.; Scott, N.; Vwalika, C.; Walter, J.; et al. Zambia Exclusive Breastfeeding Study. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N. Engl. J. Med. 2008, 359, 130–141. [Google Scholar] [CrossRef]
- Gaur, A.H.; Dominguez, K.L.; Kalish, M.L.; Rivera-Hernandez, D.; Donohoe, M.; Brooks, J.T.; Mitchell, C.D. Practice of feeding premasticated food to infants: A potential risk factor for HIV transmission. Pediatrics 2009, 124, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Maritz, E.R.; Kidd, M.; Cotton, M.F. Premastication food for weaning African infants: A possible vehicle for transmission of HIV. Pediatrics 2011, 128, e579–e590. [Google Scholar] [CrossRef]
- Evans, C.; Jones, C.E.; Prendergast, A.J. HIV-exposed, uninfected infants: New global challenges in the era of paediatric HIV elimination. Lancet Infect. Dis. 2016, 16, e92–e107. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardenas, M.C.; Farnan, S.; Hamel, B.L.; Mejia Plazas, M.C.; Sintim-Aboagye, E.; Littlefield, D.R.; Behl, S.; Punia, S.; Enninga, E.A.L.; Johnson, E.; et al. Prevention of the Vertical Transmission of HIV; A Recap of the Journey so Far. Viruses 2023, 15, 849. https://doi.org/10.3390/v15040849
Cardenas MC, Farnan S, Hamel BL, Mejia Plazas MC, Sintim-Aboagye E, Littlefield DR, Behl S, Punia S, Enninga EAL, Johnson E, et al. Prevention of the Vertical Transmission of HIV; A Recap of the Journey so Far. Viruses. 2023; 15(4):849. https://doi.org/10.3390/v15040849
Chicago/Turabian StyleCardenas, Maria Camila, Sheila Farnan, Benjamin L. Hamel, Maria Camila Mejia Plazas, Elise Sintim-Aboagye, Dawn R. Littlefield, Supriya Behl, Sohan Punia, Elizabeth Ann L Enninga, Erica Johnson, and et al. 2023. "Prevention of the Vertical Transmission of HIV; A Recap of the Journey so Far" Viruses 15, no. 4: 849. https://doi.org/10.3390/v15040849
APA StyleCardenas, M. C., Farnan, S., Hamel, B. L., Mejia Plazas, M. C., Sintim-Aboagye, E., Littlefield, D. R., Behl, S., Punia, S., Enninga, E. A. L., Johnson, E., Temesgen, Z., Theiler, R., Gray, C. M., & Chakraborty, R. (2023). Prevention of the Vertical Transmission of HIV; A Recap of the Journey so Far. Viruses, 15(4), 849. https://doi.org/10.3390/v15040849





