Canine Distemper Virus Alters Defense Responses in an Ex Vivo Model of Pulmonary Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Lung Tissue
2.2. Virus
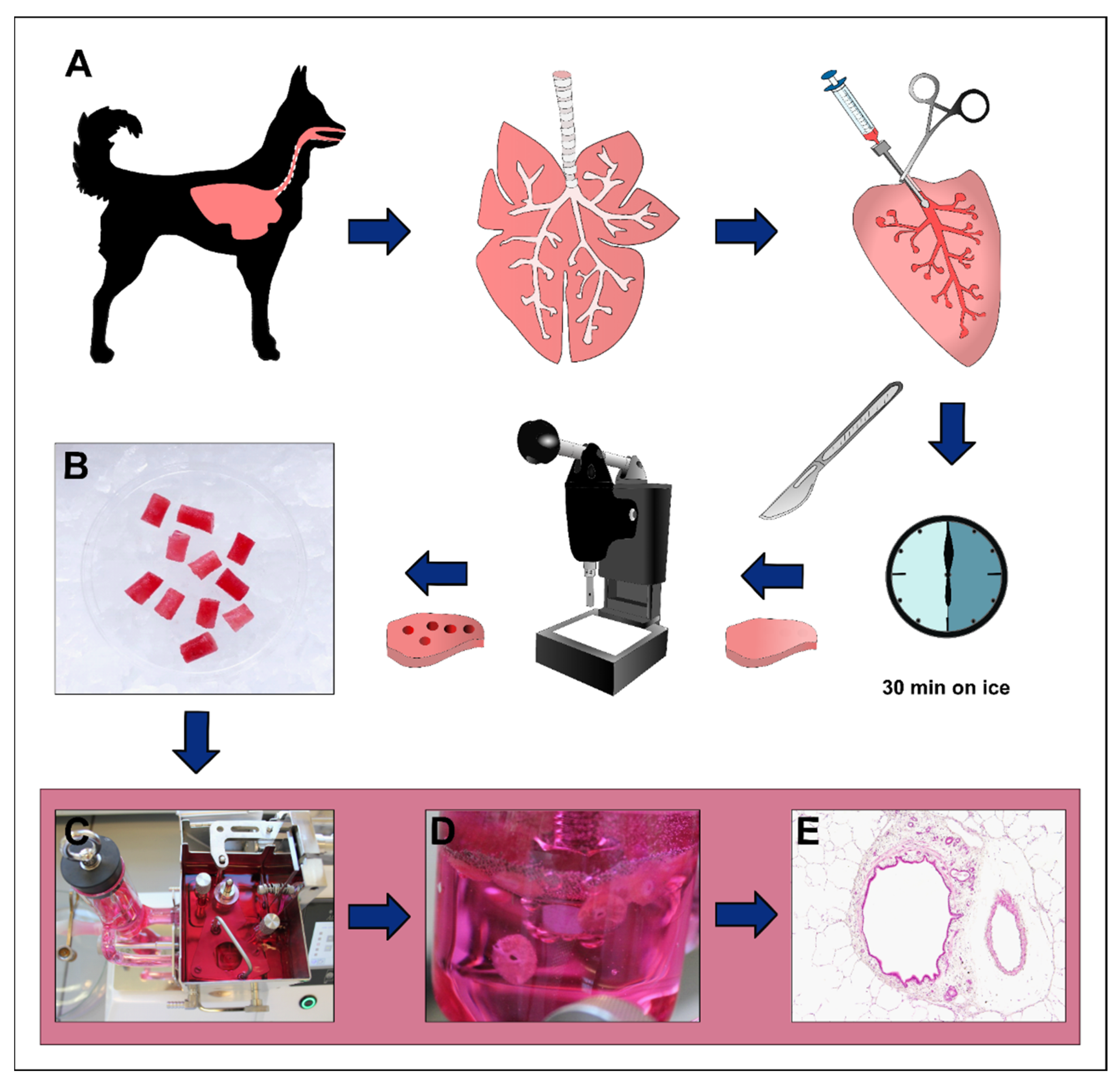
2.3. Preparation of Precision-Cut Lung Slices
2.4. Evaluation of Ciliary Activity
2.5. Infection of Precision-Cut Lung Slices with Canine Distemper Virus
2.6. Immunohistochemistry
2.7. Immunofluorescence Double-Labelling
2.8. In Situ Hybridization
2.9. Transmission Electron Microscopy
2.10. Lactate Dehydrogenase (LDH) Assay
2.11. Molecular Analysis
2.12. Statistical Analysis
3. Results
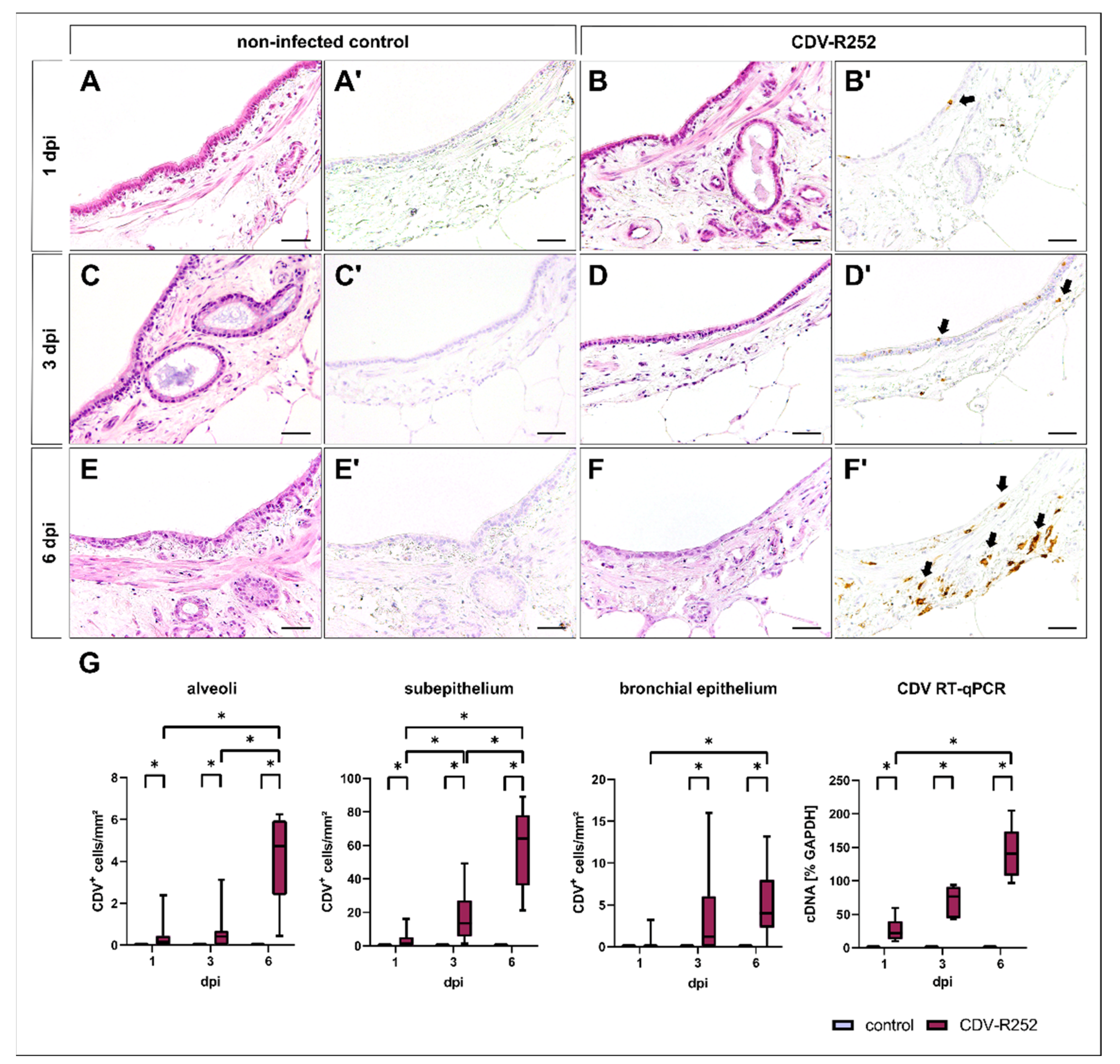
3.1. Morphology and Viral Loads
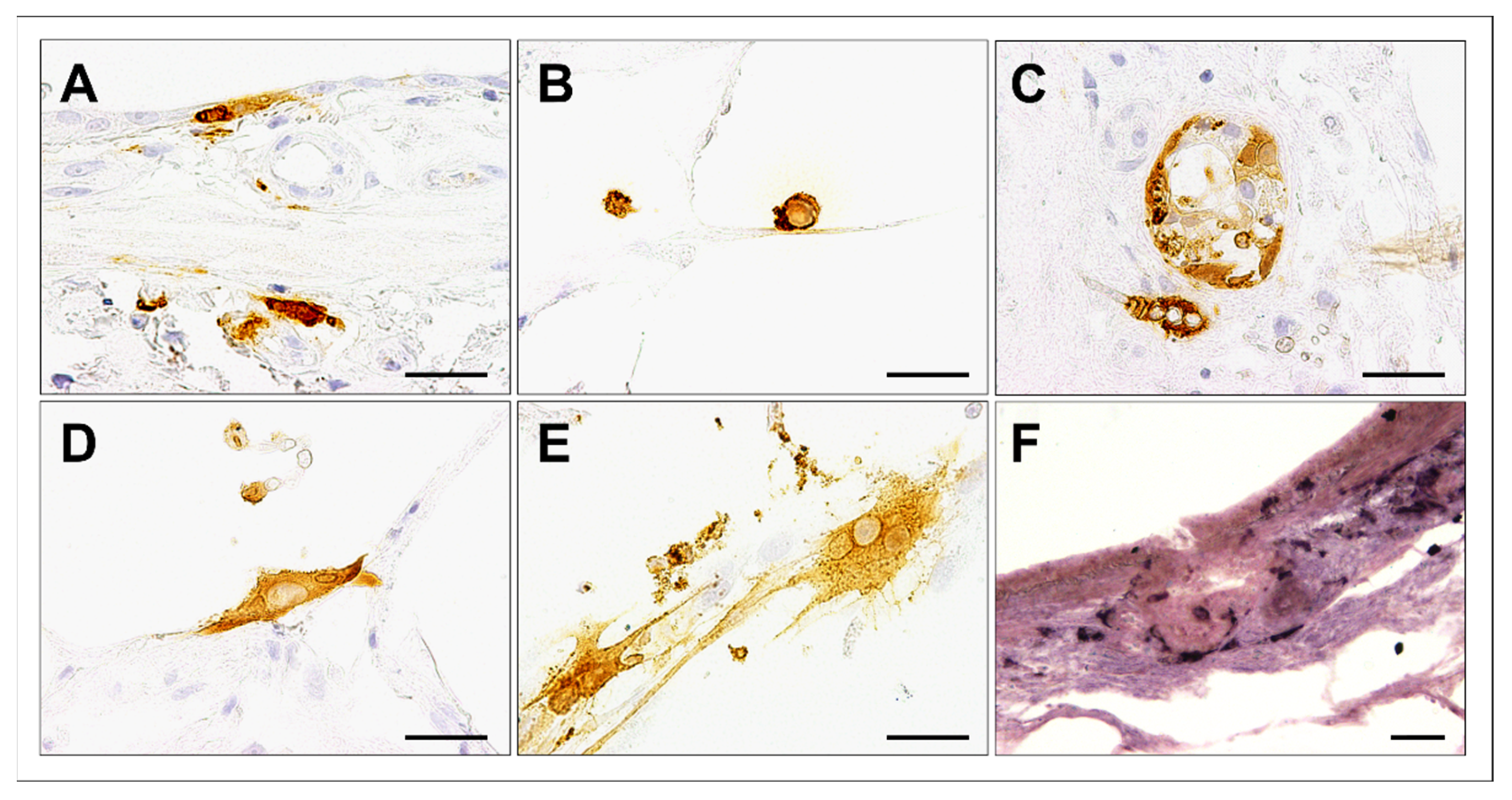
3.2. Cell Tropism and Ultrastructural Detection of Canine Distemper Virus
3.3. Ciliary Activity and Viability
3.4. Major Histocompatibility Complex Class II Expression
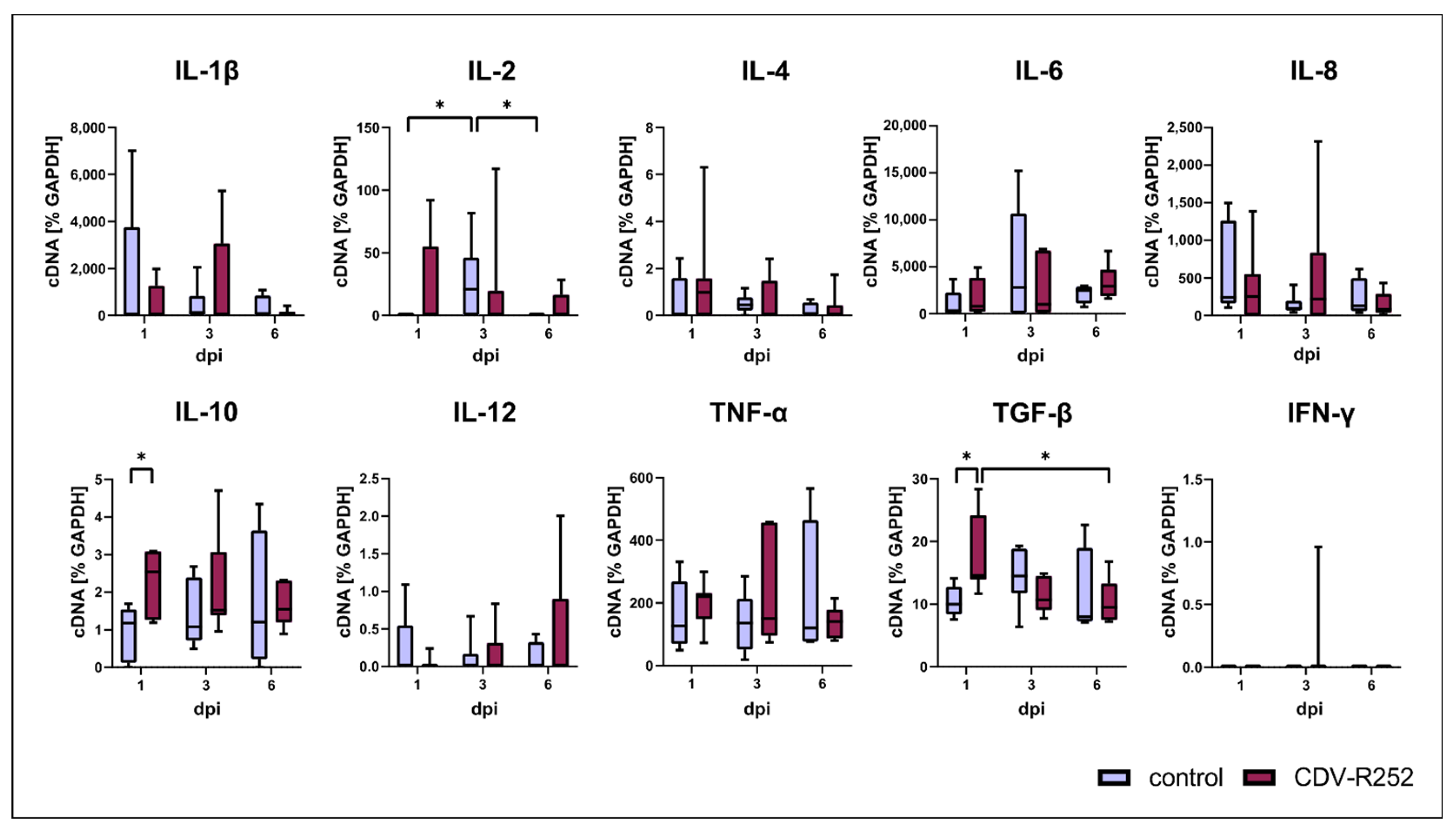
3.5. Cytokine Responses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Vries, R.D.; Duprex, W.P.; de Swart, R.L. Morbillivirus infections: An introduction. Viruses 2015, 7, 699–706. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses. Available online: https://ictv.global/report/chapter/paramyxoviridae/paramyxoviridae/morbillivirus (accessed on 25 December 2022).
- Moss, W.J.; Ota, M.O.; Griffin, D.E. Measles: Immune suppression and immune responses. Int. J. Biochem. Cell Biol. 2004, 36, 1380–1385. [Google Scholar] [CrossRef]
- Sato, H.; Yoneda, M.; Honda, T.; Kai, C. Morbillivirus receptors and tropism: Multiple pathways for infection. Front. Microbiol. 2012, 3, 75. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, M.; Ruiz-Saenz, J. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet. Res. 2016, 12, 78. [Google Scholar] [CrossRef]
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgartner, W. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunopathol. 2009, 127, 1–18. [Google Scholar] [CrossRef]
- Tatsuo, H.; Ono, N.; Yanagi, Y. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J. Virol. 2001, 75, 5842–5850. [Google Scholar] [CrossRef]
- de Vries, R.D.; Ludlow, M.; de Jong, A.; Rennick, L.J.; Verburgh, R.J.; van Amerongen, G.; van Riel, D.; van Run, P.; Herfst, S.; Kuiken, T.; et al. Delineating morbillivirus entry, dissemination and airborne transmission by studying in vivo competition of multicolor canine distemper viruses in ferrets. PLoS Pathog. 2017, 13, e1006371. [Google Scholar] [CrossRef]
- Sawatsky, B.; Cattaneo, R.; von Messling, V. Canine Distemper Virus Spread and Transmission to Naive Ferrets: Selective Pressure on Signaling Lymphocyte Activation Molecule-Dependent Entry. J. Virol. 2018, 92, e00669-18. [Google Scholar] [CrossRef]
- Lempp, C.; Spitzbarth, I.; Puff, C.; Cana, A.; Kegler, K.; Techangamsuwan, S.; Baumgartner, W.; Seehusen, F. New aspects of the pathogenesis of canine distemper leukoencephalitis. Viruses 2014, 6, 2571–2601. [Google Scholar] [CrossRef]
- von Messling, V.; Milosevic, D.; Cattaneo, R. Tropism illuminated: Lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc. Natl. Acad. Sci. USA 2004, 101, 14216–14221. [Google Scholar] [CrossRef]
- Ludlow, M.; Rennick, L.J.; Nambulli, S.; de Swart, R.L.; Duprex, W.P. Using the ferret model to study morbillivirus entry, spread, transmission and cross-species infection. Curr. Opin. Virol. 2014, 4, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Pratakpiriya, W.; Seki, F.; Otsuki, N.; Sakai, K.; Fukuhara, H.; Katamoto, H.; Hirai, T.; Maenaka, K.; Techangamsuwan, S.; Lan, N.T.; et al. Nectin4 is an epithelial cell receptor for canine distemper virus and involved in neurovirulence. J. Virol. 2012, 86, 10207–10210. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Marsland, B.J. Lung Homeostasis: Influence of Age, Microbes, and the Immune System. Immunity 2017, 46, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Foxman, E.F.; Molony, R.D. Early local immune defences in the respiratory tract. Nat. Rev. Immunol. 2017, 17, 7–20. [Google Scholar] [CrossRef]
- Shenoy, A.T.; Lyon De Ana, C.; Arafa, E.I.; Salwig, I.; Barker, K.A.; Korkmaz, F.T.; Ramanujan, A.; Etesami, N.S.; Soucy, A.M.; Martin, I.M.C.; et al. Antigen presentation by lung epithelial cells directs CD4(+) T(RM) cell function and regulates barrier immunity. Nat. Commun. 2021, 12, 5834. [Google Scholar] [CrossRef]
- Castellheim, A.; Brekke, O.L.; Espevik, T.; Harboe, M.; Mollnes, T.E. Innate immune responses to danger signals in systemic inflammatory response syndrome and sepsis. Scand. J. Immunol. 2009, 69, 479–491. [Google Scholar] [CrossRef]
- Martinic, M.M.; von Herrath, M.G. Novel strategies to eliminate persistent viral infections. Trends Immunol. 2008, 29, 116–124. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Bogdan, C.; Paik, J.; Vodovotz, Y.; Nathan, C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J. Biol. Chem. 1992, 267, 23301–23308. [Google Scholar] [CrossRef]
- de Swart, R.L.; Ludlow, M.; de Witte, L.; Yanagi, Y.; van Amerongen, G.; McQuaid, S.; Yüksel, S.; Geijtenbeek, T.B.; Duprex, W.P.; Osterhaus, A.D. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 2007, 3, e178. [Google Scholar] [CrossRef]
- Carsillo, T.; Huey, D.; Levinsky, A.; Obojes, K.; Schneider-Schaulies, J.; Niewiesk, S. Cotton rat (Sigmodon hispidus) signaling lymphocyte activation molecule (CD150) is an entry receptor for measles virus. PLoS ONE 2014, 9, e110120. [Google Scholar] [CrossRef]
- Gillespie, J.H.; Baker, J.A.; Burgher, J.; Robson, D.; Gilman, B. The immune response of dogs to distemper virus. Cornell Vet. 1958, 48, 103–126. [Google Scholar]
- Dunkin, G.W.; Laidlaw, P.P. Studies in dog-distemper: I.—Dog-distemper in the ferret. J. Comp. Pathol. Ther. 1926, 39, 201–212. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef]
- EU. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 53, 33–79. [Google Scholar] [CrossRef]
- Lin, W.W.; Tsay, A.J.; Lalime, E.N.; Pekosz, A.; Griffin, D.E. Primary differentiated respiratory epithelial cells respond to apical measles virus infection by shedding multinucleated giant cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2013264118. [Google Scholar] [CrossRef]
- Shin, D.L.; Chludzinski, E.; Wu, N.H.; Peng, J.Y.; Ciurkiewicz, M.; Sawatsky, B.; Pfaller, C.K.; Baechlein, C.; von Messling, V.; Haas, L.; et al. Overcoming the Barrier of the Respiratory Epithelium during Canine Distemper Virus Infection. mBio 2022, 13, e0304321. [Google Scholar] [CrossRef]
- Rosales Gerpe, M.C.; van Vloten, J.P.; Santry, L.A.; de Jong, J.; Mould, R.C.; Pelin, A.; Bell, J.C.; Bridle, B.W.; Wootton, S.K. Use of Precision-Cut Lung Slices as an Ex Vivo Tool for Evaluating Viruses and Viral Vectors for Gene and Oncolytic Therapy. Mol. Ther. Methods Clin. Dev. 2018, 10, 245–256. [Google Scholar] [CrossRef]
- Viana, F.; O’Kane, C.M.; Schroeder, G.N. Precision-cut lung slices: A powerful ex vivo model to investigate respiratory infectious diseases. Mol. Microbiol. 2022, 117, 578–588. [Google Scholar] [CrossRef]
- Krumdieck, C.L.; dos Santos, J.E.; Ho, K.J. A new instrument for the rapid preparation of tissue slices. Anal. Biochem. 1980, 104, 118–123. [Google Scholar] [CrossRef]
- Nguyen, D.T.; de Vries, R.D.; Ludlow, M.; van den Hoogen, B.G.; Lemon, K.; van Amerongen, G.; Osterhaus, A.D.; de Swart, R.L.; Duprex, W.P. Paramyxovirus infections in ex vivo lung slice cultures of different host species. J. Virol. Methods 2013, 193, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Viera, O.; Woolard, K.D.; Keel, M.K. Lung and lymph node explants to study the interaction between host cells and canine distemper virus. Res. Vet. Sci. 2022, 154, 44–51. [Google Scholar] [CrossRef] [PubMed]
- De Vries, R.D.; Rennick, L.J.; Duprex, W.P.; De Swart, R.L. Paramyxovirus Infections in Ex Vivo Lung Slice Cultures of Different Host Species. Methods Protoc. 2018, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Confer, A.W.; Kahn, D.E.; Koestner, A.; Krakowka, S. Biological properties of a canine distemper virus isolate associated with demyelinating encephalomyelitis. Infect. Immun. 1975, 11, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Punyadarsaniya, D.; Uhlenbruck, S.; Hennig-Pauka, I.; Schwegmann-Wessels, C.; Ren, X.; Dürrwald, R.; Herrler, G. Replication characteristics of swine influenza viruses in precision-cut lung slices reflect the virulence properties of the viruses. Vet. Res. 2013, 44, 110. [Google Scholar] [CrossRef]
- Punyadarsaniya, D.; Liang, C.H.; Winter, C.; Petersen, H.; Rautenschlein, S.; Hennig-Pauka, I.; Schwegmann-Wessels, C.; Wu, C.Y.; Wong, C.H.; Herrler, G. Infection of differentiated porcine airway epithelial cells by influenza virus: Differential susceptibility to infection by porcine and avian viruses. PLoS ONE 2011, 6, e28429. [Google Scholar] [CrossRef]
- Chludzinski, E.; Klemens, J.; Ciurkiewicz, M.; Geffers, R.; Pöpperl, P.; Stoff, M.; Shin, D.L.; Herrler, G.; Beineke, A. Phenotypic and Transcriptional Changes of Pulmonary Immune Responses in Dogs Following Canine Distemper Virus Infection. Int. J. Mol. Sci. 2022, 23, 10019. [Google Scholar] [CrossRef]
- Gröters, S.; Alldinger, S.; Baumgärtner, W. Up-regulation of mRNA for matrix metalloproteinases-9 and -14 in advanced lesions of demyelinating canine distemper leukoencephalitis. Acta Neuropathol. 2005, 110, 369–382. [Google Scholar] [CrossRef]
- Gaedke, K.; Zurbriggen, A.; Baumgärtner, W. In vivo and in vitro detection of canine distemper virus nucleoprotein gene with digoxigenin-labelled RNA, double-stranded DNA probes and oligonucleotides by in situ hybridization. J. Vet. Med. Ser. B 1997, 44, 329–340. [Google Scholar] [CrossRef]
- Zurbriggen, A.; Müller, C.; Vandevelde, M. In situ hybridization of virulent canine distemper virus in brain tissue, using digoxigenin-labeled probes. Am. J. Vet. Res. 1993, 54, 1457–1461. [Google Scholar]
- Baumgärtner, W.; Krakowka, S.; Blakeslee, J.R. Persistent infection of Vero cells by paramyxoviruses. A morphological and immunoelectron microscopic investigation. Intervirology 1987, 27, 218–223. [Google Scholar] [CrossRef]
- Coffin, D.L.; Liu, C. Studies of canine distemper infection by means of fluorescein-labeled antibody. II. The pathology and diagnosis of the naturally occurring disease in dogs and the antigenic nature of the inclusion body. Virology 1957, 3, 132–145. [Google Scholar] [CrossRef]
- de Vries, R.D.; Lemon, K.; Ludlow, M.; McQuaid, S.; Yüksel, S.; van Amerongen, G.; Rennick, L.J.; Rima, B.K.; Osterhaus, A.D.; de Swart, R.L.; et al. In vivo tropism of attenuated and pathogenic measles virus expressing green fluorescent protein in macaques. J. Virol. 2010, 84, 4714–4724. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Frenzke, M.; Leonard, V.H.; Welstead, G.G.; Richardson, C.D.; Cattaneo, R. Measles virus infection of alveolar macrophages and dendritic cells precedes spread to lymphatic organs in transgenic mice expressing human signaling lymphocytic activation molecule (SLAM, CD150). J. Virol. 2010, 84, 3033–3042. [Google Scholar] [CrossRef]
- Lemon, K.; de Vries, R.D.; Mesman, A.W.; McQuaid, S.; van Amerongen, G.; Yuksel, S.; Ludlow, M.; Rennick, L.J.; Kuiken, T.; Rima, B.K.; et al. Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog. 2011, 7, e1001263. [Google Scholar] [CrossRef]
- von Messling, V.; Svitek, N.; Cattaneo, R. Receptor (SLAM [CD150]) recognition and the V protein sustain swift lymphocyte-based invasion of mucosal tissue and lymphatic organs by a morbillivirus. J. Virol. 2006, 80, 6084–6092. [Google Scholar] [CrossRef]
- Wenzlow, N.; Plattet, P.; Wittek, R.; Zurbriggen, A.; Gröne, A. Immunohistochemical demonstration of the putative canine distemper virus receptor CD150 in dogs with and without distemper. Vet. Pathol. 2007, 44, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, H.; Tanaka, K.; Ono, N.; Tatsuo, H.; Yanagi, Y. Induction of the measles virus receptor SLAM (CD150) on monocytes. J. Gen. Virol. 2001, 82, 2913–2917. [Google Scholar] [CrossRef]
- Gradauskaite, V.; Inglebert, M.; Doench, J.; Scherer, M.; Dettwiler, M.; Wyss, M.; Shrestha, N.; Rottenberg, S.; Plattet, P. LRP6 Is a Functional Receptor for Attenuated Canine Distemper Virus. mBio 2023, 14, e03114-22. [Google Scholar] [CrossRef]
- Schreiner, T.; Allnoch, L.; Beythien, G.; Marek, K.; Becker, K.; Schaudien, D.; Stanelle-Bertram, S.; Schaumburg, B.; Mounogou Kouassi, N.; Beck, S.; et al. SARS-CoV-2 Infection Dysregulates Cilia and Basal Cell Homeostasis in the Respiratory Epithelium of Hamsters. Int. J. Mol. Sci. 2022, 23, 5124. [Google Scholar] [CrossRef]
- Anderson, C.S.; Chirkova, T.; Slaunwhite, C.G.; Qiu, X.; Walsh, E.E.; Anderson, L.J.; Mariani, T.J. CX3CR1 Engagement by Respiratory Syncytial Virus Leads to Induction of Nucleolin and Dysregulation of Cilia-related Genes. J. Virol. 2021, 95, e00095-21. [Google Scholar] [CrossRef] [PubMed]
- Geiser, J.; Boivin, G.; Huang, S.; Constant, S.; Kaiser, L.; Tapparel, C.; Essaidi-Laziosi, M. RSV and HMPV Infections in 3D Tissue Cultures: Mechanisms Involved in Virus-Host and Virus-Virus Interactions. Viruses 2021, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Kulkarni, H.; Radhakrishnan, P.; Rutman, A.; Bankart, M.J.; Williams, G.; Hirst, R.A.; Easton, A.J.; Andrew, P.W.; O’Callaghan, C. Ciliary dyskinesia is an early feature of respiratory syncytial virus infection. Eur. Respir. J. 2014, 43, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Kantar, A.; Oggiano, N.; Giorgi, P.L.; Braga, P.C.; Fiorini, R. Polymorphonuclear leukocyte-generated oxygen metabolites decrease beat frequency of human respiratory cilia. Lung 1994, 172, 215–222. [Google Scholar] [CrossRef]
- Mata, M.; Sarrion, I.; Armengot, M.; Carda, C.; Martinez, I.; Melero, J.A.; Cortijo, J. Respiratory syncytial virus inhibits ciliagenesis in differentiated normal human bronchial epithelial cells: Effectiveness of N-acetylcysteine. PLoS ONE 2012, 7, e48037. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Ikegawa, M.; Kawai, T. Antigen Presentation in the Lung. Front. Immunol. 2022, 13, 860915. [Google Scholar] [CrossRef]
- Díaz-Delgado, J.; Groch, K.R.; Ressio, R.; Riskallah, I.P.J.; Sierra, E.; Sacchini, S.; Quesada-Canales, Ó.; Arbelo, M.; Fernández, A.; Santos-Neto, E.; et al. Comparative Immunopathology of Cetacean morbillivirus Infection in Free-Ranging Dolphins From Western Mediterranean, Northeast-Central, and Southwestern Atlantic. Front. Immunol. 2019, 10, 485. [Google Scholar] [CrossRef]
- Qeska, V.; Barthel, Y.; Herder, V.; Stein, V.M.; Tipold, A.; Urhausen, C.; Günzel-Apel, A.R.; Rohn, K.; Baumgärtner, W.; Beineke, A. Canine distemper virus infection leads to an inhibitory phenotype of monocyte-derived dendritic cells in vitro with reduced expression of co-stimulatory molecules and increased interleukin-10 transcription. PLoS ONE 2014, 9, e96121. [Google Scholar] [CrossRef]
- Toews, G.B. Cytokines and the lung. Eur. Respir. J. Suppl. 2001, 34, 3s–17s. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Koppelman, B.; Neefjes, J.J.; de Vries, J.E.; de Waal Malefyt, R. Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity 1997, 7, 861–871. [Google Scholar] [CrossRef]
- Herring, A.C.; Hernández, Y.; Huffnagle, G.B.; Toews, G.B. Role and development of TH1/TH2 immune responses in the lungs. Semin. Respir. Crit. Care Med. 2004, 25, 3–10. [Google Scholar] [CrossRef]
- Brooks, D.G.; Trifilo, M.J.; Edelmann, K.H.; Teyton, L.; McGavern, D.B.; Oldstone, M.B. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 2006, 12, 1301–1309. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Jarry, A.; Bossard, C.; Bou-Hanna, C.; Masson, D.; Espaze, E.; Denis, M.G.; Laboisse, C.L. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J. Clin. Investig. 2008, 118, 1132–1142. [Google Scholar] [CrossRef]
- Chen, Q.; Daniel, V.; Maher, D.W.; Hersey, P. Production of IL-10 by melanoma cells: Examination of its role in immunosuppression mediated by melanoma. Int. J. Cancer 1994, 56, 755–760. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Gabryšová, L.; Howes, A.; Saraiva, M.; O’Garra, A. The regulation of IL-10 expression. Curr. Top. Microbiol. Immunol. 2014, 380, 157–190. [Google Scholar] [CrossRef]
- Demangel, C.; Bertolino, P.; Britton, W.J. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur. J. Immunol. 2002, 32, 994–1002. [Google Scholar] [CrossRef]
- Mamilos, A.; Winter, L.; Schmitt, V.H.; Barsch, F.; Grevenstein, D.; Wagner, W.; Babel, M.; Keller, K.; Schmitt, C.; Gürtler, F.; et al. Macrophages: From Simple Phagocyte to an Integrative Regulatory Cell for Inflammation and Tissue Regeneration—A Review of the Literature. Cells 2023, 12, 276. [Google Scholar] [CrossRef]
- Muñoz, M.; Hegazy, A.N.; Brunner, T.M.; Holecska, V.; Marek, R.M.; Fröhlich, A.; Löhning, M. Th2 cells lacking T-bet suppress naive and memory T cell responses via IL-10. Proc. Natl. Acad. Sci. USA 2021, 118, e2002787118. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.M.; Hygino, J.; Kasahara, T.M.; Vieira, M.M.; Xavier, L.F.; Blanco, B.; Damasco, P.V.; Silva, R.M.; Lima, D.B.; Oliveira, A.L.; et al. High IL-10 production by aged AIDS patients is related to high frequency of Tr-1 phenotype and low in vitro viral replication. Clin. Immunol. 2012, 145, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Kavanagh, D.; Rihn, S.; Luteijn, R.; Brooks, D.; Oldstone, M.; van Lunzen, J.; Altfeld, M. IL-10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. J. Clin. Investig. 2010, 120, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Herder, V.; Hansmann, F.; Stangel, M.; Schaudien, D.; Rohn, K.; Baumgärtner, W.; Beineke, A. Cuprizone inhibits demyelinating leukomyelitis by reducing immune responses without virus exacerbation in an infectious model of multiple sclerosis. J. Neuroimmunol. 2012, 244, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Chigbu, D.I.; Loonawat, R.; Sehgal, M.; Patel, D.; Jain, P. Hepatitis C Virus Infection: Host-Virus Interaction and Mechanisms of Viral Persistence. Cells 2019, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef]
- Gibbs, J.D.; Ornoff, D.M.; Igo, H.A.; Zeng, J.Y.; Imani, F. Cell cycle arrest by transforming growth factor beta1 enhances replication of respiratory syncytial virus in lung epithelial cells. J. Virol. 2009, 83, 12424–12431. [Google Scholar] [CrossRef]
- Valli, J.L.; Williamson, A.; Sharif, S.; Rice, J.; Shewen, P.E. In vitro cytokine responses of peripheral blood mononuclear cells from healthy dogs to distemper virus, Malassezia and Toxocara. Vet. Immunol. Immunopathol. 2010, 134, 218–229. [Google Scholar] [CrossRef]
- Frisk, A.L.; Baumgärtner, W.; Gröne, A. Dominating interleukin-10 mRNA expression induction in cerebrospinal fluid cells of dogs with natural canine distemper virus induced demyelinating and non-demyelinating CNS lesions. J. Neuroimmunol. 1999, 97, 102–109. [Google Scholar] [CrossRef]
- Grone, A.; Frisk, A.L.; Baumgartner, W. Cytokine mRNA expression in whole blood samples from dogs with natural canine distemper virus infection. Vet. Immunol. Immunopathol. 1998, 65, 11–27. [Google Scholar] [CrossRef]
- Yu, X.L.; Cheng, Y.M.; Shi, B.S.; Qian, F.X.; Wang, F.B.; Liu, X.N.; Yang, H.Y.; Xu, Q.N.; Qi, T.K.; Zha, L.J.; et al. Measles virus infection in adults induces production of IL-10 and is associated with increased CD4+ CD25+ regulatory T cells. J. Immunol. 2008, 181, 7356–7366. [Google Scholar] [CrossRef]
- Moss, W.J.; Ryon, J.J.; Monze, M.; Griffin, D.E. Differential regulation of interleukin (IL)-4, IL-5, and IL-10 during measles in Zambian children. J. Infect. Dis. 2002, 186, 879–887. [Google Scholar] [CrossRef]
- Okada, H.; Sato, T.A.; Katayama, A.; Higuchi, K.; Shichijo, K.; Tsuchiya, T.; Takayama, N.; Takeuchi, Y.; Abe, T.; Okabe, N.; et al. Comparative analysis of host responses related to immunosuppression between measles patients and vaccine recipients with live attenuated measles vaccines. Arch. Virol. 2001, 146, 859–874. [Google Scholar] [CrossRef]



| Epitope | Source | Cat. No. | Species | Clone | Dilution |
|---|---|---|---|---|---|
| CDV-N | Santa Cruz Biotechnology, Dallas, TX, USA | sc-57660 | Mouse | DV2-12 | 1:10,000 * 1:100 ** |
| MHC-II | Dako, Glostrup, Denmark | M0746 | Mouse | TAL.1B5 | 1:80 * |
| CK | Dako, Glostrup, Denmark | Z0622 | Rabbit | Polyclonal | 1:50 ** |
| Iba-1 | Invitrogen™, Thermo Fisher Scientific, Langenselbold, Germany | PA5-27436 | Rabbit | Polyclonal | 1:200 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chludzinski, E.; Ciurkiewicz, M.; Stoff, M.; Klemens, J.; Krüger, J.; Shin, D.-L.; Herrler, G.; Beineke, A. Canine Distemper Virus Alters Defense Responses in an Ex Vivo Model of Pulmonary Infection. Viruses 2023, 15, 834. https://doi.org/10.3390/v15040834
Chludzinski E, Ciurkiewicz M, Stoff M, Klemens J, Krüger J, Shin D-L, Herrler G, Beineke A. Canine Distemper Virus Alters Defense Responses in an Ex Vivo Model of Pulmonary Infection. Viruses. 2023; 15(4):834. https://doi.org/10.3390/v15040834
Chicago/Turabian StyleChludzinski, Elisa, Małgorzata Ciurkiewicz, Melanie Stoff, Johanna Klemens, Johannes Krüger, Dai-Lun Shin, Georg Herrler, and Andreas Beineke. 2023. "Canine Distemper Virus Alters Defense Responses in an Ex Vivo Model of Pulmonary Infection" Viruses 15, no. 4: 834. https://doi.org/10.3390/v15040834
APA StyleChludzinski, E., Ciurkiewicz, M., Stoff, M., Klemens, J., Krüger, J., Shin, D.-L., Herrler, G., & Beineke, A. (2023). Canine Distemper Virus Alters Defense Responses in an Ex Vivo Model of Pulmonary Infection. Viruses, 15(4), 834. https://doi.org/10.3390/v15040834






