Importance of Cellular Immunity and IFN-γ Concentration in Preventing SARS-CoV-2 Infection and Reinfection: A Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants, Data Collection, and Retrospective Analysis
2.2. Cellular Immunity Analysis
2.3. Humoral Immunity Analysis
2.4. Ethics Approval and Informed Consent
2.5. Statistical Analysis
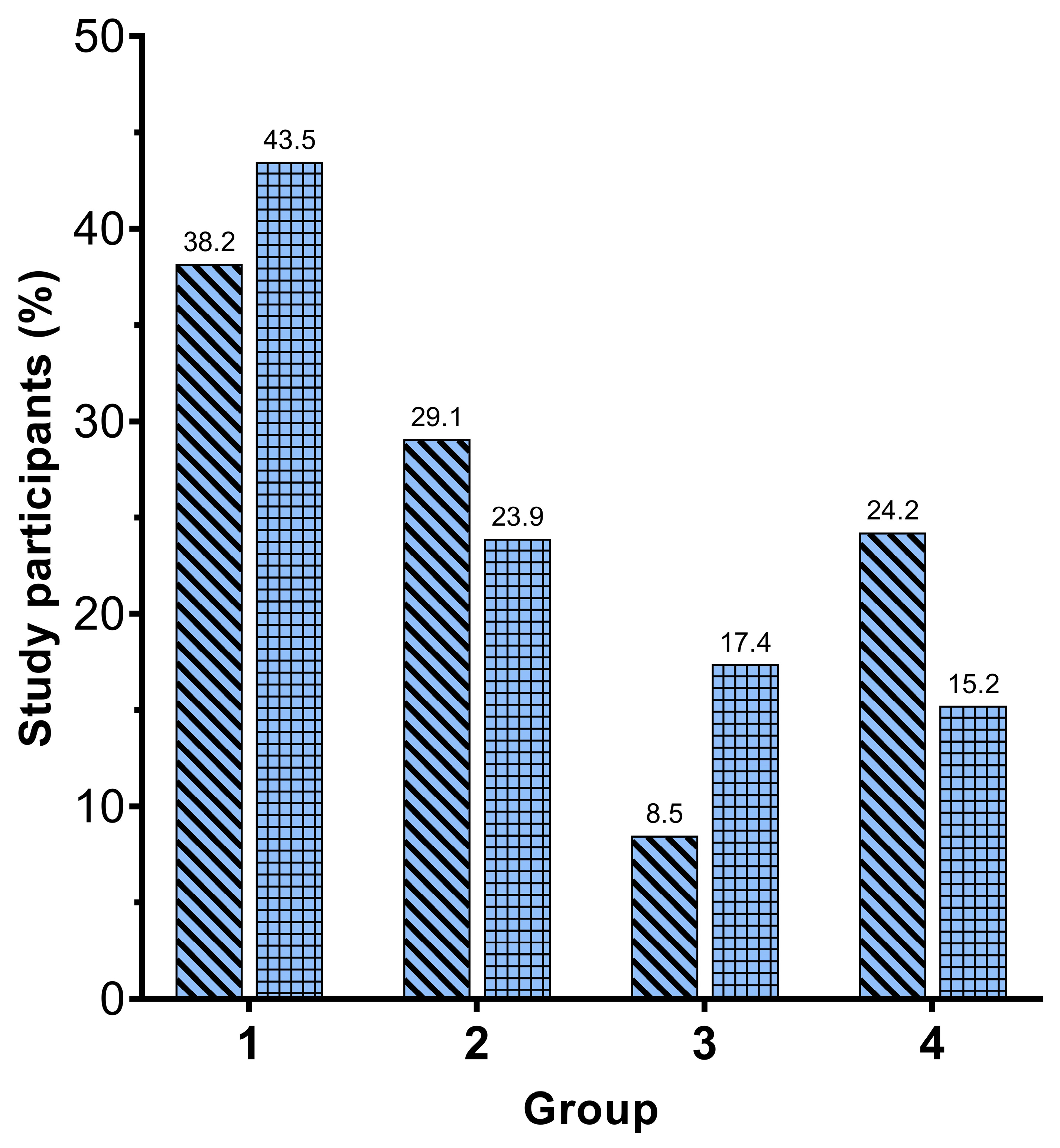
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Limitations of the Study
References
- De Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Clapham, H.E.; Tan, A.T.; Chia, W.N.; Tham, C.Y.L.; Lim, J.M.; Kunasegaran, K.; Tan, L.W.L.; Dutertre, C.A.; Shankar, N.; et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J. Exp. Med. 2021, 218, e20202617. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Muntjewerff, E.M.; Meesters, L.D.; van den Bogaart, G. Antigen Cross-Presentation by Macrophages. Front. Immunol. 2020, 11, 1276. [Google Scholar] [CrossRef]
- De Marco, L.; D’Orso, S.; Pirronello, M.; Verdiani, A.; Termine, A.; Fabrizio, C.; Capone, A.; Sabatini, A.; Guerrera, G.; Placido, R.; et al. Assessment of T-cell Reactivity to the SARS-CoV-2 Omicron Variant by Immunized Individuals. JAMA Netw. Open 2022, 5, e2210871. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Novak, T.; Hecker, J.; Grubbs, G.; Zahra, F.T.; Bellusci, L.; Pourhashemi, S.; Chou, J.; Moffitt, K.; Halasa, N.B.; et al. Author Correction: Cross-reactive immunity against the SARS-CoV-2 Omicron variant is low in pediatric patients with prior COVID-19 or MIS-C. Nat. Commun. 2022, 13, 4732. [Google Scholar] [CrossRef]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Brasu, N.; Elia, I.; Russo, V.; Montacchiesi, G.; Stabile, S.A.; De Intinis, C.; Fesi, F.; Gizzi, K.; Macagno, M.; Montone, M.; et al. Memory CD8+ T cell diversity and B cell responses correlate with protection against SARS-CoV-2 following mRNA vaccination. Nat. Immunol. 2022, 23, 1445–1456. [Google Scholar] [CrossRef]
- Michlmayr, D.; Hansen, C.H.; Gubbels, S.M.; Valentiner-Branth, P.; Bager, P.; Obel, N.; Drewes, B.; Møller, C.H.; Møller, F.T.; Legarth, R.; et al. Observed protection against SARS-CoV-2 reinfection following a primary infection: A Danish cohort study among unvaccinated using two years of nationwide PCR-test data. Lancet Reg. Health Eur. 2022, 20, 100452. [Google Scholar] [CrossRef]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P.A. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 2022, 209, 112911. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Alsafi, R.T. Lessons from SARS-CoV, MERS-CoV, and SARS-CoV-2 Infections: What We Know So Far. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 1156273. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Vrdoljak, K.; Brlek, P.; Pavelić, E.; Molnar, V.; Matišić, V.; Ivkošić, I.E.; Parčina, M. Adaptive Immune Responses and Immunity to SARS-CoV-2. Front. Immunol. 2022, 13, 848582. [Google Scholar] [CrossRef]
- Primorac, D.; Brlek, P.; Matišić, V.; Molnar, V.; Vrdoljak, K.; Zadro, R.; Parčina, M. Cellular Immunity-The Key to Long-Term Protection in Individuals Recovered from SARS-CoV-2 and after Vaccination. Vaccines 2022, 10, 442. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Wirsching, S.; Harder, L.; Heymanns, M.; Groendahl, B.; Hilbert, K.; Kowalzik, F.; Meyer, C.; Gehring, S. Long-Term, CD4+ Memory T Cell Response to SARS-CoV-2. Front. Immunol. 2022, 13, 800070. [Google Scholar] [CrossRef]
- Ng, O.W.; Chia, A.; Tan, A.T.; Jadi, R.S.; Leong, H.N.; Bertoletti, A.; Tan, Y.J. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine 2016, 34, 2008–2014. [Google Scholar] [CrossRef]
- Todorović-Raković, N.; Whitfield, J.R. Between immunomodulation and immunotolerance: The role of IFNγ in SARS-CoV-2 disease. Cytokine 2021, 146, 155637. [Google Scholar] [CrossRef]
- von Massow, G.; Oh, S.; Lam, A.; Gustafsson, K. Gamma Delta T Cells and Their Involvement in COVID-19 Virus Infections. Front. Immunol. 2021, 12, 741218. [Google Scholar] [CrossRef] [PubMed]

| Group 1 (N = 165) | Group 2 (N = 138) | p Value (Group 1 vs. Group 2) | ||
|---|---|---|---|---|
| Cellular immunity (mIU/mL) (N = 303) | MD | 768.0 | 958.5 | 0.012 * |
| IQR | 1906.1 | 2553.8 | ||
| Antibodies (IU/mL) (N = 303) | MD | 120.9 | 156.9 | 0.010 * |
| IQR | 369.2 | 747.8 | ||
| Age (years) (N = 303) | MD | 50.0 | 51.0 | 0.127 |
| IQR | 18.0 | 19.25 | ||
| Sex, No. (N = 303) | M | 72/165 (43.6%) | 67/138 (48.6%) | 0.419 |
| F | 93/165 (53.4%) | 71/138 (51.4%) | ||
| No symptoms during (re)infection | 26/165 (15.8%) | / | / | |
| Mild symptoms during (re)infection | 134/165 (81.2%) | / | / | |
| Severe symptoms during (re)infection | 5/165 (3.0%) | / | / | |
| SARS-CoV-2-positive family members | 141/165 (85.5%) | 86/138 (62.3%) | <0.001 ** | |
| Negative family history of SARS-CoV-2 | 24/165 (14.5%) | 52/138 (37.7%) | ||
| Vaccinated participants (before the IFN-γ test) | 62/165 (37.6%) | 57/138 (41.3%) | / | |
| Infected participants (before the IFN-γ test) | 77/165 (46.7%) | 84/138 (60.9%) | / | |
| Additionally vaccinated (after the IFN-γ test) | 34/165 (20.6%) | 41/138 (29.7%) | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primorac, D.; Brlek, P.; Pavelić, E.S.; Mešić, J.; Glavaš Weinberger, D.; Matišić, V.; Molnar, V.; Srića, S.; Zadro, R. Importance of Cellular Immunity and IFN-γ Concentration in Preventing SARS-CoV-2 Infection and Reinfection: A Cohort Study. Viruses 2023, 15, 792. https://doi.org/10.3390/v15030792
Primorac D, Brlek P, Pavelić ES, Mešić J, Glavaš Weinberger D, Matišić V, Molnar V, Srića S, Zadro R. Importance of Cellular Immunity and IFN-γ Concentration in Preventing SARS-CoV-2 Infection and Reinfection: A Cohort Study. Viruses. 2023; 15(3):792. https://doi.org/10.3390/v15030792
Chicago/Turabian StylePrimorac, Dragan, Petar Brlek, Eduard Stjepan Pavelić, Jana Mešić, David Glavaš Weinberger, Vid Matišić, Vilim Molnar, Saša Srića, and Renata Zadro. 2023. "Importance of Cellular Immunity and IFN-γ Concentration in Preventing SARS-CoV-2 Infection and Reinfection: A Cohort Study" Viruses 15, no. 3: 792. https://doi.org/10.3390/v15030792
APA StylePrimorac, D., Brlek, P., Pavelić, E. S., Mešić, J., Glavaš Weinberger, D., Matišić, V., Molnar, V., Srića, S., & Zadro, R. (2023). Importance of Cellular Immunity and IFN-γ Concentration in Preventing SARS-CoV-2 Infection and Reinfection: A Cohort Study. Viruses, 15(3), 792. https://doi.org/10.3390/v15030792









