Molecular Mechanisms of Antiviral Agents against Dengue Virus
Abstract
1. Introduction
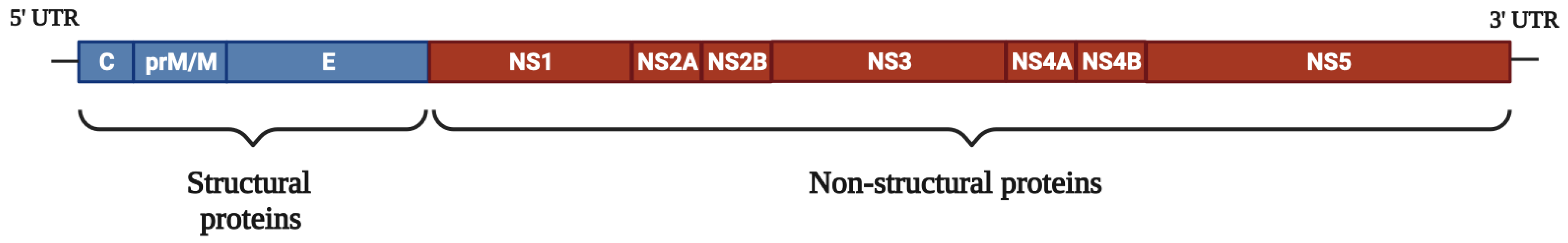
2. Structure and Genome Organization of DENV
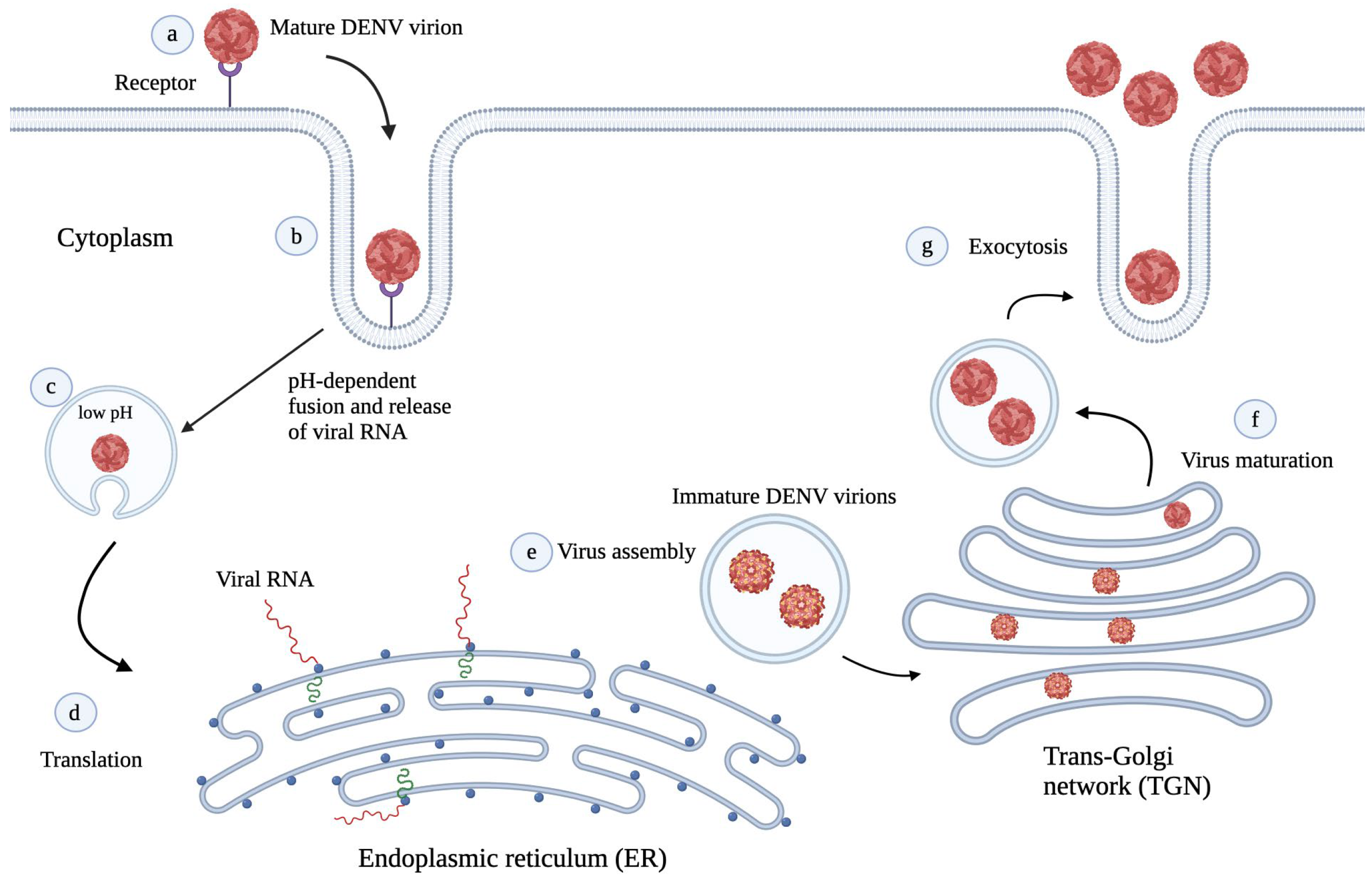
3. DENV Life Cycle
4. Preclinical and Clinical Status of DENV Antivirals
5. Modes of Action of Antivirals
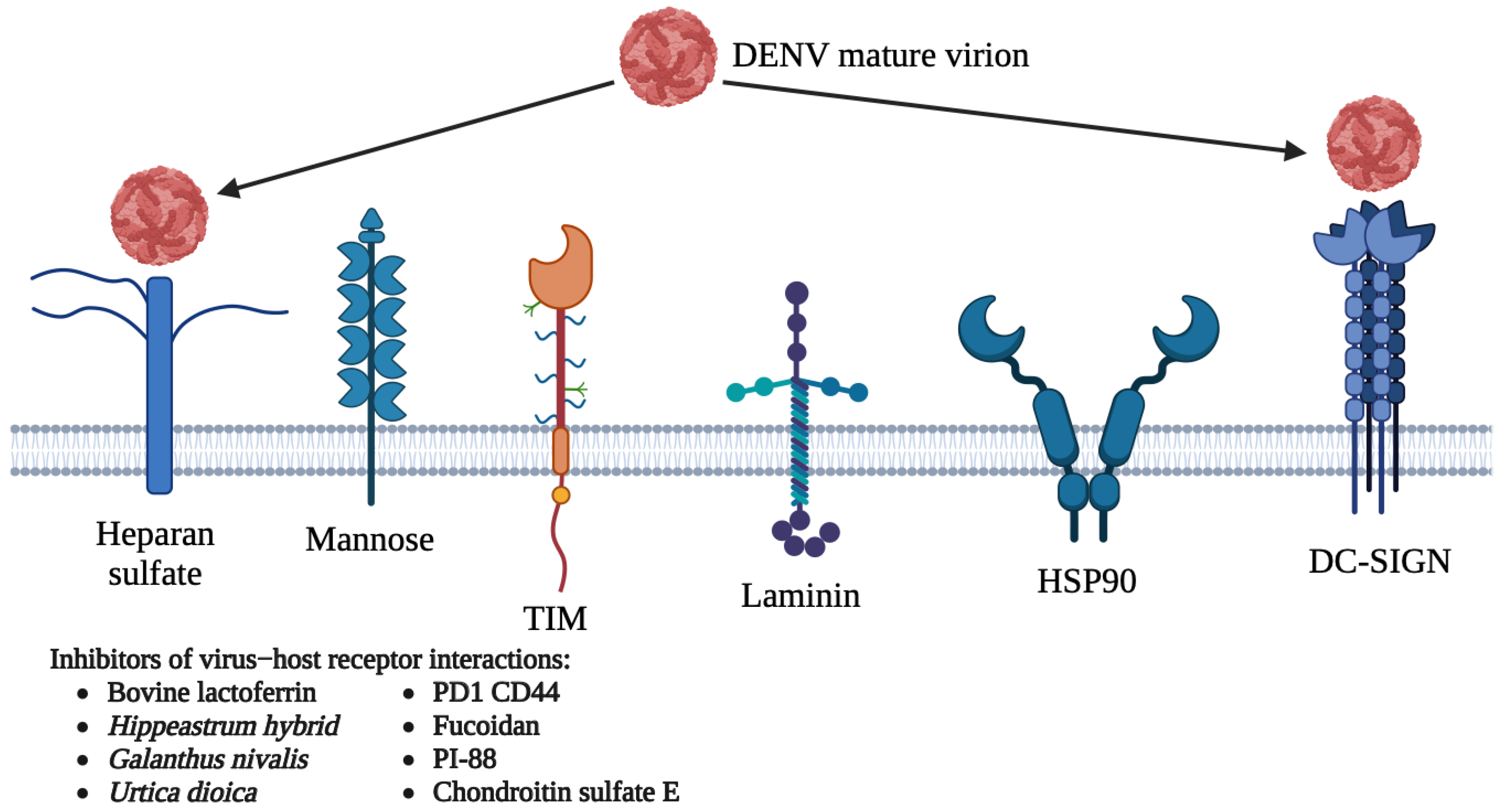
5.1. Host-Directed Antivirals
5.1.1. DC-SIGN
5.1.2. Heparan Sulfate
5.1.3. Other Receptors
| Drug | Target(s) | Mechanism(s) of Action | Inhibitory Activities (IC50/EC50 Values) | Reference |
|---|---|---|---|---|
| Bovine lactoferrin | DC-SIGN, heparan sulfate | Inhibition of the binding of DENV to DC-SIGN and heparan sulfate | IC50: D1-165.8 ± 35.1 μM D2-40.7 ± 8.6 μM D3-166.7 ± 30.6 μM D4-164.5 ± 41.0 μM | [70] |
| Hippeastrum hybrid (HHA) | DC-SIGN | Inhibition of the binding of DENV to DC-SIGN | EC50 against DENV-2: HHA-4.6 nM UDA-3.8 nM GNA-480 nM | [71] |
| Urtica dioica (UDA) | ||||
| Galanthus nivalis (GNA) | ||||
| PD1 CD44 | Heparan sulfate | Inhibition of the binding of DENV to heparan sulfate | IC50 against DENV-2: 13.8 μM | [76] |
| PG545 | IC50 against DENV-2: 25 nM | [52] | ||
| Fucoidan | IC50: D1->1000 μg/mL D2-4.7 μg/mL D3-500 μg/mL D4-365 μg/mL | [90] | ||
| PI-88 | EC50 against DENV-2: 200 μg/mL | [91] | ||
| dl-galactan hybrid C2S-3 | IC50: D2-1 μg/mL D3-13.9–14.2 μg/mL D4-29.3->50 μg/mL | [92] | ||
| iota-carrageenan G3d | ||||
| CF-238 | IC50: D1-24 μM D2-46 μM D3-14 μM D4-47 μM | [93] | ||
| Sulfated galactomannan | Heparan sulfate | Inhibition of the binding of DENV to heparan sulfate | IC50 against DENV-2: 0.12-20 μg/mL | [94] |
| Sulfated galactan | ||||
| Curdlan sulfate | EC50: D1->0.262 μg/mL D2-7 μg/mL D3-0.01 μg/mL D4-0.069 μg/mL | [95] | ||
| Chondroitin sulfate E | EC50: D1-0.53 ± 0.10 μg/mL D2- 3.80 ± 0.68 μg/mL D3-1.38 ± 0.33 μg/mL D4-0.30 ± 0.06 μg/mL | [96] | ||
| P4 | β3 integrin | Inhibition of the binding of DENV to β3 integrin | IC50 against DENV-2: 19.08 ± 2.52 μM | [78] |
| P7 | IC50 against DENV-2: 12.86 ± 5.96 μM |
5.2. Direct-Acting Antivirals
5.2.1. Targeting DENV E Protein
5.2.2. Targeting DENV prM/M and C Proteins
| Drug | Target(s) | Mechanism(s) of Action | Inhibitory Activities (IC50/IC90/EC50 Values) | Reference |
|---|---|---|---|---|
| 1662G07 and analogs | E protein | Fusion inhibitor | IC90: D2-0.89–2.0 μM D4-1.3–2.3 μM | [110] |
| DN59 | IC50 against DENV-2: ~10 μM | [109] | ||
| NITD448 | IC50 against DENV-2: 6.8 μM EC50 against DENV-2: 9.8 μM | [111] | ||
| DV2419–447 | IC90: D1-0.1 μM D2-0.3 μM D3-2 μM D4-0.7 μM | [123,124] | ||
| DN57opt | IC50 against DENV-2: 8 ± 1 μM | [125] | ||
| 1OAN1 | IC50 against DENV-2: 7 ± 4 μM | |||
| Rolitetracycline | IC50 against DENV-2: 67.1 μM | [126] | ||
| Doxycycline | IC50 against DENV-2: 55.6 μM | |||
| A5 | IC50 against DENV-2: 1.2 ± 0.7 μM | [127] | ||
| Compound 6 | EC50: D1-0.108 ± 0.08 μM D2- 0.068 ± 0.01 μM D3-0.496 ± 0.09 μM D4-0.334 ± 0.12 μM | [128] | ||
| P02 | E protein | Inhibition of virus entry | N/D | [129] |
| gg-ww | IC50 against DENV-2: 77 and 91 μmol L−1, determined using plaque assay and RT-PCR respectively. | [130] | ||
| EF | Inhibition of virus binding and entry | IC50 against DENV-2: 96 μM | [108] | |
| Geraniin | IC50 against DENV-2: 1.75 μM | [50,131] | ||
| DET2 | IC50 against DENV-2: >500 μM | [132] | ||
| DET4 | IC50 against DENV-2: 35 μM | |||
| Peptide 1 | N/D | [133] | ||
| MLH40 | prM/M protein | Inhibition of interactions between DENV M and E proteins | IC50: D1-30.35 ± 1.25 μM D2-31.41 ± 1.09 μM D3-27.95 ± 1.41 μM D4-24.45 ± 1.20 μM | [115] |
| pr | Fusion inhibitor | N/D | [116] | |
| Pep14-23 | C protein | Inhibition of interactions between the DENV C protein and host intracellular lipid droplets | EC50 against DENV-2: 0.016 μM | [119] |
| VGTI-A3 | IC90 against DENV-2: 112 nM | [122] | ||
| VGTI-A3-03 | IC90 against DENV-2: 25 nM |
5.2.3. Targeting DENV Non-Structural Proteins
Targeting NS1 Protein
Targeting NS3 Protein
Targeting NS4 Protein
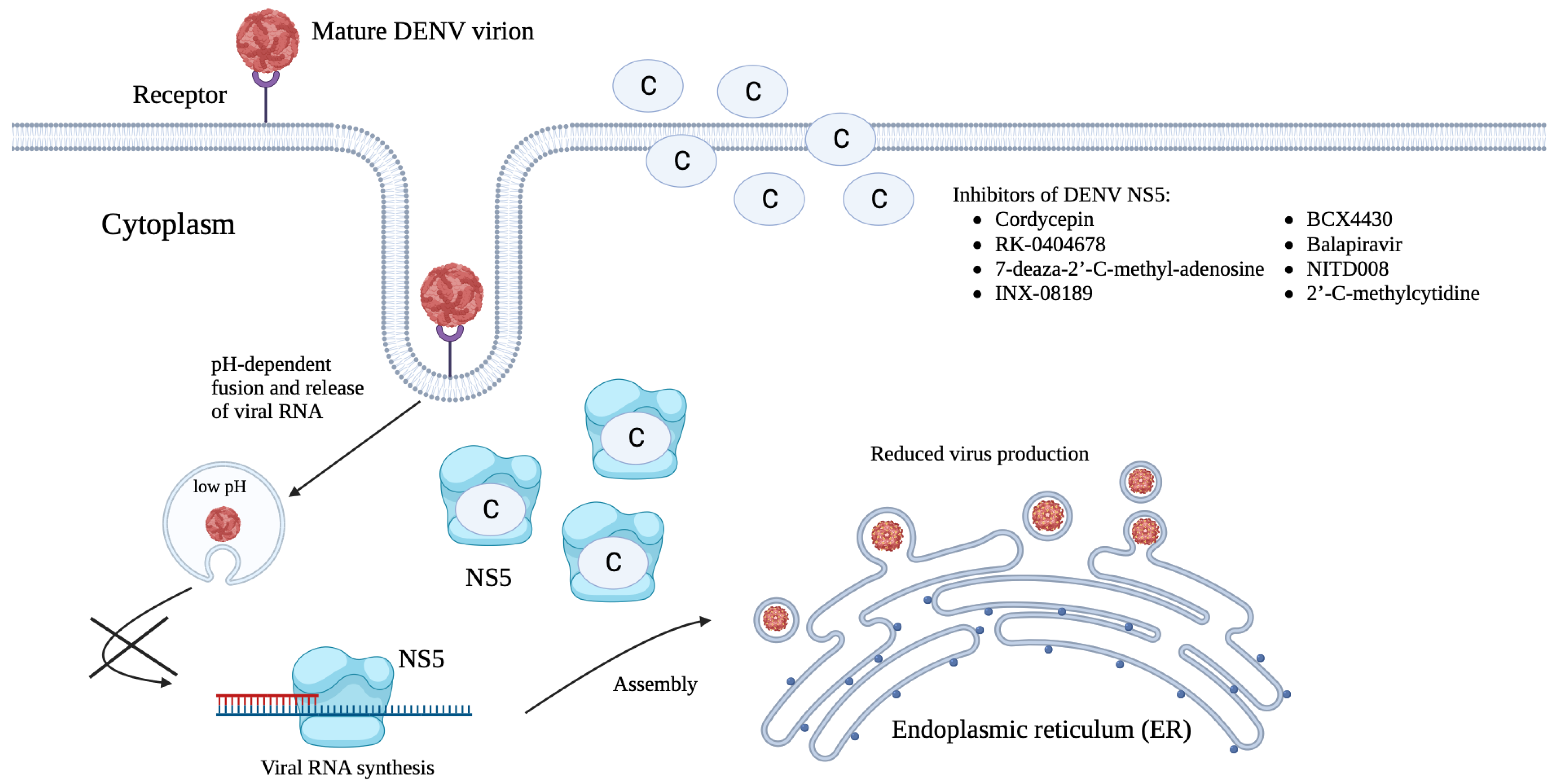
Targeting NS5 Protein
| Drug | Target(s) | Mechanism(s) of Action | Inhibitory Activities (IC50/EC50/EC90 Values) | Reference |
|---|---|---|---|---|
| Peptide 3 | NS1 protein | NS1 inhibition | N/D | [138] |
| Peptide 4 | ||||
| Peptide 10 | ||||
| Peptide 11 | ||||
| Honeysuckle (Lonicera japonica Thunb.) extracts | Inhibition of NS1 protein expression and viral replication | [139] | ||
| Ivermectin | NS3 helicase and NS2B-NS3 protease | NS3 helicase and NS2B-NS3 protease inhibition | IC50 against DENV-2: 0.50 ± 0.07 μM EC50 against DENV-2: 0.70 μM | [144,166] |
| ST-610 | NS3 helicase | NS3 helicase inhibition | EC50 against DENV-2: 0.272 μM EC90 against DENV-2: 3.59 μM | [145] |
| Suramin | IC50 against DENV-4: 0.80 μg/mL | [167] | ||
| Compound 25 | IC50 against DENV-2: 78 ± 23 μM EC50 against DENV-2: 36 ± 6 μM | [168] | ||
| Compound 7 | IC50 against DENV-2: 6 ± 5.4 μM | [169] | ||
| Protegrin-1 | NS2B-NS3 protease | NS2B-NS3 protease inhibition | IC50 against DENV-2: 11.7 μM | [146] |
| Retrocyclin-1 | IC50 against DENV-2: 21.4 μM at 37 °C and 14.1 μM at 40 °C | [147] | ||
| Nelfinavir | NS2B-NS3 protease | NS2B-NS3 protease inhibition | EC50 against DENV-2: 3.5 ± 0.4 μM | [148] |
| Carnosine | IC50 against DENV-2: 63.7 μM | [170] | ||
| Palmatine | N/D | [171] | ||
| Thiazolidinone-peptide hybrids | [172] | |||
| Compound 32 | [173] | |||
| Compound 1 | IC50: D1-36.4 μM D2-6.0 ± 2.6 μM D3-17.5 μM D4-32.8 μM | [174] | ||
| 166347 | IC50: D1-3 ± 1 μM D2-5 ± 2 μM D3-5 ± 2 μM D4-11 ± 3 μM | [175] | ||
| ARDP0006 | EC50 against DENV-2: 4.2 ± 1.9 μM | [176] | ||
| ARDP0009 | EC50 against DENV-2: 35 ± 8 μM | |||
| Compound 7n | IC50 against DENV-2: 3.75 ± 0.06 μM | [177] | ||
| Diaryl(thio)ethers | NS2B-NS3 protease | NS2B-NS3 protease inhibition | IC50: D2-4.2–98 μM D3-0.99–31.8 μM | [178] |
| Compound C | IC50: D1-4.06 ± 0.21 μM D2-4.05 ± 0.18 μM D3-2.94 ± 0.18 μM D4-3.40 ± 0.11 μM | [179] | ||
| Compound D | IC50: D1-10.83 ± 0.37 μM D2-10.45 ± 0.40 μM D3-11.14 ± 0.38 μM D4-11.04 ± 0.37 μM | |||
| Compound F (tolcapone) | IC50: D1-1.15 ± 0.1 μM D2-0.98 ± 0.06 μM D3-0.91 ± 0.06 μM D4-0.64 ± 0.03 μM | |||
| SK-12 | IC50 against DENV-1 to 4: 0.74–4.92 μM | [180] | ||
| Compound 104 | IC50 against DENV-2: 0.176 μM | [181] | ||
| Ltc1 | NS2B-NS3 protease | NS2B-NS3 protease inhibition | IC50 against DENV-2: 12.68 ± 3.2 μM at 37 °C and 6.58 ± 4.1 μM at 40 °C | [182] |
| BP13944 | IC50 against DENV-2: 22.63 ± 0.74 μM EC50 against DENV-2: 0.23 ± 0.01 μM | [183] | ||
| Policresulen | IC50 against DENV-2: 0.48 μg/mL | [184] | ||
| BP2109 | IC50 against DENV-2: 15.43 ± 2.12 μM EC50 against DENV-2: 0.17 ± 0.01 μM | [185] | ||
| MB21 | IC50 against DENV-2: 5.95 μM | [186] | ||
| Compound 45a | IC50 against DENV-2: 0.26 ± 0.03 μM | [187] | ||
| Compound 14 | N/D | [188] | ||
| AM404 | NS4B | NS4B inhibition | EC50 against DENV-2: 3.6 μM | [155] |
| Compound 1a | EC50: D1->1 μM D2-0.012 ± 0.004 μM D3-0.032 ± 0.011 μM D4->1 μM | [156] | ||
| Compound 14a | NS4B | NS4B inhibition | EC50: D1->20 μM D2-0.042 ± 0.016 μM D3-0.076 ± 0.019 μM D4->20 μM | [156] |
| NITD-618 | IC50: D1-1.5 μM D2-1.6 μM D3-1.6 μM D4-4.1 μM | [51] | ||
| AZD0530 | N/D | [189] | ||
| Dasatinib | ||||
| JNJ-1A | EC50 against DENV-1, 2, and 4: ~1 μM | [190] | ||
| NITD-688 | N/D | [191] | ||
| JNJ-A07 | Inhibition of interactions between NS3 and NS4B proteins | EC50 against DENV-2: 0.035 μM | [192] | |
| Compound B | NS4A | Inhibition of viral replication | IC50: D1-1.81 μM D2-1.32 μM D3-2.66 μM D4-4.12 μM | [193] |
| Cordycepin | NS5 MTase and NS5 RdRp | Inhibition of viral replication | EC50 against DENV-2: 26.94 μM | [163] |
| Azidothymidine-based triazoles | NS5 MTase | Inhibition of viral RNA capping | EC50 against DENV-2: 7.3-14 μM | [194] |
| Compound 10 | N/D | [195] | ||
| BG-323 | [196] | |||
| NSC 12155 | EC50 against DENV-2: 7.0 μM | [197] | ||
| Myrtopsis corymbose extracts | NS5 RdRp | NS5 RdRp inhibition | N/D | [164] |
| RK-0404678 | IC50: D1-46.2 ± 2.8 μM D2-201 ± 4.9 μM D3-287 ± 11 μM D4-445 ± 23 μM EC50: D1-29.5 ± 4.2 μM D2-6.0 ± 0.30 μM D3-29.4 ± 1.8 μM D4-31.9 ± 2.8 μM | [165] | ||
| Trigocherrins | NS5 RdRp | NS5 RdRp inhibition | IC50 against DENV-2: 3.1-16 μM | [198] |
| Trigocherriolides | ||||
| Chartaceones | IC50 against DENV-2: 1.8-4.2 μM | [199] | ||
| Avicularin | IC50 against DENV-2: 1.7 μM | [200] | ||
| Quercitrin | IC50 against DENV-2: 2.1 μM | |||
| Betulinic acid | IC50 against DENV-2: 1.7 μM | |||
| Spiraeoside | IC50 against DENV-2: 1.9 μM | |||
| Rutin | IC50 against DENV-2: 2.1 μM | |||
| Pyridobenzothiazolones | IC50 against DENV-2: 9.164-81.29 μM EC50 against DENV-2: 1.8-3.7 μM | [201] | ||
| (E)-tridec-2-en-4-ynedioic | NS5 RdRp | NS5 RdRp inhibition | IC50 against DENV-2: ~3 μM | [202] |
| Octadeca-9,11,13-triynoic acid | ||||
| Octadic-13-en-9,11-diynoic acid | ||||
| Octadic-13-en-11-ynoic acid | ||||
| 7-deaza-2′-C-methyl-adenosine | Inhibitor of viral replication | EC50 against DENV-2: 15 μM | [203] | |
| INX-08189 | N/D | [204] | ||
| BCX4430 | EC50 against DENV-2: 32.8 μM EC90 against DENV-2: 89.3 μM | [205] | ||
| Balapiravir | N/D | [206] | ||
| NITD008 | IC50 against DENV-2: 0.31 μM | [207] | ||
| 2′-C-methylcytidine | IC50 against DENV-2: 11.2 ± 0.3 μM | [208] |
| Drug | Structure | Class of Compound | Reference |
|---|---|---|---|
| Compound 6 |  | Thiophene pyrimidine | [128] |
| Compound 25 (PubChem CID: 45382104) | 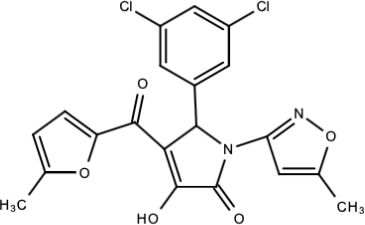 | Pyrrolone | [168] |
| Compound 7 | 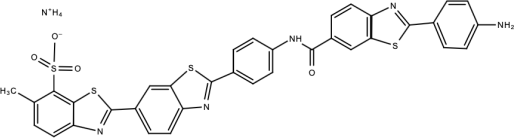 | Benzothiazole | [169] |
| Compound 32 |  | α-ketoamides | [173] |
| Compound 1 | 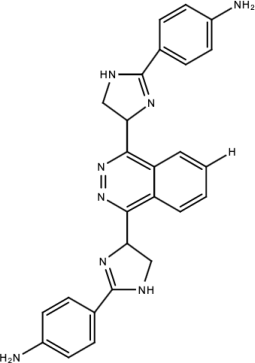 | Phthalazine | [174] |
| Compound 7n | 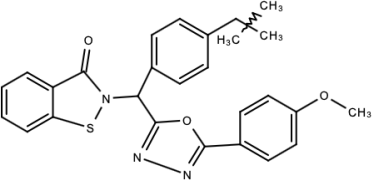 | 1,2-benzisothiazol-3(2H)-one—1,3,4-oxadiazole hybrid derivative | [177] |
| Compound C | 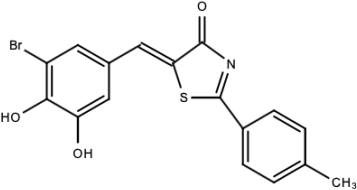 | Catechols | [179] |
| Compound D | 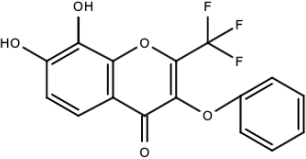 | ||
| Compound F (tolcapone) | 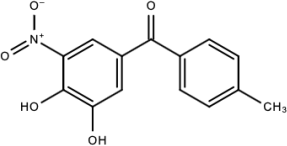 | Catechols | [179] |
| Compound 104 |  | Bithiophene cap, 3-OCH3-benzyl ether | [181] |
| Compound 45a | 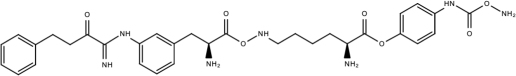 | Phenylalanine-phenylglycine analogues | [187] |
| Compound 14 | 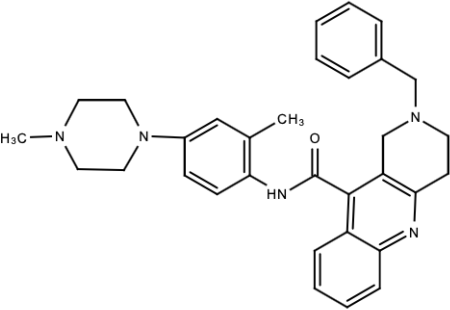 | N/S | [188] |
| Compound 1a | 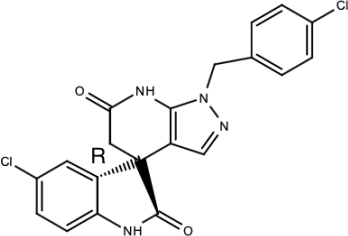 | Spiropyrazolopyridone | [156] |
| Compound 14a |  | ||
| Compound B | 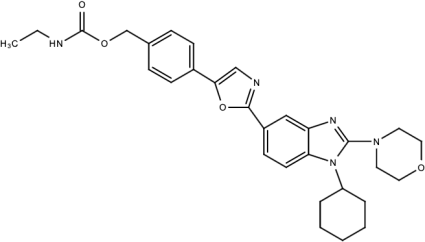 | Benzimidazole | [193] |
| Compound 10 | 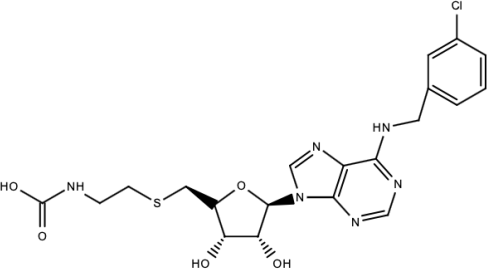 | S-adenosyl-homocysteine derivatives | [195] |
5.3. Targeting during Post-Infection Stages
5.3.1. Inhibition of Viral RNA Synthesis and Viral Translation
5.3.2. Inhibition of Virus Assembly, Maturation, and Release
| Drug | Mechanism(s) of Action | Reference |
|---|---|---|
| Protegrin-1 | Inhibition of viral RNA synthesis | [146] |
| Ltc 1 | [182] | |
| 7-deaza-2′-C-acetylene-adenosine | [210] | |
| Mycophenolic acid | [211] | |
| NITD-451 | Inhibition of viral translation | [215] |
| Narasin | [216] | |
| Lactimidomycin | [217] | |
| ST081006 | [218] | |
| Bromocriptine | [219] | |
| Peptide-conjugated phosphorodiamidate morpholino oligomers | [220] | |
| Lovastatin | Inhibition of viral assembly | [237] |
| Dasatinib | [238] | |
| Hirsutine | [239] | |
| Castanospermine | Inhibition of virus release | [240] |
| Brefeldin A | Inhibition of virus maturation and release | [241] |
6. Combination Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitra, A.K.; Mawson, A.R. Neglected Tropical Diseases: Epidemiology and Global Burden. Trop. Med. Infect. Dis. 2017, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Obi, J.O.; Gutiérrez-Barbosa, H.; Chua, J.V.; Deredge, D.J. Current Trends and Limitations in Dengue Antiviral Research. Trop. Med. Infect. Dis. 2021, 6, 180. [Google Scholar] [CrossRef]
- Montoya, M.; Gresh, L.; Mercado, J.C.; Williams, K.L.; Vargas, M.J.; Gutierrez, G.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Symptomatic Versus Inapparent Outcome in Repeat Dengue Virus Infections Is Influenced by the Time Interval between Infections and Study Year. PLoS Negl. Trop. Dis. 2013, 7, e2357. [Google Scholar] [CrossRef]
- Snow, G.E.; Ooi, E.E.; Haaland, B.; Gubler, D.J. Research on Dengue During World War II Revisited. Am. J. Trop. Med. Hyg. 2014, 91, 1203–1217. [Google Scholar] [CrossRef]
- Flipse, J.; Diosa-Toro, M.A.; Hoornweg, T.E.; van de Pol, D.P.I.; Urcuqui-Inchima, S.; Smit, J.M. Antibody-Dependent Enhancement of Dengue Virus Infection in Primary Human Macrophages; Balancing Higher Fusion against Antiviral Responses. Sci. Rep. 2016, 6, 29201. [Google Scholar] [CrossRef]
- Midgley, C.M.; Bajwa-Joseph, M.; Vasanawathana, S.; Limpitikul, W.; Wills, B.; Flanagan, A.; Waiyaiya, E.; Tran, H.B.; Cowper, A.E.; Chotiyarnwong, P.; et al. An In-Depth Analysis of Original Antigenic Sin in Dengue Virus Infection. J. Virol. 2011, 85, 410–421. [Google Scholar] [CrossRef]
- Zompi, S.; Harris, E. Original antigenic sin in dengue revisited. Proc. Natl. Acad. Sci. USA 2013, 110, 8761–8762. [Google Scholar] [CrossRef]
- Bouri, N.; Sell, T.K.; Franco, C.; Adalja, A.A.; Henderson, D.; Hynes, N.A. Return of Epidemic Dengue in the United States: Implications for the Public Health Practitioner. Public Health Rep. 2012, 127, 259–266. [Google Scholar] [CrossRef]
- Frentiu, F.D.; Zakir, T.; Walker, T.; Popovici, J.; Pyke, A.T.; Hurk, A.V.D.; McGraw, E.A.; O’Neill, S.L. Limited Dengue Virus Replication in Field-Collected Aedes aegypti Mosquitoes Infected with Wolbachia. PLoS Negl. Trop. Dis. 2014, 8, e2688. [Google Scholar] [CrossRef]
- Phuc, H.K.; Andreasen, M.H.; Burton, R.S.; Vass, C.; Epton, M.J.; Pape, G.; Fu, G.; Condon, K.C.; Scaife, S.; Donnelly, C.A.; et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007, 5, 11. [Google Scholar] [CrossRef]
- Capeding, M.R.; Tran, N.H.; Hadinegoro, S.R.S.; Ismail, H.I.H.M.; Chotpitayasunondh, T.; Chua, M.N.; Luong, C.Q.; Rusmil, K.; Wirawan, D.N.; Nallusamy, R.; et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014, 384, 1358–1365. [Google Scholar] [CrossRef]
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Ismail, H.H.M.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef]
- Villar, L.; Dayan, G.H.; Arredondo-García, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramírez, J.O.; Carrasquilla, G.; et al. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef]
- Lim, S.P.; Wang, Q.-Y.; Noble, C.G.; Chen, Y.-L.; Dong, H.; Zou, B.; Yokokawa, F.; Nilar, S.; Smith, P.; Beer, D.; et al. Ten years of dengue drug discovery: Progress and prospects. Antivir. Res. 2013, 100, 500–519. [Google Scholar] [CrossRef]
- Low, J.G.; Sung, C.; Wijaya, L.; Wei, Y.; Rathore, A.P.S.; Watanabe, S.; Tan, B.H.; Toh, L.; Chua, L.T.; Hou, Y.; et al. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): A phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect. Dis. 2014, 14, 706–715. [Google Scholar] [CrossRef]
- Tam, D.T.H.; Ngoc, T.V.; Tien, N.T.H.; Kieu, N.T.T.; Thuy, T.T.T.; Thanh, L.T.C.; Tam, C.T.; Truong, N.T.; Dung, N.T.; Qui, P.T.; et al. Effects of Short-Course Oral Corticosteroid Therapy in Early Dengue Infection in Vietnamese Patients: A Randomized, Placebo-Controlled Trial. Clin. Infect. Dis. 2012, 55, 1216–1224. [Google Scholar] [CrossRef]
- Tricou, V.; Minh, N.N.; Van, T.P.; Lee, S.J.; Farrar, J.; Wills, B.; Tran, H.T.; Simmons, C.P. A Randomized Controlled Trial of Chloroquine for the Treatment of Dengue in Vietnamese Adults. PLoS Negl. Trop. Dis. 2010, 4, e785. [Google Scholar] [CrossRef]
- Whitehorn, J.; Nguyen, C.V.V.; Khanh, L.P.; Kien, D.T.H.; Quyen, N.T.H.; Tran, N.T.T.; Hang, N.T.; Truong, N.T.; Tai, L.T.H.; Huong, N.T.C.; et al. Lovastatin for the Treatment of Adult Patients with Dengue: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2015, 62, 468–476. [Google Scholar] [CrossRef]
- Kuno, G.; Chang, G.-J.J.; Tsuchiya, K.R.; Karabatsos, N.; Cropp, C.B. Phylogeny of the Genus Flavivirus. J. Virol. 1998, 72, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 2003, 100, 6986–6991. [Google Scholar] [CrossRef] [PubMed]
- Gamarnik, A.V. Role of the dengue virus 5′ and 3′ untranslated regions in viral replication. In Frontiers in Dengue Virus Research, 1st ed.; Hanley, K.A., Weaver, S.C., Eds.; Academic Press: London, UK, 2010; Volume 1, pp. 55–78. [Google Scholar]
- Padmanabhan, R.; Strongin, A.Y. Translation and processing of the dengue virus polyprotein. In Frontiers in Dengue Virus Research, 1st ed.; Hanley, K.A., Weaver, S.C., Eds.; Academic Press: London, UK, 2010; Volume 1, pp. 14–33. [Google Scholar]
- Ng, W.C.; Soto-Acosta, R.; Bradrick, S.S.; Garcia-Blanco, M.A.; Ooi, E.E. The 5′ and 3′ Untranslated Regions of the Flaviviral Genome. Viruses 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Tuiskunen Bäck, A.; Lundkvist, Å. Dengue viruses—An overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Rice, C.M. Molecular biology of flaviviruses. Adv. Virus Res. 2003, 59, 23–61. [Google Scholar] [CrossRef]
- Chew, M.-F.; Poh, K.-S.; Poh, C.-L. Peptides as Therapeutic Agents for Dengue Virus. Int. J. Med. Sci. 2017, 14, 1342–1359. [Google Scholar] [CrossRef]
- Kuhn, R.J.; Zhang, W.; Rossmann, M.G.; Pletnev, S.V.; Corver, J.; Lenches, E.; Jones, C.T.; Mukhopadhyay, S.; Chipman, P.R.; Strauss, E.G.; et al. Structure of Dengue Virus: Implications for Flavivirus Organization, Maturation, and Fusion. Cell 2014, 108, 317–325. [Google Scholar] [CrossRef]
- El Sahili, A.; Lescar, J. Dengue Virus Non-Structural Protein 5. Viruses 2017, 9, 91. [Google Scholar] [CrossRef]
- Reddy, S.B.G.; Chin, W.-X.; Shivananju, N.S. Dengue virus NS2 and NS4: Minor proteins, mammoth roles. Biochem. Pharmacol. 2018, 154, 54–63. [Google Scholar] [CrossRef]
- Ramirez, R.R.; Ludert, J.E. The Dengue Virus Nonstructural Protein 1 (NS1) Is Secreted from Mosquito Cells in Association with the Intracellular Cholesterol Transporter Chaperone Caveolin Complex. J. Virol. 2019, 93, e01985-18. [Google Scholar] [CrossRef]
- Silva, E.M.; Conde, J.N.; Allonso, D.; Ventura, G.T.; Coelho, D.R.; Carneiro, P.H.; Silva, M.L.; Paes, M.V.; Rabelo, K.; Weissmuller, G.; et al. Dengue virus nonstructural 3 protein interacts directly with human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and reduces its glycolytic activity. Sci. Rep. 2019, 9, 2651. [Google Scholar] [CrossRef]
- Huerta, V.; Chinea, G.; Fleitas, N.; Sarría, M.; Sánchez, J.; Toledo, P.; Padrón, G. Characterization of the interaction of domain III of the envelope protein of dengue virus with putative receptors from CHO cells. Virus Res. 2008, 137, 225–234. [Google Scholar] [CrossRef]
- Swaminathan, S.; Khanna, N. Dengue: Recent advances in biology and current status of translational research. Curr. Mol. Med. 2009, 9, 152–173. [Google Scholar] [CrossRef]
- Chu, J.J.H.; Ng, M.L. Infectious Entry of West Nile Virus Occurs through a Clathrin-Mediated Endocytic Pathway. J. Virol. 2004, 78, 10543–10555. [Google Scholar] [CrossRef]
- Chu, J.J.; Leong, P.W.; Ng, M.L. Analysis of the endocytic pathway mediating the infectious entry of mosquito-borne flavivirus West Nile into Aedes albopictus mosquito (C6/36) cells. Virology 2006, 349, 463–475. [Google Scholar] [CrossRef]
- Krishnan, M.N.; Sukumaran, B.; Pal, U.; Agaisse, H.; Murray, J.L.; Hodge, T.W.; Fikrig, E. Rab 5 Is Required for the Cellular Entry of Dengue and West Nile Viruses. J. Virol. 2007, 81, 4881–4885. [Google Scholar] [CrossRef]
- Klein, D.E.; Choi, J.L.; Harrison, S.C. Structure of a Dengue Virus Envelope Protein Late-Stage Fusion Intermediate. J. Virol. 2013, 87, 2287–2293. [Google Scholar] [CrossRef]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Structure of the dengue virus envelope protein after membrane fusion. Nature 2004, 427, 313–319. [Google Scholar] [CrossRef]
- Stiasny, K.; Kössl, C.; Lepault, J.; Rey, F.A.; Heinz, F.X. Characterization of a Structural Intermediate of Flavivirus Membrane Fusion. PLoS Pathog. 2007, 3, e20. [Google Scholar] [CrossRef]
- Liao, M.; Martín, C.S.-S.; Zheng, A.; Kielian, M. In Vitro Reconstitution Reveals Key Intermediate States of Trimer Formation by the Dengue Virus Membrane Fusion Protein. J. Virol. 2010, 84, 5730–5740. [Google Scholar] [CrossRef]
- Koschinski, A.; Wengler, G.; Wengler, G.; Repp, H. The membrane proteins of flaviviruses form ion-permeable pores in the target membrane after fusion: Identification of the pores and analysis of their possible role in virus infection. J. Gen. Virol. 2003, 84, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Clyde, K.; Kyle, J.L.; Harris, E. Recent Advances in Deciphering Viral and Host Determinants of Dengue Virus Replication and Pathogenesis. J. Virol. 2006, 80, 11418–11431. [Google Scholar] [CrossRef] [PubMed]
- Stadler, K.; Allison, S.L.; Schalich, J.; Heinz, F.X. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 1997, 71, 8475–8481. [Google Scholar] [CrossRef] [PubMed]
- Azami, N.A.M.; Takasaki, T.; Kurane, I.; Moi, M.L. Non-Human Primate Models of Dengue Virus Infection: A Comparison of Viremia Levels and Antibody Responses during Primary and Secondary Infection among Old World and New World Monkeys. Pathogens 2020, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Kayesh, M.E.H.; Tsukiyama-Kohara, K. Mammalian animal models for dengue virus infection: A recent overview. Arch. Virol. 2021, 167, 31–44. [Google Scholar] [CrossRef]
- Watanabe, S.; Low, J.G.-H.; Vasudevan, S.G. Preclinical Antiviral Testing for Dengue Virus Infection in Mouse Models and Its Association with Clinical Studies. ACS Infect. Dis. 2018, 4, 1048–1057. [Google Scholar] [CrossRef]
- Chan, K.W.K.; Watanabe, S.; Kavishna, R.; Alonso, S.; Vasudevan, S.G. Animal models for studying dengue pathogenesis and therapy. Antivir. Res. 2015, 123, 5–14. [Google Scholar] [CrossRef]
- Ahmad, S.A.A.; Palanisamy, U.D.; Khoo, J.J.; Dhanoa, A.; Hassan, S.S. Efficacy of geraniin on dengue virus type-2 infected BALB/c mice. Virol. J. 2019, 16, 26. [Google Scholar] [CrossRef]
- Xie, X.; Wang, Q.-Y.; Xu, H.Y.; Qing, M.; Kramer, L.; Yuan, Z.; Shi, P.-Y. Inhibition of Dengue Virus by Targeting Viral NS4B Protein. J. Virol. 2011, 85, 11183–11195. [Google Scholar] [CrossRef]
- Modhiran, N.; Gandhi, N.; Wimmer, N.; Cheung, S.; Stacey, K.; Young, P.R.; Ferro, V.; Watterson, D. Dual targeting of dengue virus virions and NS1 protein with the heparan sulfate mimic PG545. Antivir. Res. 2019, 168, 121–127. [Google Scholar] [CrossRef]
- Monteiro, J.M.; Oliveira, M.D.; Dias, R.S.; Nacif-Marçal, L.; Feio, R.N.; Ferreira, S.O.; Oliveira, L.L.; Silva, C.C.; Paula, S.O. The antimicrobial peptide HS-1 inhibits dengue virus infection. Virology 2018, 514, 79–87. [Google Scholar] [CrossRef]
- Che, P.; Tang, H.; Li, Q. The interaction between claudin-1 and dengue viral prM/M protein for its entry. Virology 2013, 446, 303–313. [Google Scholar] [CrossRef]
- Hung, J.-J.; Hsieh, M.-T.; Young, M.-J.; Kao, C.-L.; King, C.-C.; Chang, W. An External Loop Region of Domain III of Dengue Virus Type 2 Envelope Protein Is Involved in Serotype-Specific Binding to Mosquito but Not Mammalian Cells. J. Virol. 2004, 78, 378–388. [Google Scholar] [CrossRef]
- Meertens, L.; Carnec, X.; Lecoin, M.P.; Ramdasi, R.; Guivel-Benhassine, F.; Lew, E.; Lemke, G.; Schwartz, O.; Amara, A. The TIM and TAM Families of Phosphatidylserine Receptors Mediate Dengue Virus Entry. Cell Host Microb. 2012, 12, 544–557. [Google Scholar] [CrossRef]
- Miller, J.L.; de Wet, B.J.; Martinez-Pomares, L.; Radcliffe, C.M.; Dwek, R.A.; Rudd, P.M.; Gordon, S. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008, 4, e17. [Google Scholar] [CrossRef]
- Reyes-del Valle, J.; Chávez-Salinas, S.; Medina, F.; del Angel, R.M. Heat Shock Protein 90 and Heat Shock Protein 70 Are Components of Dengue Virus Receptor Complex in Human Cells. J. Virol. 2005, 79, 4557–4567. [Google Scholar] [CrossRef]
- Tassaneetrithep, B.; Burgess, T.H.; Granelli-Piperno, A.; Trumpfheller, C.; Finke, J.; Sun, W.; Eller, M.A.; Pattanapanyasat, K.; Sarasombath, S.; Birx, D.L.; et al. DC-SIGN (CD209) Mediates Dengue Virus Infection of Human Dendritic Cells. J. Exp. Med. 2003, 197, 823–829. [Google Scholar] [CrossRef]
- Thepparit, C.; Smith, D.R. Serotype-Specific Entry of Dengue Virus into Liver Cells: Identification of the 37-Kilodalton/67-Kilodalton High-Affinity Laminin Receptor as a Dengue Virus Serotype 1 Receptor. J. Virol. 2004, 78, 12647–12656. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Chuang, Y.-J.; Lin, Y.-S.; Wan, S.-W.; Chen, C.-L.; Lin, C.-F. An emerging role for the anti-inflammatory cytokine interleukin-10 in dengue virus infection. J. Biomed. Sci. 2013, 20, 40. [Google Scholar] [CrossRef]
- Wan, S.-W.; Lin, C.-F.; Lu, Y.-T.; Lei, H.-Y.; Anderson, R.; Lin, Y.-S. Endothelial cell surface expression of protein disulfide isomerase activates β1 and β3 integrins and facilitates dengue virus infection. J. Cell. Biochem. 2011, 113, 1681–1691. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wang, J.L.; Gao, N.; Chen, Z.T.; Tian, Y.P.; An, J. Up-regulated expression of beta3 integrin induced by dengue virus serotype 2 infection associated with virus entry into human dermal microvascular endothelial cells. Biochem. Biophys. Res. Commun. 2007, 356, 763–768. [Google Scholar] [CrossRef]
- Mahajan, S.; Choudhary, S.; Kumar, P.; Tomar, S. Antiviral strategies targeting host factors and mechanisms obliging +ssRNA viral pathogens. Bioorganic Med. Chem. 2021, 46, 116356. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.H.; Van Kooyk, Y. Pathogens target DC-SIGN to influence their fate DC-SIGN functions as a pathogen receptor with broad specificity. Apmis 2003, 111, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Sanchez, E.; Altmeyer, R.; Amara, A.; Schwartz, O.; Fieschi, F.; Virelizier, J.L.; Arenzana-Seisdedos, F.; Desprès, P. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003, 4, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Lozach, P.-Y.; Burleigh, L.; Staropoli, I.; Navarro-Sanchez, E.; Harriague, J.; Virelizier, J.-L.; Rey, F.A.; Desprès, P.; Arenzana-Seisdedos, F.; Amara, A. Dendritic Cell-specific Intercellular Adhesion Molecule 3-grabbing Non-integrin (DC-SIGN)-mediated Enhancement of Dengue Virus Infection Is Independent of DC-SIGN Internalization Signals. J. Biol. Chem. 2005, 280, 23698–23708. [Google Scholar] [CrossRef]
- Mondotte, J.A.; Lozach, P.-Y.; Amara, A.; Gamarnik, A.V. Essential Role of Dengue Virus Envelope Protein N Glycosylation at Asparagine-67 during Viral Propagation. J. Virol. 2007, 81, 7136–7148. [Google Scholar] [CrossRef]
- Shah, M.; Wadood, A.; Rahman, Z.; Husnain, T. Interaction and Inhibition of Dengue Envelope Glycoprotein with Mammalian Receptor DC-Sign, an In-Silico Approach. PLoS ONE 2013, 8, e59211. [Google Scholar] [CrossRef]
- Chen, J.-M.; Fan, Y.-C.; Lin, J.-W.; Chen, Y.-Y.; Hsu, W.-L.; Chiou, S.-S. Bovine Lactoferrin Inhibits Dengue Virus Infectivity by Interacting with Heparan Sulfate, Low-Density Lipoprotein Receptor, and DC-SIGN. Int. J. Mol. Sci. 2017, 18, 1957. [Google Scholar] [CrossRef]
- Alen, M.M.F.; De Burghgraeve, T.; Kaptein, S.J.F.; Balzarini, J.; Neyts, J.; Schols, D. Broad Antiviral Activity of Carbohydrate-Binding Agents against the Four Serotypes of Dengue Virus in Monocyte-Derived Dendritic Cells. PLoS ONE 2011, 6, e21658. [Google Scholar] [CrossRef]
- Esko, J.D.; Selleck, S.B. Order Out of Chaos: Assembly of Ligand Binding Sites in Heparan Sulfate. Annu. Rev. Biochem. 2002, 71, 435–471. [Google Scholar] [CrossRef]
- Hilgard, P.; Stockert, R. Heparan sulfate proteoglycans initiate dengue virus infection of hepatocytes. Hepatology 2000, 32, 1069–1077. [Google Scholar] [CrossRef]
- Talarico, L.B.; Noseda, M.D.; Ducatti, D.R.B.; Duarte, M.E.R.; Damonte, E.B. Differential inhibition of dengue virus infection in mammalian and mosquito cells by iota-carrageenan. J. Gen. Virol. 2011, 92, 1332–1342. [Google Scholar] [CrossRef]
- Watterson, D.; Kobe, B.; Young, P. Residues in domain III of the dengue virus envelope glycoprotein involved in cell-surface glycosaminoglycan binding. J. Gen. Virol. 2012, 93, 72–82. [Google Scholar] [CrossRef]
- Recalde-Reyes, D.P.; Rodríguez-Salazar, C.A.; Castaño-Osorio, J.C.; Giraldo, M.I. PD1 CD44 antiviral peptide as an inhibitor of the protein-protein interaction in dengue virus invasion. Peptides 2022, 153, 170797. [Google Scholar] [CrossRef]
- Hussein, H.; Walker, L.R.; Abdel-Raouf, U.M.; Desouky, S.A.; Montasser, A.K.M.; Akula, S.M. Beyond RGD: Virus interactions with integrins. Arch. Virol. 2015, 160, 2669–2681. [Google Scholar] [CrossRef]
- Cui, X.; Wu, Y.; Fan, D.; Gao, N.; Ming, Y.; Wang, P.; An, J. Peptides P4 and P7 derived from E protein inhibit entry of dengue virus serotype 2 via interacting with β3 integrin. Antivir. Res. 2018, 155, 20–27. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Zagórska, A.; Lew, E.D.; Shrestha, B.; Rothlin, C.V.; Naughton, J.; Diamond, M.S.; Lemke, G.; Young, J.A. Enveloped Viruses Disable Innate Immune Responses in Dendritic Cells by Direct Activation of TAM Receptors. Cell Host Microbe 2013, 14, 136–147. [Google Scholar] [CrossRef]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus Entry Receptors: An Update. Viruses 2013, 6, 69–88. [Google Scholar] [CrossRef]
- Acosta, E.G.; Castilla, V.; Damonte, E.B. Functional entry of dengue virus into Aedes albopictus mosquito cells is dependent on clathrin-mediated endocytosis. J. Gen. Virol. 2008, 89, 474–484. [Google Scholar] [CrossRef]
- Acosta, E.G.; Castilla, V.; Damonte, E.B. Alternative infectious entry pathways for dengue virus serotypes into mammalian cells. Cell. Microbiol. 2009, 11, 1533–1549. [Google Scholar] [CrossRef]
- van der Schaar, H.M.; Rust, M.J.; Chen, C.; van der Ende-Metselaar, H.; Wilschut, J.; Zhuang, X.; Smit, J.M. Dissecting the Cell Entry Pathway of Dengue Virus by Single-Particle Tracking in Living Cells. PLoS Pathog. 2008, 4, e1000244. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Ng, M.L. A different mode of entry by dengue-2 neutralisation escape mutant virus. Arch. Virol. 1999, 144, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C.; Hidari, K.I.; Itonori, S.; Yamada, A.; Takahashi, N.; Kasama, T.; Hasebe, F.; Islam, M.A.; Hatano, K.; Matsuoka, K.; et al. Identification and Characterization of Carbohydrate Molecules in Mammalian Cells Recognized by Dengue Virus Type 2. J. Biochem. 2006, 139, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Germi, R.; Crance, J.-M.; Garin, D.; Guimet, J.; Lortat-Jacob, H.; Ruigrok, R.W.; Zarski, J.-P.; Drouet, E. Heparan Sulfate-Mediated Binding of Infectious Dengue Virus Type 2 and Yellow Fever Virus. Virology 2002, 292, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Sakoonwatanyoo, P.; Boonsanay, V.; Smith, D.R. Growth and production of the dengue virus in C6/36 cells and identification of a laminin-binding protein as a candidate serotype 3 and 4 receptor protein. Intervirology 2006, 49, 161–172. [Google Scholar] [CrossRef]
- Takada, A.; Kawaoka, Y. Antibody-dependent enhancement of viral infection: Molecular mechanisms andin vivo implications. Rev. Med. Virol. 2003, 13, 387–398. [Google Scholar] [CrossRef]
- Wichit, S.; Jittmittraphap, A.; Hidari, K.I.; Thaisomboonsuk, B.; Petmitr, S.; Ubol, S.; Aoki, C.; Itonori, S.; Morita, K.; Suzuki, T.; et al. Dengue virus type 2 recognizes the carbohydrate moiety of neutral glycosphingolipids in mammalian and mosquito cells. Microbiol. Immunol. 2011, 55, 135–140. [Google Scholar] [CrossRef]
- Hidari, K.I.P.J.; Takahashi, N.; Arihara, M.; Nagaoka, M.; Morita, K.; Suzuki, T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem. Biophys. Res. Commun. 2008, 376, 91–95. [Google Scholar] [CrossRef]
- Lee, E.; Pavy, M.; Young, N.; Freeman, C.; Lobigs, M. Antiviral effect of the heparan sulfate mimetic, PI-88, against dengue and encephalitic flaviviruses. Antivir. Res. 2006, 69, 31–38. [Google Scholar] [CrossRef]
- Talarico, L.B.; Pujol, C.A.; Zibetti, R.G.M.; Faría, P.C.S.; Noseda, M.; Duarte, M.E.R.; Damonte, E.B. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antivir. Res. 2005, 66, 103–110. [Google Scholar] [CrossRef]
- Rees, C.R.; Costin, J.M.; Fink, R.C.; McMichael, M.; Fontaine, K.A.; Isern, S.; Michael, S.F. In vitro inhibition of dengue virus entry by p-sulfoxy-cinnamic acid and structurally related combinatorial chemistries. Antivir. Res. 2008, 80, 135–142. [Google Scholar] [CrossRef]
- Pujol, C.A.; Ray, S.; Ray, B.; Damonte, E.B. Antiviral activity against dengue virus of diverse classes of algal sulfated polysaccharides. Int. J. Biol. Macromol. 2012, 51, 412–416. [Google Scholar] [CrossRef]
- Ichiyama, K.; Reddy, S.B.G.; Zhang, L.F.; Chin, W.-X.; Muschin, T.; Heinig, L.; Suzuki, Y.; Nanjundappa, H.; Yoshinaka, Y.; Ryo, A.; et al. Sulfated Polysaccharide, Curdlan Sulfate, Efficiently Prevents Entry/Fusion and Restricts Antibody-Dependent Enhancement of Dengue Virus Infection In Vitro: A Possible Candidate for Clinical Application. PLoS Negl. Trop. Dis. 2013, 7, e2188. [Google Scholar] [CrossRef]
- Kato, D.; Era, S.; Watanabe, I.; Arihara, M.; Sugiura, N.; Kimata, K.; Suzuki, Y.; Morita, K.; Hidari, K.I.; Suzuki, T. Antiviral activity of chondroitin sulphate E targeting dengue virus envelope protein. Antivir. Res. 2010, 88, 236–243. [Google Scholar] [CrossRef]
- Low, J.G.; Gatsinga, R.; Vasudevan, S.G.; Sampath, A. Dengue Antiviral Development: A Continuing Journey. Adv. Exp. Med. Biol. 2018, 1062, 319–332. [Google Scholar] [CrossRef]
- Boldescu, V.; Behnam, M.A.M.; Vasilakis, N.; Klein, C.D. Broad-spectrum agents for flaviviral infections: Dengue, Zika and beyond. Nat. Rev. Drug Discov. 2017, 16, 565–586. [Google Scholar] [CrossRef]
- Troost, B.; Smit, J.M. Recent advances in antiviral drug development towards dengue virus. Curr. Opin. Virol. 2020, 43, 9–21. [Google Scholar] [CrossRef]
- Kostyuchenko, V.A.; Zhang, Q.; Tan, J.L.; Ng, T.-S.; Lok, S.-M. Immature and Mature Dengue Serotype 1 Virus Structures Provide Insight into the Maturation Process. J. Virol. 2013, 87, 7700–7707. [Google Scholar] [CrossRef]
- Tang, C.-T.; Liao, M.-Y.; Chiu, C.-Y.; Shen, W.-F.; Chiu, C.-Y.; Cheng, P.-C.; Chang, G.-J.J.; Wu, H.-C. Generation of Monoclonal Antibodies against Dengue Virus Type 4 and Identification of Enhancing Epitopes on Envelope Protein. PLoS ONE 2015, 10, e0136328. [Google Scholar] [CrossRef]
- Yu, K.; Sheng, Z.; Huang, B.; Ma, X.; Li, Y.; Yuan, X.; Qin, Z.; Wang, D.; Chakravarty, S.; Li, F.; et al. Structural, Antigenic, and Evolutionary Characterizations of the Envelope Protein of Newly Emerging Duck Tembusu Virus. PLoS ONE 2013, 8, e71319. [Google Scholar] [CrossRef]
- Crill, W.D.; Roehrig, J. Monoclonal Antibodies That Bind to Domain III of Dengue Virus E Glycoprotein Are the Most Efficient Blockers of Virus Adsorption to Vero Cells. J. Virol. 2001, 75, 7769–7773. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Gromowski, G.D.; Li, L.; Barrett, A.D.T. Characterization of a dengue type-specific epitope on dengue 3 virus envelope protein domain III. J. Gen. Virol. 2010, 91, 2249–2253. [Google Scholar] [CrossRef] [PubMed]
- Roehrig, J.T.; Butrapet, S.; Liss, N.M.; Bennett, S.L.; Luy, B.E.; Childers, T.; Boroughs, K.L.; Stovall, J.L.; Calvert, A.E.; Blair, C.D.; et al. Mutation of the dengue virus type 2 envelope protein heparan sulfate binding sites or the domain III lateral ridge blocks replication in Vero cells prior to membrane fusion. Virology 2013, 441, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, W.; Ogata, S.; Clements, D.; Strauss, J.H.; Baker, T.S.; Kuhn, R.J.; Rossmann, M.G. Conformational Changes of the Flavivirus E Glycoprotein. Structure 2004, 12, 1607–1618. [Google Scholar] [CrossRef]
- Fritz, R.; Blazevic, J.; Taucher, C.; Pangerl, K.; Heinz, F.X.; Stiasny, K. The Unique Transmembrane Hairpin of Flavivirus Fusion Protein E Is Essential for Membrane Fusion. J. Virol. 2011, 85, 4377–4385. [Google Scholar] [CrossRef]
- Panya, A.; Bangphoomi, K.; Choowongkomon, K.; Yenchitsomanus, P.-T. Peptide Inhibitors Against Dengue Virus Infection. Chem. Biol. Drug Des. 2014, 84, 148–157. [Google Scholar] [CrossRef]
- Hrobowski, Y.M.; Garry, R.F.; Michael, S.F. Peptide inhibitors of dengue virus and West Nile virus infectivity. Virol. J. 2005, 2, 49. [Google Scholar] [CrossRef]
- Schmidt, A.G.; Lee, K.; Yang, P.L.; Harrison, S.C. Small-Molecule Inhibitors of Dengue-Virus Entry. PLoS Pathog. 2012, 8, e1002627. [Google Scholar] [CrossRef]
- Poh, M.K.; Yip, A.; Zhang, S.; Priestle, J.P.; Ma, N.L.; Smit, J.M.; Wilschut, J.; Shi, P.-Y.; Wenk, M.R.; Schul, W. A small molecule fusion inhibitor of dengue virus. Antivir. Res. 2009, 84, 260–266. [Google Scholar] [CrossRef]
- Li, L.; Lok, S.-M.; Yu, I.-M.; Zhang, Y.; Kuhn, R.J.; Chen, J.; Rossmann, M.G. The Flavivirus Precursor Membrane-Envelope Protein Complex: Structure and Maturation. Science 2008, 319, 1830–1834. [Google Scholar] [CrossRef]
- Rodenhuis-Zybert, I.A.; Wilschut, J.; Smit, J.M. Dengue virus life cycle: Viral and host factors modulating infectivity. Cell. Mol. Life Sci. CMLS 2010, 67, 2773–2786. [Google Scholar] [CrossRef]
- Tomlinson, S.M.; Malmstrom, R.D.; Watowich, S.J. New Approaches to Structure-Based Discovery of Dengue Protease Inhibitors. Infect. Disord. Drug Targ. 2009, 9, 327–343. [Google Scholar] [CrossRef]
- Panya, A.; Sawasdee, N.; Junking, M.; Srisawat, C.; Choowongkomon, K.; Yenchitsomanus, P.-T. A Peptide Inhibitor Derived from the Conserved Ectodomain Region of DENV Membrane (M) Protein with Activity Against Dengue Virus Infection. Chem. Biol. Drug Des. 2015, 86, 1093–1104. [Google Scholar] [CrossRef]
- Zheng, A.; Umashankar, M.; Kielian, M. In Vitro and In Vivo Studies Identify Important Features of Dengue Virus pr-E Protein Interactions. PLoS Pathog. 2010, 6, e1001157. [Google Scholar] [CrossRef]
- Balinsky, C.A.; Schmeisser, H.; Ganesan, S.; Singh, K.; Pierson, T.C.; Zoon, K.C. Nucleolin Interacts with the Dengue Virus Capsid Protein and Plays a Role in Formation of Infectious Virus Particles. J. Virol. 2013, 87, 13094–13106. [Google Scholar] [CrossRef]
- Ma, L.; Jones, C.T.; Groesch, T.D.; Kuhn, R.J.; Post, C.B. Solution structure of dengue virus capsid protein reveals another fold. Proc. Natl. Acad. Sci. USA 2004, 101, 3414–3419. [Google Scholar] [CrossRef]
- Faustino, A.F.; Guerra, G.M.; Huber, R.G.; Hollmann, A.; Domingues, M.M.; Barbosa, G.M.; Enguita, F.J.; Bond, P.J.; Castanho, M.A.R.B.; Da Poian, A.T.; et al. Understanding Dengue Virus Capsid Protein Disordered N-Terminus and pep14-23-Based Inhibition. ACS Chem. Biol. 2014, 10, 517–526. [Google Scholar] [CrossRef]
- Byrd, C.M.; Dai, D.; Grosenbach, D.W.; Berhanu, A.; Jones, K.F.; Cardwell, K.B.; Schneider, C.; Wineinger, K.A.; Page, J.M.; Harver, C.; et al. A Novel Inhibitor of Dengue Virus Replication That Targets the Capsid Protein. Antimicrob. Agents Chemother. 2013, 57, 15–25. [Google Scholar] [CrossRef]
- Scaturro, P.; Trist, I.M.L.; Paul, D.; Kumar, A.; Acosta, E.G.; Byrd, C.M.; Jordan, R.; Brancale, A.; Bartenschlager, R. Characterization of the Mode of Action of a Potent Dengue Virus Capsid Inhibitor. J. Virol. 2014, 88, 11540–11555. [Google Scholar] [CrossRef]
- Smith, J.L.; Sheridan, K.; Parkins, C.J.; Frueh, L.; Jemison, A.; Strode, K.; Dow, G.; Nilsen, A.; Hirsch, A.J. Characterization and structure-activity relationship analysis of a class of antiviral compounds that directly bind dengue virus capsid protein and are incorporated into virions. Antivir. Res. 2018, 155, 12–19. [Google Scholar] [CrossRef]
- Schmidt, A.G.; Yang, P.L.; Harrison, S.C. Peptide Inhibitors of Flavivirus Entry Derived from the E Protein Stem. J. Virol. 2010, 84, 12549–12554. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.G.; Yang, P.L.; Harrison, S.C. Peptide Inhibitors of Dengue-Virus Entry Target a Late-Stage Fusion Intermediate. PLoS Pathog. 2010, 6, e1000851. [Google Scholar] [CrossRef] [PubMed]
- Costin, J.M.; Jenwitheesuk, E.; Lok, S.-M.; Hunsperger, E.; Conrads, K.A.; Fontaine, K.A.; Rees, C.R.; Rossmann, M.G.; Isern, S.; Samudrala, R.; et al. Structural Optimization and De Novo Design of Dengue Virus Entry Inhibitory Peptides. PLoS Negl. Trop. Dis. 2010, 4, e721. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-M.; Chen, Y.-F.; Tu, Y.-Y.; Yen, K.-R.; Yang, Y.-L. Combinatorial Computational Approaches to Identify Tetracycline Derivatives as Flavivirus Inhibitors. PLoS ONE 2007, 2, e428. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, T.; Yennamalli, R.; Campbell, P.; Stoermer, M.J.; Fairlie, D.P.; Kobe, B.; Young, P.R. In silico screening of small molecule libraries using the dengue virus envelope E protein has identified compounds with antiviral activity against multiple flaviviruses. Antivir. Res. 2009, 84, 234–241. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Patel, S.J.; Vangrevelinghe, E.; Xu, H.Y.; Rao, R.; Jaber, D.; Schul, W.; Gu, F.; Heudi, O.; Ma, N.L.; et al. A Small-Molecule Dengue Virus Entry Inhibitor. Antimicrob. Agents Chemother. 2009, 53, 1823–1831. [Google Scholar] [CrossRef]
- Zhou, Z.; Khaliq, M.; Suk, J.-E.; Patkar, C.; Li, L.; Kuhn, R.J.; Post, C.B. Antiviral Compounds Discovered by Virtual Screening of Small–Molecule Libraries against Dengue Virus E Protein. ACS Chem. Biol. 2008, 3, 765–775. [Google Scholar] [CrossRef]
- Chew, M.-F.; Tham, H.-W.; Rajik, M.; Sharifah, S. Anti-dengue virus serotype 2 activity and mode of action of a novel peptide. J. Appl. Microbiol. 2015, 119, 1170–1180. [Google Scholar] [CrossRef]
- Ahmad, S.A.A.; Palanisamy, U.D.; Tejo, B.A.; Chew, M.F.; Tham, H.W.; Hassan, S.S. Geraniin extracted from the rind of Nephelium lappaceum binds to dengue virus type-2 envelope protein and inhibits early stage of virus replication. Virol. J. 2017, 14, 229. [Google Scholar] [CrossRef]
- Alhoot, M.A.; Rathinam, A.K.; Wang, S.M.; Manikam, R.; Sekaran, S.D. Inhibition of Dengue Virus Entry into Target Cells Using Synthetic Antiviral Peptides. Int. J. Med. Sci. 2013, 10, 719–729. [Google Scholar] [CrossRef]
- Bai, F.; Town, T.; Pradhan, D.; Cox, J.; Ashish; Ledizet, M.; Anderson, J.F.; Flavell, R.A.; Krueger, J.K.; Koski, R.A.; et al. Antiviral Peptides Targeting the West Nile Virus Envelope Protein. J. Virol. 2007, 81, 2047–2055. [Google Scholar] [CrossRef]
- Muller, D.A.; Young, P.R. The flavivirus NS1 protein: Molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antivir. Res. 2013, 98, 192–208. [Google Scholar] [CrossRef]
- Mackenzie, J.; Jones, M.; Young, P.R. Immunolocalization of the Dengue Virus Nonstructural Glycoprotein NS1 Suggests a Role in Viral RNA Replication. Virology 1996, 220, 232–240. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Flaviviruses and flavivirus vaccines. Vaccine 2012, 30, 4301–4306. [Google Scholar] [CrossRef]
- Libraty, D.H.; Young, P.; Pickering, D.; Endy, T.P.; Kalayanarooj, S.; Green, S.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; Rothman, A. High Circulating Levels of the Dengue Virus Nonstructural Protein NS1 Early in Dengue Illness Correlate with the Development of Dengue Hemorrhagic Fever. J. Infect. Dis. 2002, 186, 1165–1168. [Google Scholar] [CrossRef]
- Songprakhon, P.; Thaingtamtanha, T.; Limjindaporn, T.; Puttikhunt, C.; Srisawat, C.; Luangaram, P.; Dechtawewat, T.; Uthaipibull, C.; Thongsima, S.; Yenchitsomanus, P.-T.; et al. Peptides targeting dengue viral nonstructural protein 1 inhibit dengue virus production. Sci. Rep. 2020, 10, 12933. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Yeh, S.-F.; Ruan, X.-M.; Zhang, H.; Hsu, S.-D.; Huang, H.-D.; Hsieh, C.-C.; Lin, Y.-S.; Yeh, T.-M.; Liu, H.-S.; et al. Honeysuckle aqueous extract and induced let-7a suppress dengue virus type 2 replication and pathogenesis. J. Ethnopharmacol. 2017, 198, 109–121. [Google Scholar] [CrossRef]
- Apte-Sengupta, S.; Sirohi, D.; Kuhn, R.J. Coupling of replication and assembly in flaviviruses. Curr. Opin. Virol. 2014, 9, 134–142. [Google Scholar] [CrossRef]
- Luo, D.; Vasudevan, S.G.; Lescar, J. The flavivirus NS2B–NS3 protease–helicase as a target for antiviral drug development. Antivir. Res. 2015, 118, 148–158. [Google Scholar] [CrossRef]
- Luo, D.; Xu, T.; Watson, R.P.; Scherer-Becker, D.; Sampath, A.; Jahnke, W.; Yeong, S.S.; Wang, C.H.; Lim, S.P.; Strongin, A.; et al. Insights into RNA unwinding and ATP hydrolysis by the flavivirus NS3 protein. EMBO J. 2008, 27, 3209–3219. [Google Scholar] [CrossRef]
- Noble, C.G.; Chen, Y.-L.; Dong, H.; Gu, F.; Lim, S.P.; Schul, W.; Wang, Q.-Y.; Shi, P.-Y. Strategies for development of dengue virus inhibitors. Antivir. Res. 2010, 85, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, E.; Pezzullo, M.; De Burghgraeve, T.; Kaptein, S.; Pastorino, B.; Dallmeier, K.; de Lamballerie, X.; Neyts, J.; Hanson, A.M.; Frick, D.N.; et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug. J. Antimicrob. Chemother. 2012, 67, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Byrd, C.M.; Grosenbach, D.W.; Berhanu, A.; Dai, D.; Jones, K.F.; Cardwell, K.B.; Schneider, C.; Yang, G.; Tyavanagimatt, S.; Harver, C.; et al. Novel Benzoxazole Inhibitor of Dengue Virus Replication That Targets the NS3 Helicase. Antimicrob. Agents Chemother. 2013, 57, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Abdulrahman, A.Y.; Sasikumer, P.G.; Othman, S.; Rahman, N.A.; Yusof, R. Protegrin-1 Inhibits Dengue NS2B-NS3 Serine Protease and Viral Replication in MK2 Cells. J. Biomed. Biotechnol. 2012, 2012, 251482. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Han, H.C.; Ramasamy, T.S.; Othman, S.; Rahman, N.A.; Yusof, R. Inhibition of dengue NS2B-NS3 protease and viral replication in Vero cells by recombinant retrocyclin-1. BMC Infect. Dis. 2012, 12, 314. [Google Scholar] [CrossRef]
- Bhakat, S.; Delang, L.; Kaptein, S.; Neyts, J.; Leyssen, P.; Jayaprakash, V. Reaching beyond HIV/HCV: Nelfinavir as a potential starting point for broad-spectrum protease inhibitors against dengue and chikungunya virus. RSC Adv. 2015, 5, 85938–85949. [Google Scholar] [CrossRef]
- Miller, S.; Kastner, S.; Krijnse-Locker, J.; Bühler, S.; Bartenschlager, R. The Non-structural Protein 4A of Dengue Virus Is an Integral Membrane Protein Inducing Membrane Alterations in a 2K-regulated Manner. J. Biol. Chem. 2007, 282, 8873–8882. [Google Scholar] [CrossRef]
- Roosendaal, J.; Westaway, E.G.; Khromykh, A.; Mackenzie, J.M. Regulated Cleavages at the West Nile Virus NS4A-2K-NS4B Junctions Play a Major Role in Rearranging Cytoplasmic Membranes and Golgi Trafficking of the NS4A Protein. J. Virol. 2006, 80, 4623–4632. [Google Scholar] [CrossRef]
- McLean, J.E.; Wudzinska, A.; Datan, E.; Quaglino, D.; Zakeri, Z. Flavivirus NS4A-induced Autophagy Protects Cells against Death and Enhances Virus Replication. J. Biol. Chem. 2011, 286, 22147–22159. [Google Scholar] [CrossRef]
- Muñoz-Jordán, J.L.; Sánchez-Burgos, G.G.; Laurent-Rolle, M.; García-Sastre, A. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. USA 2003, 100, 14333–14338. [Google Scholar] [CrossRef]
- Tajima, S.; Takasaki, T.; Kurane, I. Restoration of replication-defective dengue type 1 virus bearing mutations in the N-terminal cytoplasmic portion of NS4A by additional mutations in NS4B. Arch. Virol. 2010, 156, 63–69. [Google Scholar] [CrossRef]
- Nemésio, H.; Palomares-Jerez, F.; Villalaín, J. NS4A and NS4B proteins from dengue virus: Membranotropic regions. Biochim. Biophys. Acta 2012, 1818, 2818–2830. [Google Scholar] [CrossRef]
- van Cleef, K.W.R.; Overheul, G.J.; Thomassen, M.C.; Marjakangas, J.M.; van Rij, R.P. Escape Mutations in NS4B Render Dengue Virus Insensitive to the Antiviral Activity of the Paracetamol Metabolite AM404. Antimicrob. Agents Chemother. 2016, 60, 2554–2557. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Dong, H.; Zou, B.; Karuna, R.; Wan, K.F.; Zou, J.; Susila, A.; Yip, A.; Shan, C.; Yeo, K.L.; et al. Discovery of Dengue Virus NS4B Inhibitors. J. Virol. 2015, 89, 8233–8244. [Google Scholar] [CrossRef]
- Bollati, M.; Alvarez, K.; Assenberg, R.; Baronti, C.; Canard, B.; Cook, S.; Coutard, B.; Decroly, E.; de Lamballerie, X.; Gould, E.A.; et al. Structure and functionality in flavivirus NS-proteins: Perspectives for drug design. Antivir. Res. 2010, 87, 125–148. [Google Scholar] [CrossRef]
- Zou, G.; Chen, Y.-L.; Dong, H.; Lim, C.C.; Yap, L.J.; Yau, Y.H.; Shochat, S.G.; Lescar, J.; Shi, P.-Y. Functional Analysis of Two Cavities in Flavivirus NS5 Polymerase. J. Biol. Chem. 2011, 286, 14362–14372. [Google Scholar] [CrossRef]
- Zhao, Y.; Soh, T.S.; Zheng, J.; Chan, K.W.K.; Phoo, W.W.; Lee, C.C.; Tay, M.; Swaminathan, K.; Cornvik, T.C.; Lim, S.P.; et al. A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication. PLoS Pathog. 2015, 11, e1004682. [Google Scholar] [CrossRef]
- Bujalowski, P.J.; Bujalowski, W.; Choi, K.H. Interactions between the Dengue Virus Polymerase NS5 and Stem-Loop A. J. Virol. 2017, 91, e00047-17. [Google Scholar] [CrossRef]
- Yang, S.N.Y.; Maher, B.; Wang, C.; Wagstaff, K.M.; Fraser, J.E.; Jans, D.A. High Throughput Screening Targeting the Dengue NS3-NS5 Interface Identifies Antivirals against Dengue, Zika and West Nile Viruses. Cells 2022, 11, 730. [Google Scholar] [CrossRef]
- Wang, B.; Thurmond, S.; Zhou, K.; Sánchez-Aparicio, M.T.; Fang, J.; Lu, J.; Gao, L.; Ren, W.; Cui, Y.; Veit, E.C.; et al. Structural basis for STAT2 suppression by flavivirus NS5. Nat. Struct. Mol. Biol. 2020, 27, 875–885. [Google Scholar] [CrossRef]
- Panya, A.; Songprakhon, P.; Panwong, S.; Jantakee, K.; Kaewkod, T.; Tragoolpua, Y.; Sawasdee, N.; Lee, V.; Nimmanpipug, P.; Yenchitsomanus, P.-T. Cordycepin Inhibits Virus Replication in Dengue Virus-Infected Vero Cells. Molecules 2021, 26, 3118. [Google Scholar] [CrossRef] [PubMed]
- Coulerie, P.; Maciuk, A.; Lebouvier, N.; Hnawia, E.; Guillemot, J.C.; Canard, B.; Figadère, B.; Nour, M. Phytochemical Study of Myrtopsis corymbosa, Perspectives for Anti-dengue Natural Compound Research. Rec. Nat. Prod. 2013, 7, 250. [Google Scholar]
- Shimizu, H.; Saito, A.; Mikuni, J.; Nakayama, E.E.; Koyama, H.; Honma, T.; Shirouzu, M.; Sekine, S.-I.; Shioda, T. Discovery of a small molecule inhibitor targeting dengue virus NS5 RNA-dependent RNA polymerase. PLoS Negl. Trop. Dis. 2019, 13, e0007894. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, S.M.; Watowich, S.J. Use of parallel validation high-throughput screens to reduce false positives and identify novel dengue NS2B-NS3 protease inhibitors. Antivir. Res. 2012, 93, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Basavannacharya, C.; Vasudevan, S.G. Suramin inhibits helicase activity of NS3 protein of dengue virus in a fluorescence-based high throughput assay format. Biochem. Biophys. Res. Commun. 2014, 453, 539–544. [Google Scholar] [CrossRef]
- Sweeney, N.L.; Hanson, A.M.; Mukherjee, S.; Ndjomou, J.; Geiss, B.J.; Steel, J.J.; Frankowski, K.J.; Li, K.; Schoenen, F.J.; Frick, D.N. Benzothiazole and Pyrrolone Flavivirus Inhibitors Targeting the Viral Helicase. ACS Infect. Dis. 2015, 1, 140–148. [Google Scholar] [CrossRef]
- Ndjomou, J.; Kolli, R.; Mukherjee, S.; Shadrick, W.R.; Hanson, A.M.; Sweeney, N.L.; Bartczak, D.; Li, K.; Frankowski, K.J.; Schoenen, F.J.; et al. Fluorescent primuline derivatives inhibit hepatitis C virus NS3-catalyzed RNA unwinding, peptide hydrolysis and viral replicase formation. Antivir. Res. 2012, 96, 245–255. [Google Scholar] [CrossRef]
- Rothan, H.A.; Abdulrahman, A.Y.; Khazali, A.S.; Rashid, N.N.; Chong, T.T.; Yusof, R. Carnosine exhibits significant antiviral activity against Dengue and Zika virus. J. Pept. Sci. 2019, 25, e3196. [Google Scholar] [CrossRef]
- Jia, F.; Zou, G.; Fan, J.; Yuan, Z. Identification of palmatine as an inhibitor of West Nile virus. Arch. Virol. 2010, 155, 1325–1329. [Google Scholar] [CrossRef]
- Nitsche, C.; Behnam, M.A.; Steuer, C.; Klein, C.D. Retro peptide-hybrids as selective inhibitors of the Dengue virus NS2B-NS3 protease. Antivir. Res. 2012, 94, 72–79. [Google Scholar] [CrossRef]
- Steuer, C.; Gege, C.; Fischl, W.; Heinonen, K.H.; Bartenschlager, R.; Klein, C.D. Synthesis and biological evaluation of α-ketoamides as inhibitors of the Dengue virus protease with antiviral activity in cell-culture. Bioorganic Med. Chem. 2011, 19, 4067–4074. [Google Scholar] [CrossRef]
- Bodenreider, C.; Beer, D.; Keller, T.; Sonntag, S.; Wen, D.; Yap, L.; Yau, Y.H.; Shochat, S.G.; Huang, D.; Zhou, T.; et al. A fluorescence quenching assay to discriminate between specific and nonspecific inhibitors of dengue virus protease. Anal. Biochem. 2009, 395, 195–204. [Google Scholar] [CrossRef]
- Cregar-Hernandez, L.; Jiao, G.-S.; Johnson, A.T.; Lehrer, A.T.; Wong, T.A.S.; Margosiak, S.A. Small Molecule Pan-Dengue and West Nile Virus NS3 Protease Inhibitors. Antivir. Chem. Chemother. 2011, 21, 209–217. [Google Scholar] [CrossRef]
- Tomlinson, S.M.; Malmstrom, R.D.; Russo, A.; Mueller, N.; Pang, Y.-P.; Watowich, S.J. Structure-based discovery of dengue virus protease inhibitors. Antivir. Res. 2009, 82, 110–114. [Google Scholar] [CrossRef]
- Lai, H.; Dou, D.; Aravapalli, S.; Teramoto, T.; Lushington, G.; Mwania, T.M.; Alliston, K.R.; Eichhorn, D.; Padmanabhan, R.; Groutas, W.C. Design, synthesis and characterization of novel 1,2-benzisothiazol-3(2H)-one and 1,3,4-oxadiazole hybrid derivatives: Potent inhibitors of Dengue and West Nile virus NS2B/NS3 proteases. Bioorganic Med. Chem. 2012, 21, 102–113. [Google Scholar] [CrossRef]
- Wu, H.; Bock, S.; Snitko, M.; Berger, T.; Weidner, T.; Holloway, S.; Kanitz, M.; Diederich, W.E.; Steuber, H.; Walter, C.; et al. Novel Dengue Virus NS2B/NS3 Protease Inhibitors. Antimicrob. Agents Chemother. 2015, 59, 1100–1109. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Manzano, M.; Teramoto, T.; Pilankatta, R.; Padmanabhan, R. High-throughput screening for the identification of small-molecule inhibitors of the flaviviral protease. Antivir. Res. 2016, 134, 6–16. [Google Scholar] [CrossRef]
- Pambudi, S.; Kawashita, N.; Phanthanawiboon, S.; Omokoko, M.D.; Masrinoul, P.; Yamashita, A.; Limkittikul, K.; Yasunaga, T.; Takagi, T.; Ikuta, K.; et al. A small compound targeting the interaction between nonstructural proteins 2B and 3 inhibits dengue virus replication. Biochem. Biophys. Res. Commun. 2013, 440, 393–398. [Google Scholar] [CrossRef]
- Behnam, M.A.M.; Graf, D.; Bartenschlager, R.; Zlotos, D.P.; Klein, C.D. Discovery of Nanomolar Dengue and West Nile Virus Protease Inhibitors Containing a 4-Benzyloxyphenylglycine Residue. J. Med. Chem. 2015, 58, 9354–9370. [Google Scholar] [CrossRef]
- Rothan, H.A.; Bahrani, H.; Rahman, N.A.; Yusof, R. Identification of natural antimicrobial agents to treat dengue infection: In vitro analysis of latarcin peptide activity against dengue virus. BMC Microbiol. 2014, 14, 140. [Google Scholar] [CrossRef]
- Yang, C.-C.; Hu, H.-S.; Wu, R.-H.; Wu, S.-H.; Lee, S.-J.; Jiaang, W.-T.; Chern, J.-H.; Huang, Z.-S.; Wu, H.-N.; Chang, C.-M.; et al. A Novel Dengue Virus Inhibitor, BP13944, Discovered by High-Throughput Screening with Dengue Virus Replicon Cells Selects for Resistance in the Viral NS2B/NS3 Protease. Antimicrob. Agents Chemother. 2014, 58, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-W.; Mao, F.; Ye, Y.; Li, J.; Xu, C.-L.; Luo, X.-M.; Chen, J.; Shen, X. Policresulen, a novel NS2B/NS3 protease inhibitor, effectively inhibits the replication of DENV2 virus in BHK-21 cells. Acta Pharmacol. Sin. 2015, 36, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Hsieh, Y.-C.; Lee, S.-J.; Wu, S.-H.; Liao, C.-L.; Tsao, C.-H.; Chao, Y.-S.; Chern, J.-H.; Wu, C.-P.; Yueh, A. Novel Dengue Virus-Specific NS2B/NS3 Protease Inhibitor, BP2109, Discovered by a High-Throughput Screening Assay. Antimicrob. Agents Chemother. 2011, 55, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Raut, R.; Beesetti, H.; Tyagi, P.; Khanna, I.; Jain, S.K.; Jeankumar, V.U.; Yogeeswari, P.; Sriram, D.; Swaminathan, S. A small molecule inhibitor of dengue virus type 2 protease inhibits the replication of all four dengue virus serotypes in cell culture. Virol. J. 2015, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Weigel, L.F.; Nitsche, C.; Graf, D.; Bartenschlager, R.; Klein, C.D. Phenylalanine and Phenylglycine Analogues as Arginine Mimetics in Dengue Protease Inhibitors. J. Med. Chem. 2015, 58, 7719–7733. [Google Scholar] [CrossRef]
- Li, L.; Basavannacharya, C.; Chan, K.W.K.; Shang, L.; Vasudevan, S.G.; Yin, Z. Structure-guided Discovery of a Novel Non-peptide Inhibitor of Dengue Virus NS2B-NS3 Protease. Chem. Biol. Drug Des. 2015, 86, 255–264. [Google Scholar] [CrossRef]
- de Wispelaere, M.; LaCroix, A.J.; Yang, P.L. The Small Molecules AZD0530 and Dasatinib Inhibit Dengue Virus RNA Replication via Fyn Kinase. J. Virol. 2013, 87, 7367–7381. [Google Scholar] [CrossRef]
- Hernandez-Morales, I.; Geluykens, P.; Clynhens, M.; Strijbos, R.; Goethals, O.; Megens, S.; Verheyen, N.; Last, S.; McGowan, D.; Coesemans, E.; et al. Characterization of a dengue NS4B inhibitor originating from an HCV small molecule library. Antivir. Res. 2017, 147, 149–158. [Google Scholar] [CrossRef]
- Moquin, S.A.; Simon, O.; Karuna, R.; Lakshminarayana, S.B.; Yokokawa, F.; Wang, F.; Saravanan, C.; Zhang, J.; Day, C.W.; Chan, K.; et al. NITD-688, a pan-serotype inhibitor of the dengue virus NS4B protein, shows favorable pharmacokinetics and efficacy in preclinical animal models. Sci. Transl. Med. 2021, 13, eabb2181. [Google Scholar] [CrossRef]
- Kaptein, S.J.F.; Goethals, O.; Kiemel, D.; Marchand, A.; Kesteleyn, B.; Bonfanti, J.-F.; Bardiot, D.; Stoops, B.; Jonckers, T.H.M.; Dallmeier, K.; et al. A pan-serotype dengue virus inhibitor targeting the NS3–NS4B interaction. Nature 2021, 598, 504–509. [Google Scholar] [CrossRef]
- Nobori, H.; Toba, S.; Yoshida, R.; Hall, W.W.; Orba, Y.; Sawa, H.; Sato, A. Identification of Compound-B, a novel anti-dengue virus agent targeting the non-structural protein 4A. Antivir. Res. 2018, 155, 60–66. [Google Scholar] [CrossRef]
- Vernekar, S.K.V.; Qiu, L.; Zhang, J.; Kankanala, J.; Li, H.; Geraghty, R.J.; Wang, Z. 5′-Silylated 3′-1,2,3-triazolyl Thymidine Analogues as Inhibitors of West Nile Virus and Dengue Virus. J. Med. Chem. 2015, 58, 4016–4028. [Google Scholar] [CrossRef]
- Lim, S.P.; Sonntag, L.S.; Noble, C.; Nilar, S.H.; Ng, R.H.; Zou, G.; Monaghan, P.; Chung, K.Y.; Dong, H.; Liu, B.; et al. Small Molecule Inhibitors That Selectively Block Dengue Virus Methyltransferase. J. Biol. Chem. 2011, 286, 6233–6240. [Google Scholar] [CrossRef]
- Bullard, K.M.; Gullberg, R.C.; Soltani, E.; Steel, J.J.; Geiss, B.J.; Keenan, S.M. Murine Efficacy and Pharmacokinetic Evaluation of the Flaviviral NS5 Capping Enzyme 2-Thioxothiazolidin-4-One Inhibitor BG-323. PLoS ONE 2015, 10, e0130083. [Google Scholar] [CrossRef]
- Brecher, M.; Chen, H.; Li, Z.; Banavali, N.K.; Jones, S.A.; Zhang, J.; Kramer, L.D.; Li, H. Identification and Characterization of Novel Broad-Spectrum Inhibitors of the Flavivirus Methyltransferase. ACS Infect. Dis. 2015, 1, 340–349. [Google Scholar] [CrossRef]
- Allard, P.-M.; Leyssen, P.; Martin, M.-T.; Bourjot, M.; Dumontet, V.; Eydoux, C.; Guillemot, J.-C.; Canard, B.; Poullain, C.; Guéritte, F.; et al. Antiviral chlorinated daphnane diterpenoid orthoesters from the bark and wood of Trigonostemon cherrieri. Phytochemistry 2012, 84, 160–168. [Google Scholar] [CrossRef]
- Allard, P.-M.; Dau, E.T.H.; Eydoux, C.; Guillemot, J.-C.; Dumontet, V.; Poullain, C.; Canard, B.; Guéritte, F.; Litaudon, M. Alkylated Flavanones from the Bark of Cryptocarya chartacea As Dengue Virus NS5 Polymerase Inhibitors. J. Nat. Prod. 2011, 74, 2446–2453. [Google Scholar] [CrossRef]
- Coulerie, P.; Maciuk, A.; Eydoux, C.; Hnawia, E.; Lebouvier, N.; Figadère, B.; Guillemot, J.-C.; Nour, M. New Inhibitors of the DENV-NS 5 RdRp from Carpolepis laurifolia as Potential Antiviral Drugs for Dengue Treatment. Rec. Nat. Prod. 2014, 8, 286. [Google Scholar]
- Cannalire, R.; Chan, K.W.K.; Burali, M.S.; Gwee, C.P.; Wang, S.; Astolfi, A.; Massari, S.; Sabatini, S.; Tabarrini, O.; Mastrangelo, E.; et al. Pyridobenzothiazolones Exert Potent Anti-Dengue Activity by Hampering Multiple Functions of NS5 Polymerase. ACS Med. Chem. Lett. 2020, 11, 773–782. [Google Scholar] [CrossRef]
- Bourjot, M.; Leyssen, P.; Eydoux, C.; Guillemot, J.-C.; Canard, B.; Rasoanaivo, P.; Guéritte, F.; Litaudon, M. Chemical constituents of Anacolosa pervilleana and their antiviral activities. Fitoterapia 2012, 83, 1076–1080. [Google Scholar] [CrossRef]
- Olsen, D.B.; Eldrup, A.B.; Bartholomew, L.; Bhat, B.; Bosserman, M.R.; Ceccacci, A.; Colwell, L.F.; Fay, J.F.; Flores, O.A.; Getty, K.L.; et al. A 7-Deaza-Adenosine Analog Is a Potent and Selective Inhibitor of Hepatitis C Virus Replication with Excellent Pharmacokinetic Properties. Antimicrob. Agents Chemother. 2004, 48, 3944–3953. [Google Scholar] [CrossRef] [PubMed]
- Vernachio, J.H.; Bleiman, B.; Bryant, K.D.; Chamberlain, S.; Hunley, D.; Hutchins, J.; Ames, B.; Gorovits, E.; Ganguly, B.; Hall, A.; et al. INX-08189, a Phosphoramidate Prodrug of 6- O -Methyl-2′- C -Methyl Guanosine, Is a Potent Inhibitor of Hepatitis C Virus Replication with Excellent Pharmacokinetic and Pharmacodynamic Properties. Antimicrob. Agents Chemother. 2011, 55, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.K.; Wells, J.; Panchal, R.G.; Stuthman, K.S.; Garza, N.L.; Van Tongeren, S.A.; Dong, L.; Retterer, C.J.; Eaton, B.P.; Pegoraro, G.; et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 2014, 508, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Ghafar, N.A.; Karuna, R.; Fu, Y.; Lim, S.P.; Schul, W.; Gu, F.; Herve, M.; Yokohama, F.; Wang, G.; et al. Activation of Peripheral Blood Mononuclear Cells by Dengue Virus Infection Depotentiates Balapiravir. J. Virol. 2014, 88, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, Y.-L.; Schul, W.; Wang, Q.-Y.; Gu, F.; Duraiswamy, J.; Kondreddi, R.R.; Niyomrattanakit, P.; Lakshminarayana, S.B.; Goh, A.; et al. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. USA 2009, 106, 20435–20439. [Google Scholar] [CrossRef]
- Lee, J.-C.; Tseng, C.-K.; Wu, Y.-H.; Kaushik-Basu, N.; Lin, C.-K.; Chen, W.-C.; Wu, H.-N. Characterization of the activity of 2′-C-methylcytidine against dengue virus replication. Antivir. Res. 2015, 116, 1–9. [Google Scholar] [CrossRef]
- Paranjape, S.M.; Harris, E. Control of Dengue Virus Translation and Replication. Poxviruses 2009, 338, 15–34. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Yin, Z.; Duraiswamy, J.; Schul, W.; Lim, C.C.; Liu, B.; Xu, H.Y.; Qing, M.; Yip, A.; Wang, G.; et al. Inhibition of Dengue Virus RNA Synthesis by an Adenosine Nucleoside. Antimicrob. Agents Chemother. 2010, 54, 2932–2939. [Google Scholar] [CrossRef]
- Diamond, M.S.; Zachariah, M.; Harris, E. Mycophenolic Acid Inhibits Dengue Virus Infection by Preventing Replication of Viral RNA. Virology 2002, 304, 211–221. [Google Scholar] [CrossRef]
- Chiu, W.-W.; Kinney, R.M.; Dreher, T.W. Control of Translation by the 5′- and 3′-Terminal Regions of the Dengue Virus Genome. J. Virol. 2005, 79, 8303–8315. [Google Scholar] [CrossRef]
- Holden, K.L.; Harris, E. Enhancement of dengue virus translation: Role of the 3′ untranslated region and the terminal 3′ stem-loop domain. Virology 2004, 329, 119–133. [Google Scholar] [CrossRef]
- Dong, H.; Ray, D.; Ren, S.; Zhang, B.; Puig-Basagoiti, F.; Takagi, Y.; Ho, C.K.; Li, H.; Shi, P.-Y. Distinct RNA Elements Confer Specificity to Flavivirus RNA Cap Methylation Events. J. Virol. 2007, 81, 4412–4421. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Kondreddi, R.R.; Xie, X.; Rao, R.; Nilar, S.; Xu, H.Y.; Qing, M.; Chang, D.; Dong, H.; Yokokawa, F.; et al. A Translation Inhibitor That Suppresses Dengue Virus In Vitro and In Vivo. Antimicrob. Agents Chemother. 2011, 55, 4072–4080. [Google Scholar] [CrossRef]
- Low, J.S.Y.; Wu, K.X.; Chen, K.C.; Ng, M.M.-L.; Chu, J.J.H. Narasin, a Novel Antiviral Compound that Blocks Dengue Virus Protein Expression. Antivir. Ther. 2011, 16, 1203–1218. [Google Scholar] [CrossRef]
- Carocci, M.; Yang, P.L. Lactimidomycin is a broad-spectrum inhibitor of dengue and other RNA viruses. Antivir. Res. 2016, 128, 57–62. [Google Scholar] [CrossRef]
- Lee, J.K.; Chui, J.L.M.; Lee, R.C.H.; Kong, H.Y.; Chin, W.-X.; Chu, J.J.H. Antiviral activity of ST081006 against the dengue virus. Antivir. Res. 2019, 171, 104589. [Google Scholar] [CrossRef]
- Kato, F.; Ishida, Y.; Oishi, S.; Fujii, N.; Watanabe, S.; Vasudevan, S.G.; Tajima, S.; Takasaki, T.; Suzuki, Y.; Ichiyama, K.; et al. Novel antiviral activity of bromocriptine against dengue virus replication. Antivir. Res. 2016, 131, 141–147. [Google Scholar] [CrossRef]
- Holden, K.L.; Stein, D.A.; Pierson, T.C.; Ahmed, A.A.; Clyde, K.; Iversen, P.; Harris, E. Inhibition of dengue virus translation and RNA synthesis by a morpholino oligomer targeted to the top of the terminal 3′ stem–loop structure. Virology 2005, 344, 439–452. [Google Scholar] [CrossRef]
- Wen, W.; He, Z.; Jing, Q.; Hu, Y.; Lin, C.; Zhou, R.; Wang, X.; Wang, X.; Su, Y.; Yuan, J.; et al. Cellular microRNA-miR-548g-3p modulates the replication of dengue virus. J. Infect. 2015, 70, 631–640. [Google Scholar] [CrossRef]
- Castillo, J.A.; Castrillón, J.C.; Diosa-Toro, M.; Betancur, J.G.; Iii, G.S.L.; Smit, J.M.; Urcuqui-Inchima, S. Complex interaction between dengue virus replication and expression of miRNA-133a. BMC Infect. Dis. 2015, 16, 29. [Google Scholar] [CrossRef]
- Castrillón-Betancur, J.C.; Urcuqui-Inchima, S. Overexpression of miR-484 and miR-744 in Vero cells alters Dengue virus replication. Memórias Inst. Oswaldo Cruz 2017, 112, 281–291. [Google Scholar] [CrossRef]
- Yeh, S.-C.; Diosa-Toro, M.; Tan, W.-L.; Rachenne, F.; Hain, A.; Yeo, C.P.X.; Bribes, I.; Xiang, B.W.W.; Kannan, G.S.; Manuel, M.C.; et al. Characterization of dengue virus 3’UTR RNA binding proteins in mosquitoes reveals that AeStaufen reduces subgenomic flaviviral RNA in saliva. PLoS Pathog. 2022, 18, e1010427. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-C.; Chuo, V.; Ng, W.C.; Neo, S.P.; Pompon, J.; Gunaratne, J.; Ooi, E.E.; Garcia-Blanco, M.A. Identification and characterization of host proteins bound to dengue virus 3′ UTR reveal an antiviral role for quaking proteins. RNA 2018, 24, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Barnard, T.R.; Abram, Q.H.; Lin, Q.F.; Wang, A.B.; Sagan, S.M. Molecular Determinants of Flavivirus Virion Assembly. Trends Biochem. Sci. 2021, 46, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zou, J.; Zhang, X.; Zhou, Y.; Routh, A.L.; Kang, C.; Popov, V.L.; Chen, X.; Wang, Q.-Y.; Dong, H.; et al. Dengue NS2A Protein Orchestrates Virus Assembly. Cell Host Microbe 2019, 26, 606–622.e8. [Google Scholar] [CrossRef]
- Patkar, C.G.; Kuhn, R.J. Yellow Fever Virus NS3 Plays an Essential Role in Virus Assembly Independent of Its Known Enzymatic Functions. J. Virol. 2008, 82, 3342–3352. [Google Scholar] [CrossRef]
- Voßmann, S.; Wieseler, J.; Kerber, R.; Kümmerer, B.M. A Basic Cluster in the N Terminus of Yellow Fever Virus NS2A Contributes to Infectious Particle Production. J. Virol. 2015, 89, 4951–4965. [Google Scholar] [CrossRef]
- Byk, L.A.; Gamarnik, A.V. Properties and Functions of the Dengue Virus Capsid Protein. Annu. Rev. Virol. 2016, 3, 263–281. [Google Scholar] [CrossRef]
- Nanaware, N.; Banerjee, A.; Bagchi, S.M.; Bagchi, P.; Mukherjee, A. Dengue Virus Infection: A Tale of Viral Exploitations and Host Responses. Viruses 2021, 13, 1967. [Google Scholar] [CrossRef]
- Bressanelli, S.; Stiasny, K.; Allison, S.L.; Stura, E.A.; Duquerroy, S.; Lescar, J.; Heinz, F.X.; Rey, F.A. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004, 23, 728–738. [Google Scholar] [CrossRef]
- Yu, I.-M.; Holdaway, H.A.; Chipman, P.R.; Kuhn, R.J.; Rossmann, M.G.; Chen, J. Association of the pr Peptides with Dengue Virus at Acidic pH Blocks Membrane Fusion. J. Virol. 2009, 83, 12101–12107. [Google Scholar] [CrossRef]
- Duan, X.; Lu, X.; Li, J.; Liu, Y. Novel binding between pre-membrane protein and vacuolar ATPase is required for efficient dengue virus secretion. Biochem. Biophys. Res. Commun. 2008, 373, 319–324. [Google Scholar] [CrossRef]
- Perera, R.; Kuhn, R.J. Structural proteomics of dengue virus. Curr. Opin. Microbiol. 2008, 11, 369–377. [Google Scholar] [CrossRef]
- Yu, I.-M.; Zhang, W.; Holdaway, H.A.; Li, L.; Kostyuchenko, V.A.; Chipman, P.R.; Kuhn, R.J.; Rossmann, M.G.; Chen, J. Structure of the Immature Dengue Virus at Low pH Primes Proteolytic Maturation. Science 2008, 319, 1834–1837. [Google Scholar] [CrossRef]
- Martínez-Gutierrez, M.; Castellanos, J.E.; Gallego-Gómez, J.C. Statins Reduce Dengue Virus Production via Decreased Virion Assembly. Intervirology 2011, 54, 202–216. [Google Scholar] [CrossRef]
- Chu, J.J.H.; Yang, P.L. c-Src protein kinase inhibitors block assembly and maturation of dengue virus. Proc. Natl. Acad. Sci. USA 2007, 104, 3520–3525. [Google Scholar] [CrossRef]
- Hishiki, T.; Kato, F.; Tajima, S.; Toume, K.; Umezaki, M.; Takasaki, T.; Miura, T. Hirsutine, an Indole Alkaloid of Uncaria rhynchophylla, Inhibits Late Step in Dengue Virus Lifecycle. Front. Microbiol. 2017, 8, 1674. [Google Scholar] [CrossRef]
- Whitby, K.; Pierson, T.C.; Geiss, B.; Lane, K.; Engle, M.; Zhou, Y.; Doms, R.W.; Diamond, M.S. Castanospermine, a Potent Inhibitor of Dengue Virus Infection In Vitro and In Vivo. J. Virol. 2005, 79, 8698–8706. [Google Scholar] [CrossRef]
- Raekiansyah, M.; Mori, M.; Nonaka, K.; Agoh, M.; Shiomi, K.; Matsumoto, A.; Morita, K. Identification of novel antiviral of fungus-derived brefeldin A against dengue viruses. Trop. Med. Health 2017, 45, 32. [Google Scholar] [CrossRef]
- Bobrowski, T.; Chen, L.; Eastman, R.T.; Itkin, Z.; Shinn, P.; Chen, C.Z.; Guo, H.; Guo, H.; Zheng, W.; Michael, S.; et al. Synergistic and Antagonistic Drug Combinations against SARS-CoV-2. Mol. Ther. 2021, 29, 873–885. [Google Scholar] [CrossRef]
- Shyr, Z.A.; Cheng, Y.-S.; Lo, D.C.; Zheng, W. Drug combination therapy for emerging viral diseases. Drug Discov. Today 2021, 26, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Schul, W.; Butters, T.D.; Yip, A.; Liu, B.; Goh, A.; Lakshminarayana, S.B.; Alonzi, D.; Reinkensmeier, G.; Pan, X.; et al. Combination of α-glucosidase inhibitor and ribavirin for the treatment of dengue virus infection in vitro and in vivo. Antivir. Res. 2011, 89, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.; de Mello, C.P.; Brown, A. Antiviral Evaluation of UV-4B and Interferon-Alpha Combination Regimens against Dengue Virus. Viruses 2021, 13, 771. [Google Scholar] [CrossRef] [PubMed]
| Drug | Clinical Trial Phase | Status | ClinicalTrials.gov Identifier * |
|---|---|---|---|
| JNJ-64281802 | II | Recruiting | NCT05048875 |
| Melatonin | Not yet recruiting | NCT05034809 | |
| AT-752 | I | Recruiting | NCT05366439 |
| Zanamivir | Not yet recruiting | NCT04597437 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.F.; Wu, Y.S.; Poh, C.L. Molecular Mechanisms of Antiviral Agents against Dengue Virus. Viruses 2023, 15, 705. https://doi.org/10.3390/v15030705
Lee MF, Wu YS, Poh CL. Molecular Mechanisms of Antiviral Agents against Dengue Virus. Viruses. 2023; 15(3):705. https://doi.org/10.3390/v15030705
Chicago/Turabian StyleLee, Michelle Felicia, Yuan Seng Wu, and Chit Laa Poh. 2023. "Molecular Mechanisms of Antiviral Agents against Dengue Virus" Viruses 15, no. 3: 705. https://doi.org/10.3390/v15030705
APA StyleLee, M. F., Wu, Y. S., & Poh, C. L. (2023). Molecular Mechanisms of Antiviral Agents against Dengue Virus. Viruses, 15(3), 705. https://doi.org/10.3390/v15030705






