Role of T Cells in Vaccine-Mediated Immunity against Marek’s Disease
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Chickens
2.2. Viruses
2.3. Hybridoma Cell Cultures
2.4. Purification of Monoclonal Antibodies
2.5. Peripheral Blood Mononuclear Cells Isolation
2.6. Monoclonal Antibodies Used in Flowcytometric Analysis of PBMC
2.7. Flowcytometric Analysis of PBMC
2.8. Characterization of Anti-CD4 and Anti-CD8 Monoclonal Antibodies
2.9. PCR Analysis of MDV-Encoded pp38 Gene
2.10. MDV Genome Copy Number Assay
2.11. Immunohistochemistry
2.12. Statistical Analysis
2.13. Experimental Design
3. Results
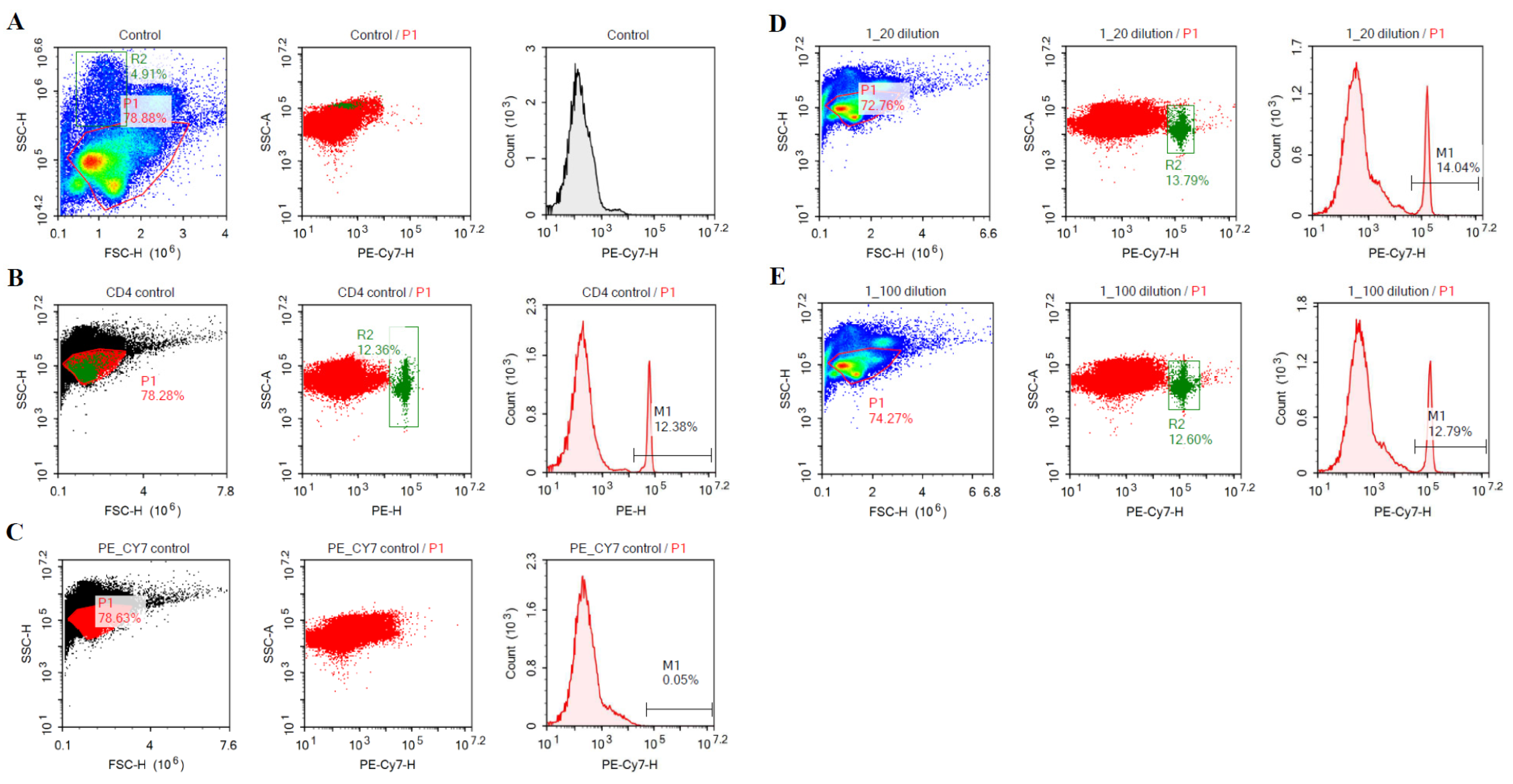
3.1. Purification and Binding Specificity of Anti-CD4 and Anti-CD-8 mAbs
3.2. T Cell Depletion
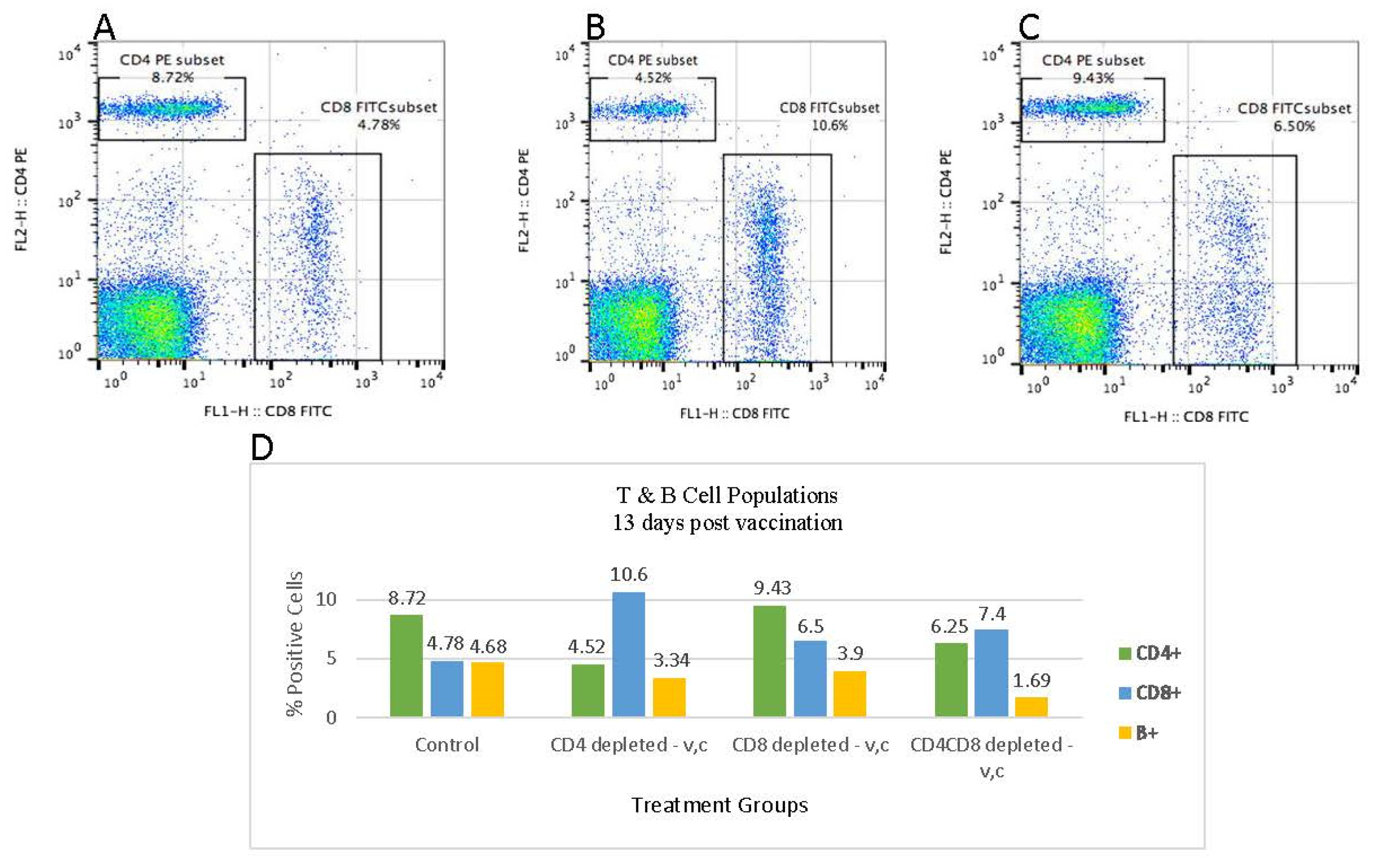
3.3. Flowcytometric Analysis of PBMC at 11 Days Post Antibody Treatment
3.4. Flowcytometric Analysis of PBMC at 13 Days Post Termination of Antibody Treatment
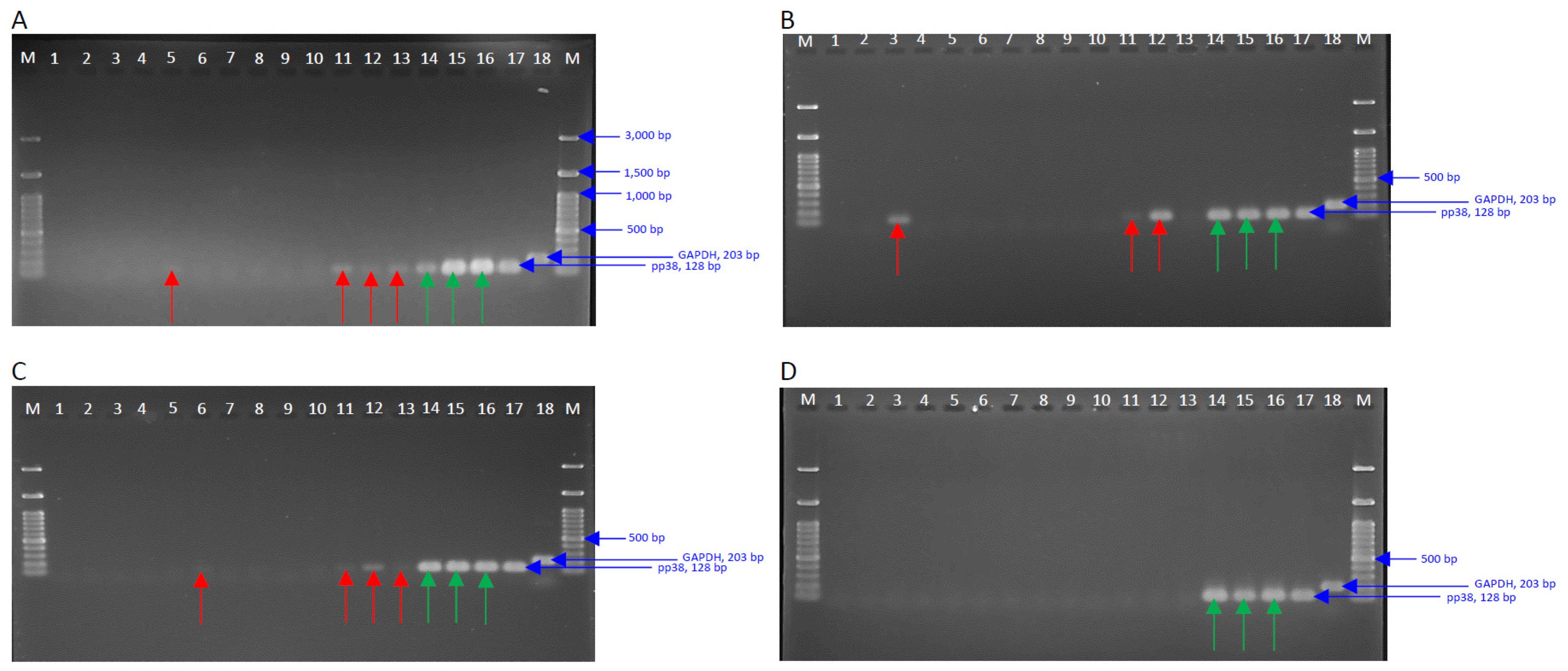
3.5. Detection of Viral Genome in the Spleen Tissues at 5-, 10-, 20-, and 57-dpi
3.6. Flow Cytometric Analysis of Binding Specificity of Anti-CD4 mAb
3.7. MDV Genomes Copy Number in the Skin Tissues
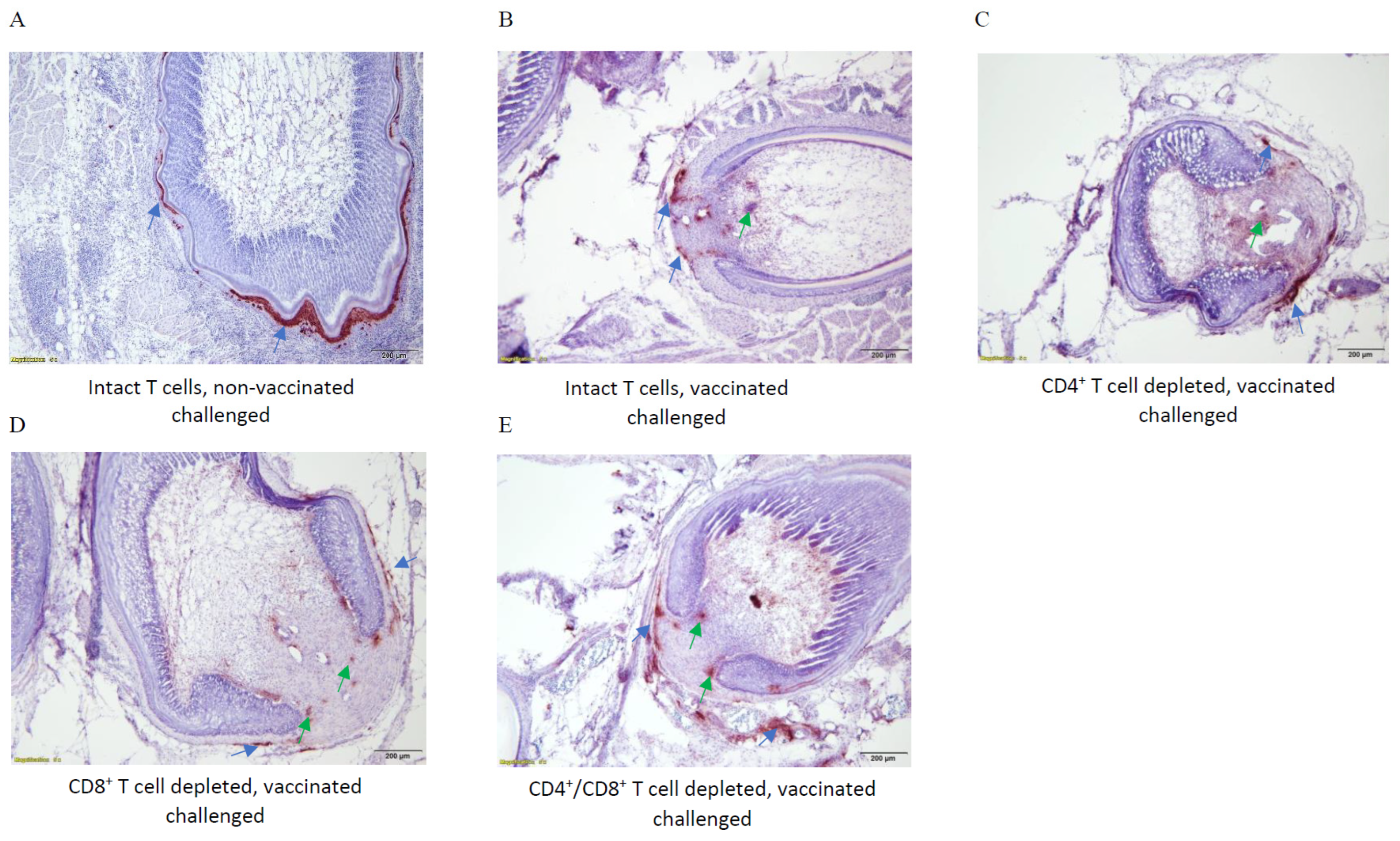
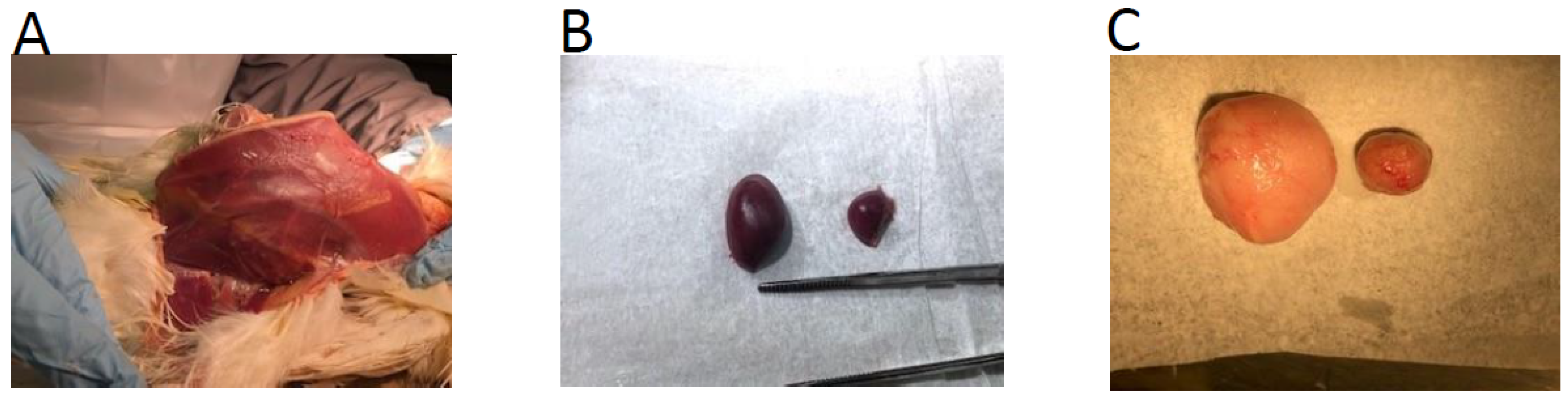
3.8. Immunohistochemical Analysis of MDV Replication
3.9. Protection Efficacy of CVI/988 Rispens in Birds with Depleted T Cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calnek, B.W.; Schat, K.A.; Peckham, M.C.; Fabricant, J. Field trials with a bivalent vaccine (HVT and SB-1) against Marek’s disease. Avian Dis. 1983, 27, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Calnek, B.W. Pathogenesis of Marek’s disease virus infection. Curr. Top. Microbiol. Immunol. 2001, 255, 25–55. [Google Scholar] [PubMed]
- Ross, N.L. T-cell transformation by Marek’s disease virus. Trends Microbiol. 1999, 7, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Parcells, M.S.; Arumugaswami, V.; Prigge, J.T.; Pandya, K.; Dienglewicz, R.L. Marek’s disease virus reactivation from latency: Changes in gene expression at the origin of replication. Poult. Sci. 2003, 82, 893–898. [Google Scholar] [CrossRef]
- Schermuly, J.; Greco, A.; Hartle, S.; Osterrieder, N.; Kaufer, B.B.; Kaspers, B. In vitro model for lytic replication, latency, and transformation of an oncogenic alphaherpesvirus. Proc. Natl. Acad. Sci. USA 2015, 112, 7279–7284. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.F.; Liu, X.; Witter, R.L. Monoclonal antibodies with specificity for three different serotypes of Marek’s disease viruses in chickens. J. Immunol. 1983, 130, 1003–1006. [Google Scholar] [CrossRef]
- Schat, K.; Nair, V. Marek’s disease. In Diseases of Poultry, 12th ed.; Saif, Y.M., Fadly, A.M., Glisson, J.R., MacDougald, L.R., Nolan, L.R., Swyane, D.E., Eds.; Blackwell Publishing Professional: Ames, IA, USA, 2008; pp. 452–514. [Google Scholar]
- Okazaki, W.; Purchase, H.G.; Burmester, B.R. Protection against Marek’s disease by vaccination with a herpesvirus of turkeys. Avian Dis. 1970, 14, 413–429. [Google Scholar] [CrossRef]
- Rispens, B.H.; van Vloten, H.; Mastenbroek, N.; Maas, J.L.; Schat, K.A. Control of Marek’s disease in the Netherlands. II. Field trials on vaccination with an avirulent strain (CVI 988) of Marek’s disease virus. Avian Dis. 1972, 16, 126–138. [Google Scholar] [CrossRef]
- Schat, K.A.; Calnek, B.W. Characterization of an apparently nononcogenic Marek’s disease virus. J. Natl. Cancer Inst. 1978, 60, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Witter, R.L.; Nazerian, K.; Purchase, H.G.; Burgoyne, G.H. Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek’s disease virus. Am. J. Vet. Res. 1970, 31, 525–538. [Google Scholar]
- Venugopal, K. Marek’s disease: An update on oncogenic mechanisms and control. Res. Vet. Sci. 2000, 69, 17–23. [Google Scholar] [CrossRef]
- Baigent, S.J.; Kgosana, L.B.; Gamawa, A.A.; Smith, L.P.; Read, A.F.; Nair, V.K. Relationship between levels of very virulent MDV in poultry dust and in feather tips from vaccinated chickens. Avian Dis. 2013, 57, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Calnek, B.W.; Adldinger, H.K.; Kahn, D.E. Feather follicle epithelium: A source of enveloped and infectious cell-free herpesvirus from Marek’s disease. Avian Dis. 1970, 14, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Haq, K.; Fear, T.; Ibraheem, A.; Abdul-Careem, M.F.; Sharif, S. Influence of vaccination with CVI988/Rispens on load and replication of a very virulent Marek’s disease virus strain in feathers of chickens. Avian Pathol. 2012, 41, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Ohashi, K.; Morimura, T.; Sugimoto, C.; Onuma, M. Re-isolation of Marek’s disease virus from T cell subsets of vaccinated and non-vaccinated chickens. Arch. Virol. 1999, 144, 45–54. [Google Scholar] [CrossRef]
- Witter, R.L.; Solomon, J.J.; Champion, L.R.; Nazerian, K. Long-term studies of Marek’s disease infection in individual chickens. Avian Dis. 1971, 15, 346–365. [Google Scholar] [CrossRef] [PubMed]
- Schat, K.A.; Xing, Z. Specific and nonspecific immune responses to Marek’s disease virus. Dev. Comp. Immunol. 2000, 24, 201–221. [Google Scholar] [CrossRef]
- Markowski-Grimsrud, C.J.; Schat, K.A. Cytotoxic T lymphocyte responses to Marek’s disease herpesvirus-encoded glycoproteins. Vet. Immunol. Immunopathol. 2002, 90, 133–144. [Google Scholar] [CrossRef]
- Lanier, L.L. NK cell recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 1989, 47, 187–376. [Google Scholar] [CrossRef]
- Trinchieri, G. Natural killer cells wear different hats: Effector cells of innate resistance and regulatory cells of adaptive immunity and of hematopoiesis. Semin. Immunol. 1995, 7, 83–88. [Google Scholar] [CrossRef]
- Heller, E.D.; Schat, K.A. Enhancement of natural killer cell activity by Marek’s disease vaccines. Avian Pathol. 1987, 16, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Lessard, M.; Hutchings, D.L.; Spencer, J.L.; Lillehoj, H.S.; Gavora, J.S. Influence of Marek’s disease virus strain AC-1 on cellular immunity in birds carrying endogenous viral genes. Avian Dis. 1996, 40, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.M.; Okazaki, W. Natural killer cell activity in chickens: Target cell analysis and effect of antithymocyte serum on effector cells. Infect. Immun. 1981, 31, 1078–1085. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, J.; Venugopal, K. The importance of MHC for Rous sarcoma virus and Marek’s disease virus—Some Payne-ful considerations. Avian Pathol. 1998, 27, S82–S87. [Google Scholar] [CrossRef] [Green Version]
- Bertzbach, L.D.; van Haarlem, D.A.; Hartle, S.; Kaufer, B.B.; Jansen, C.A. Marek’s Disease Virus Infection of Natural Killer Cells. Microorganisms 2019, 7, 588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karupiah, G.; Xie, Q.W.; Buller, R.M.; Nathan, C.; Duarte, C.; MacMicking, J.D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science 1993, 261, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Vilcek, J.; Oliveira, I.C. Recent progress in the elucidation of interferon-gamma actions: Molecular biology and biological functions. Int. Arch. Allergy Immunol. 1994, 104, 311–316. [Google Scholar] [CrossRef]
- Xing, Z.; Schat, K.A. Inhibitory effects of nitric oxide and gamma interferon on in vitro and in vivo replication of Marek’s disease virus. J. Virol. 2000, 74, 3605–3612. [Google Scholar] [CrossRef] [Green Version]
- Djeraba, A.; Musset, E.; Bernardet, N.; Le Vern, Y.; Quere, P. Similar pattern of iNOS expression, NO production and cytokine response in genetic and vaccination-acquired resistance to Marek’s disease. Vet. Immunol. Immunopathol. 2002, 85, 63–75. [Google Scholar] [CrossRef]
- Witter, R.L. Increased virulence of Marek’s disease virus field isolates. Avian Dis. 1997, 41, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Witter, R.L.; Kreager, K.S. Serotype 1 viruses modified by backpassage or insertional mutagenesis: Approaching the threshold of vaccine efficacy in Marek’s disease. Avian Dis. 2004, 48, 768–782. [Google Scholar] [CrossRef]
- Witter, R.L.; Calnek, B.W.; Buscaglia, C.; Gimeno, I.M.; Schat, K.A. Classification of Marek’s disease viruses according to pathotype: Philosophy and methodology. Avian Pathol. 2005, 34, 75–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimeno, I.M. Marek’s disease vaccines: A solution for today but a worry for tomorrow? Vaccine 2008, 26 (Suppl. S3), C31–C41. [Google Scholar] [CrossRef] [PubMed]
- Nair, V. Evolution of Marek’s disease—A paradigm for incessant race between the pathogen and the host. Vet. J. 2005, 170, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Niikura, M.; Kim, T.; Silva, R.F.; Dodgson, J.; Cheng, H.H. Virulent Marek’s disease virus generated from infectious bacterial artificial chromosome clones with complete DNA sequence and the implication of viral genetic homogeneity in pathogenesis. J. Gen. Virol. 2011, 92, 598–607. [Google Scholar] [CrossRef]
- Kondo, T.; Hattori, M.; Kodama, H.; Onuma, M.; Mikami, T. Characterization of two monoclonal antibodies which recognize different subpopulations of chicken T lymphocytes. Jpn J. Vet. Res. 1990, 38, 11–17. [Google Scholar] [PubMed]
- Kondo, T.; Hattori, M.; Kodama, H.; Onuma, M.; Mikami, T. Production and characterization of monoclonal antibodies against chicken lymphocyte surface antigens. Nihon Juigaku Zasshi 1990, 52, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Okada, K.; Tanaka, Y.; Murakami, K.; Chiba, S.; Morimura, T.; Hattori, M.; Goryo, M.; Onuma, M. Phenotype analysis of lymphoid cells in Marek’s disease of CD4(+) or CD8(+) T-cell-deficient chickens: Occurrence of double negative T-cell tumour. Avian Pathol. 1997, 26, 525–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimura, T.; Ohashi, K.; Sugimoto, C.; Onuma, M. Pathogenesis of Marek’s disease (MD) and possible mechanisms of immunity induced by MD vaccine. J. Vet. Med. Sci. 1998, 60, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umthong, S.; Dunn, J.R.; Cheng, H.H. Depletion of CD8alphabeta(+) T Cells in Chickens Demonstrates Their Involvement in Protective Immunity towards Marek’s Disease with Respect to Tumor Incidence and Vaccinal Protection. Vaccines 2020, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Morimura, T.; Cho, K.O.; Kudo, Y.; Hiramoto, Y.; Ohashi, K.; Hattori, M.; Sugimoto, C.; Onuma, M. Anti-viral and anti-tumor effects induced by an attenuated Marek’s disease virus in CD4- or CD8-deficient chickens. Arch. Virol. 1999, 144, 1809–1818. [Google Scholar] [CrossRef]
- Yamamoto, H.; Hattori, M.; Ohashi, K.; Sugimoto, C.; Onuma, M. Characterization of extrathymic T cells of chickens. Vet. Immunol. Immunopathol. 1996, 49, 375–386. [Google Scholar] [CrossRef]
- Heidari, M.; Delekta, P.C. Transcriptomic Analysis of Host Immune Response in the Skin of Chickens Infected with Marek’s Disease Virus. Viral. Immunol. 2017, 30, 377–387. [Google Scholar] [CrossRef]
- Chen, M.; Payne, W.S.; Hunt, H.; Zhang, H.; Holmen, S.L.; Dodgson, J.B. Inhibition of Marek’s disease virus replication by retroviral vector-based RNA interference. Virology 2008, 377, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidari, M.; Zhang, H.; Hearn, C.; Sunkara, L. B cells do not play a role in vaccine-mediated immunity against Marek’s disease. Vaccine X 2022, 10, 100128. [Google Scholar] [CrossRef] [PubMed]
- Davison, F.; Nair, V. Use of Marek’s disease vaccines: Could they be driving the virus to increasing virulence? Expert Rev. Vaccines 2005, 4, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Faiz, N.M.; Cortes, A.L.; Guy, J.S.; Fogle, J.E.; Gimeno, I.M. Efficacy of various Marek’s disease vaccines protocols for prevention of Marek’s disease virus-induced immunosuppression. Vaccine 2016, 34, 4180–4187. [Google Scholar] [CrossRef]
- Dunn, J.R.; Gimeno, I.M. Current status of Marek’s disease in the United States and worldwide based on a questionnaire survey. Avian Dis. 2013, 57, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.F.; Zhang, H.; Heidari, M.; Lupiani, B.; Reddy, S.M. Evaluation of factors affecting vaccine efficacy of recombinant Marek’s disease virus lacking the Meq oncogene in chickens. Avian Dis. 2011, 55, 172–179. [Google Scholar] [CrossRef]
- Djeraba, A.; Bernardet, N.; Dambrine, G.; Quere, P. Nitric oxide inhibits Marek’s disease virus replication but is not the single decisive factor in interferon-gamma-mediated viral inhibition. Virology 2000, 277, 58–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobel, T.W. Isolation and analysis of natural killer cells in chickens. Methods Mol. Biol. 2000, 121, 337–345. [Google Scholar] [CrossRef]
- Sharma, J.M. Natural killer cell activity in chickens exposed to Marek’s disease virus: Inhibition of activity in susceptible chickens and enhancement of activity in resistant and vaccinated chickens. Avian Dis. 1981, 25, 882–893. [Google Scholar] [CrossRef] [PubMed]
- den Haan, J.M.; Bevan, M.J. A novel helper role for CD4 T cells. Proc. Natl. Acad. Sci. USA 2000, 97, 12950–12952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, D.C. T cell-dependent B cell activation. Annu. Rev. Immunol. 1993, 11, 331–360. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, I.M.; Cortes, A.L.; Faiz, N.; Villalobos, T.; Badillo, H.; Barbosa, T. Efficacy of Various HVT Vaccines (Conventional and Recombinant) Against Marek’s Disease in Broiler Chickens: Effect of Dose and Age of Vaccination. Avian Dis. 2016, 60, 662–668. [Google Scholar] [CrossRef]
- Sharma, J.M.; Nazerian, K.; Witter, R.L. Reduced incidence of Marek’s disease gross lymphomas in T-cell-depleted chickens. J. Natl. Cancer Inst. 1977, 58, 689–692. [Google Scholar] [CrossRef]
- Calnek, B.W.; Fabricant, J.; Schat, K.A.; Murthy, K.K. Rejection of a transplantable Marek’s disease lymphoma in normal versus immunologically deficient chickens. J. Natl. Cancer Inst. 1978, 60, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Pamer, E.G. CD8 T cell responses to infectious pathogens. Annu. Rev. Immunol. 2003, 21, 29–70. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama-Kato, A.; Shojadoost, B.; Boodhoo, N.; Raj, S.; Alizadeh, M.; Fazel, F.; Fletcher, C.; Zheng, J.; Gupta, B.; Abdul-Careem, M.F.; et al. Activated Chicken Gamma Delta T Cells Are Involved in Protective Immunity against Marek’s Disease. Viruses 2023, 15, 285. [Google Scholar] [CrossRef]
- Hao, X.; Li, S.; Li, J.; Yang, Y.; Qin, A.; Shang, S. An Anti-Tumor Vaccine Against Marek’s Disease Virus Induces Differential Activation and Memory Response of gammadelta T Cells and CD8 T Cells in Chickens. Front. Immunol. 2021, 12, 645426. [Google Scholar] [CrossRef] [PubMed]
- Schusser, B.; Collarini, E.J.; Yi, H.; Izquierdo, S.M.; Fesler, J.; Pedersen, D.; Klasing, K.C.; Kaspers, B.; Harriman, W.D.; van de Lavoir, M.C.; et al. Immunoglobulin knockout chickens via efficient homologous recombination in primordial germ cells. Proc. Natl. Acad. Sci. USA 2013, 110, 20170–20175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertzbach, L.D.; Laparidou, M.; Hartle, S.; Etches, R.J.; Kaspers, B.; Schusser, B.; Kaufer, B.B. Unraveling the role of B cells in the pathogenesis of an oncogenic avian herpesvirus. Proc. Natl. Acad. Sci. USA 2018, 115, 11603–11607. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Chicken Line 15I5 × 71 Hatch day 26 Birds/Isolator | T Cell Depletion IP Day of Hatch & 3 dph * | T Cell Depletion IP/IV 6 & 9 dph | Flow to Verify T Cell Depletion 11 dph | Vaccination Rispens 2000 pfu 12 dph | T Cell Depletion IP/IV 12 & 15 dph | Flow to Verify T Cell Recovery 28 dph | Challenge rMd5 1000 pfu 29 dph | Samples Collection: 5, 10, 20, & 57 dpi |
|---|---|---|---|---|---|---|---|---|
| A: Control No Treatment | √ | √ | √ | |||||
| B: Birds with Intact T Cells | √ | √ | √ | |||||
| C: Birds with Depleted CD4+ T Cells | √ | √ | √ | √ | √ | √ | √ | √ |
| D: Birds with Depleted CD8+ T Cells | √ | √ | √ | √ | √ | √ | √ | √ |
| E: Birds with Depleted CD4+/CD8+ T Cells | √ | √ | √ | √ | √ | √ | √ | √ |
| F: Birds with Intact T Cells | √ | √ |
| Ab Treatment | Day of Hatch | 3 dph * | 6 dph | 9 dph | 11 dpi Flowcytometry to Verify T Cell Depletion | 12 dph Vaccination CVI/Rispens 2000 pfu/bird | 12 dph | 15 dph | 28 dph Flowcytometry to Verify the Recovery of T Cells | 29 dph Challenge rMd5 1000 pfu/bird |
|---|---|---|---|---|---|---|---|---|---|---|
| Anti-CD4 Injection | 200 μL a IP b | 200 μL IP | 100 μL IV c 200 μL IP | 100 μL IV 200 μL IP | √ | √ | 100 μL IV 200 μL IP | 100 μL IV 200 μL IP | √ | √ |
| Anti-CD8 Injection | 200 μL IP | 200 μL IP | 100 μL IV 200 μL IP | 100 μL IV 200 μL IP | √ | √ | 100 μL IV 200 μL IP | 100 μL IV 200 μL IP | √ | √ |
| Anti-CD4/CD8 Injection | 200 μL anti-CD4 IP 200 μL anti-CD8 IP | 200 μL anti-CD4 IP 200 μL anti-CD8 IP | 100 μL anti-CD4 IV 100 μL anti-CD8 IV 200 μL anti-CD4 IP 200 μL anti-CD8 IP | 100 μL anti-CD4 IV 100 μL anti-CD8 IV 200 μL anti-CD4 IP 200 μL anti-CD8 IP | √ | √ | 100 μL anti-CD4 IV 100 μL anti-CD8 IV 200 μL anti-CD4 IP 200 μL anti-CD8 IP | 100 μL anti-CD4 IV 100 μL anti-CD8 IV 200 μL anti-CD4 IP 200 μL anti-CD8 IP | √ | √ |
| Group * | Genome Copy Number at 57 dpi ** |
|---|---|
| Control | 0 |
| Intact T Cell—Vaccinated, Challenged | 78.44 |
| CD4-Depleted—Vaccinated, Challenged | 71.32 |
| CD8-Depleted—Vaccinated, Challenged | 0 |
| CD4-CD8-Depleted—Vaccinated, Challenged | 0 |
| Intact T Cell—Challenged | 1124.22 |
| Chicken Line 15I5 × 71 (Ab-) | Antibody Treatment | Vaccination CVI988/Rispens (2000 pfu) | Challenge rMd5 (1000 pfu) | MD Incidence | Protection Efficacy |
|---|---|---|---|---|---|
| Control Birds, Non-Treated | None | None | None | N/A | N/A |
| Birds with Intact T Cells, Vaccinated/Challenged | None | √ | √ | None | 100% |
| Birds with CD4+ T Cells Depleted, Vaccinated/Challenged | Anti-CD4 | √ | √ | None | 100% |
| Birds with CD8+ T Cells Depleted, Vaccinated/Challenged | Anti-CD8 | √ | √ | None | 100% |
| Birds with CD4+/CD8+ T Cells Depleted, Vaccinated/Challenged | Anti-CD4/CD8 | √ | √ | None | ?? |
| Intact T Cells Birds, Non-Vaccinated, Challenged | None | None | √ | 100% | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidari, M.; Zhang, H.; Sunkara, L.T.; Ahmad, S.M. Role of T Cells in Vaccine-Mediated Immunity against Marek’s Disease. Viruses 2023, 15, 648. https://doi.org/10.3390/v15030648
Heidari M, Zhang H, Sunkara LT, Ahmad SM. Role of T Cells in Vaccine-Mediated Immunity against Marek’s Disease. Viruses. 2023; 15(3):648. https://doi.org/10.3390/v15030648
Chicago/Turabian StyleHeidari, Mohammad, Huanmin Zhang, Lakshmi T Sunkara, and Syed Mudasir Ahmad. 2023. "Role of T Cells in Vaccine-Mediated Immunity against Marek’s Disease" Viruses 15, no. 3: 648. https://doi.org/10.3390/v15030648
APA StyleHeidari, M., Zhang, H., Sunkara, L. T., & Ahmad, S. M. (2023). Role of T Cells in Vaccine-Mediated Immunity against Marek’s Disease. Viruses, 15(3), 648. https://doi.org/10.3390/v15030648






