Tropism of Puumala orthohantavirus and Endoparasite Coinfection in the Bank Vole Reservoir
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Study Design
2.2. Indirect IgG ELISA and S Segment-Specific RT-PCR for Detection of Anti-PUUV-IgG Antibodies and PUUV RNA
2.3. Phylogenetic Analyses of PUUV and Cytochrome b Sequences
2.4. Hematoxylin and Eosin Staining (H&E) and Immunohistochemistry (IHC) for Detection of Histopathological Changes and PUUV N-Protein
2.5. In Situ Hybridization (ISH) for Detection of Positive-Strand RNA
3. Results
3.1. Serological Analyses for Anti-PUUV-IgG, Demonstration of PUUV RNA by S RT-PCR and Phylogenetic Analyses of PUUV and Cytochrome b Sequences
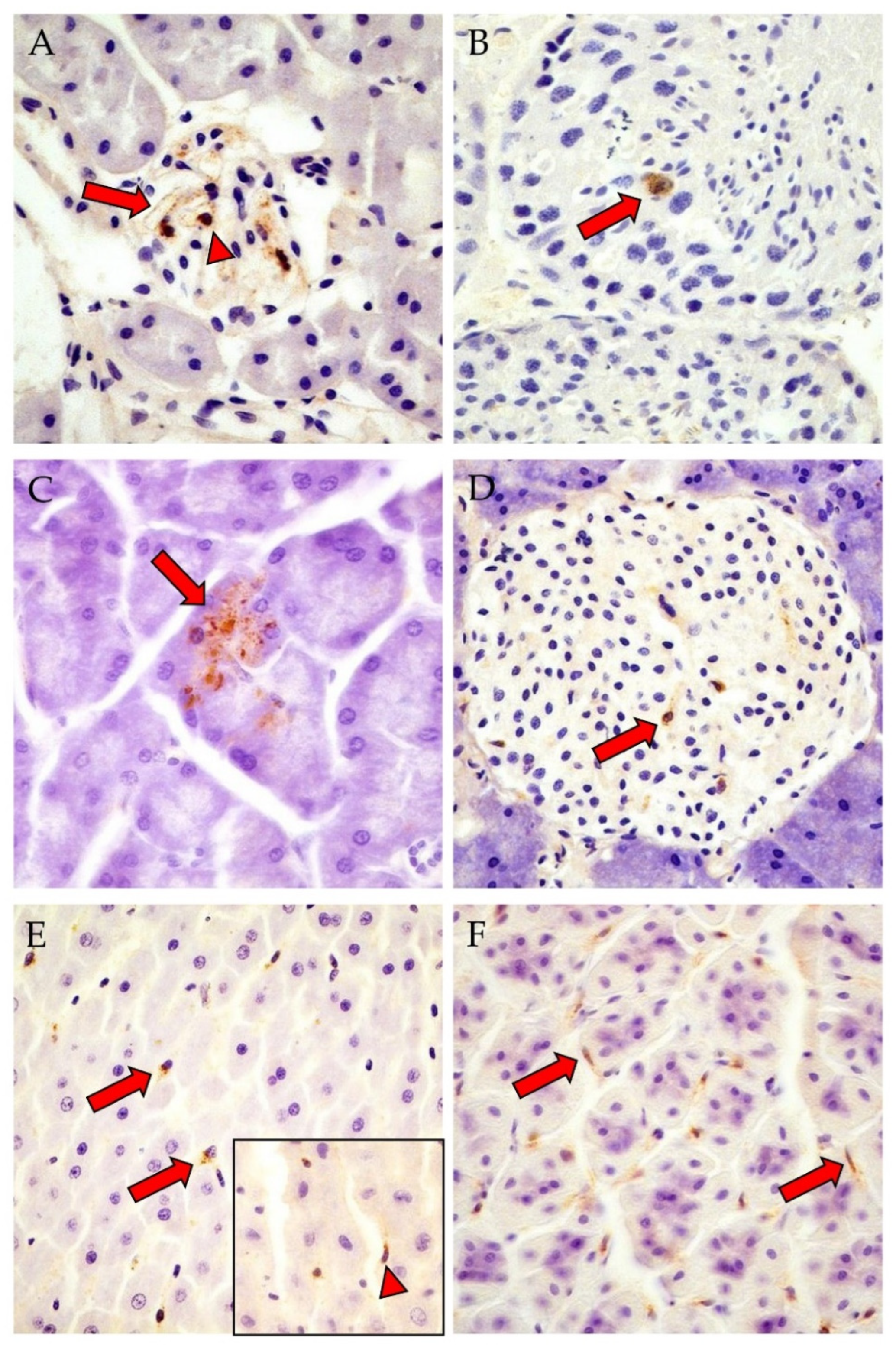
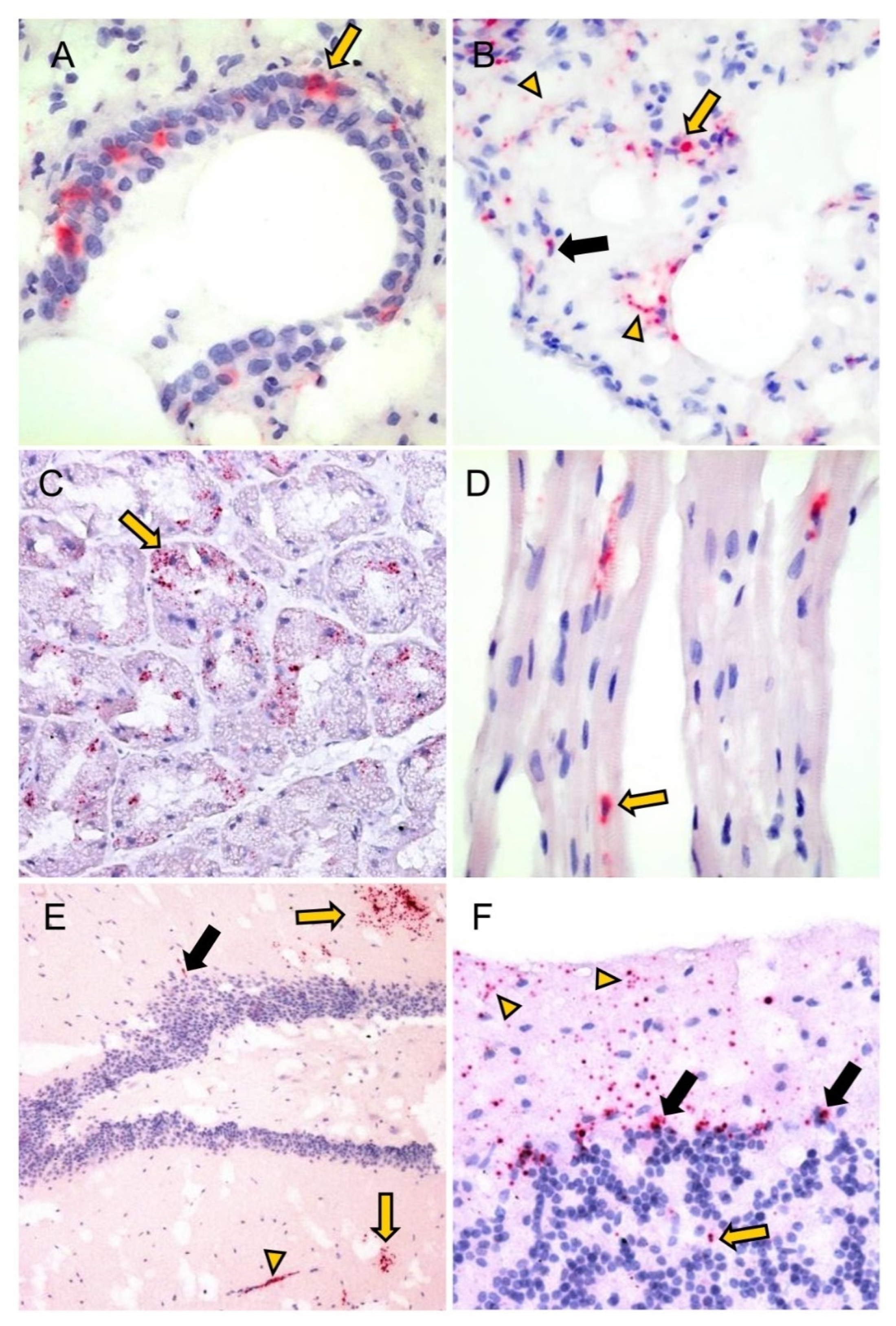
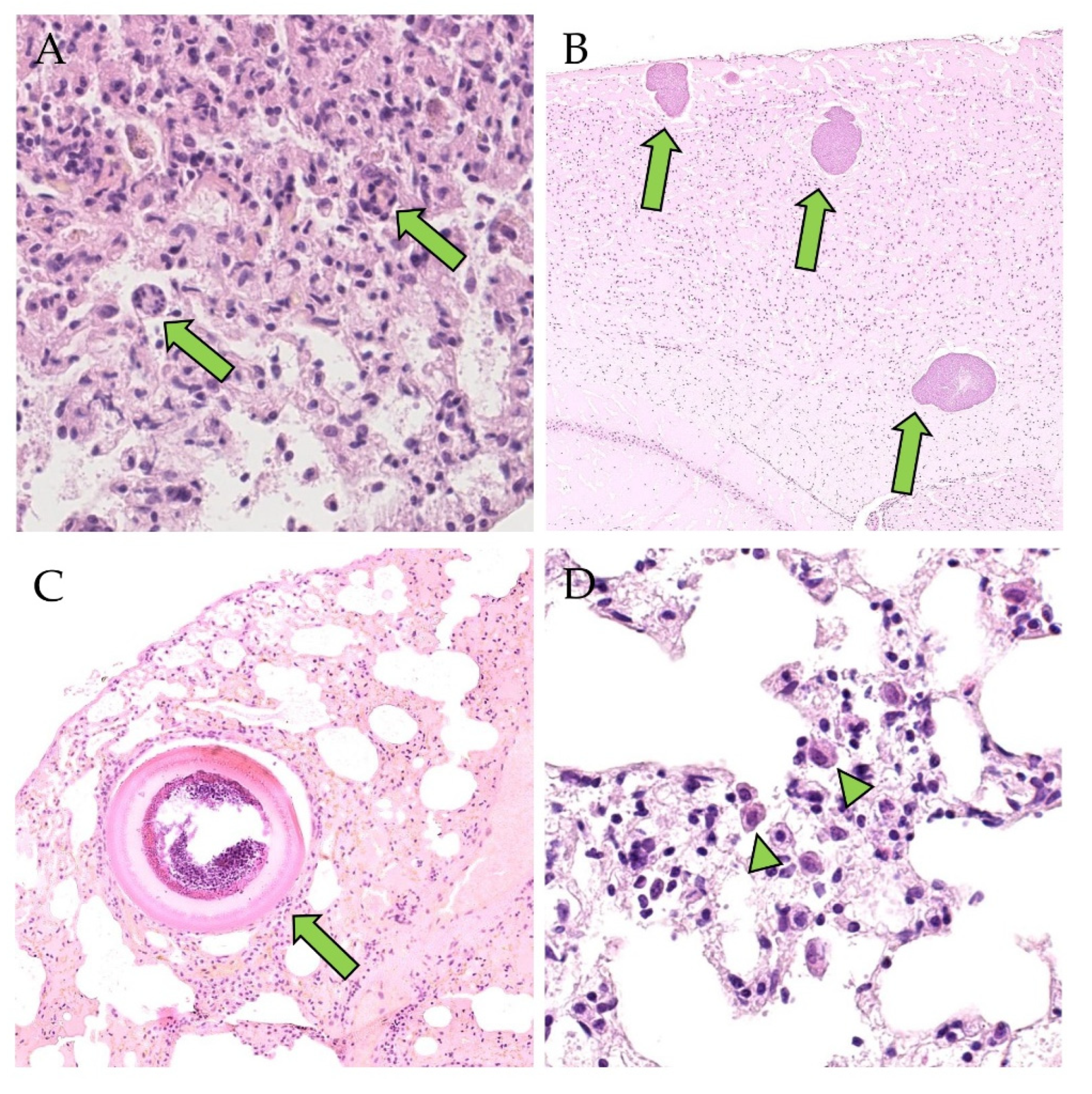
3.2. Detection of PUUV N-Protein by IHC, Viral Positive-Strand RNA by ISH, Gross and Histologic Findings and Endoparasite Coinfections
| No. of Positive/Total Number of Investigated Voles | |||
| RT-PCR Pos ELISA Pos (Persistent Infection) | RT-PCR Neg ELISA Pos | ||
| BF | BF | ||
| 12/13 | 1/13 | ||
| Detection of Viral Positive-Strand RNA by ISH | |||
| Total | 3/4 (75%) | 1/1 (100%) | |
| Cerebrum (neurons, glia cells, endothelial cells, plexus choroideus [cell with lancet-shaped nucleus in the pia mater]) | 1/3 (33%) | 0/1 (0%) | |
| Cerebellum (Stratum moleculare, -ganglionare and -granulosum) | 1/3 (33%) | 0/1 (0%) | |
| Lung | 3/3 (100%) | 1/1 (100%) | |
| (a) Bronchiolar epithelial cells | 1/3 (33%) | 0/1 (0%) | |
| (b) Pneumocytes type I and II (c) Endothelial cells | 3/3 (100%) | 1/1 (100%) | |
| 1/3 (33%) | 0/1 (0%) | ||
| (d) IC with spindle-shaped and round-oval nuclei | 3/3 (100%) | 1/1 (100%) | |
| Glandula submandibularis (acini) | 1/2 (50%) | n.i. | |
| Glandula mandibularis (acini) | 1/1 (100%) | n.i. | |
| Glandula parotidea (acini) | 1/1 (100%) | n.i. | |
| Tongue (myocytes, IC with lancet to spindle-shaped nuclei) | 1/1 (100%) | n.i. | |
| Liver (Kupffer cells) | 2/3 (66%) | 0/1 | |
| Pancreas (a) Acini (b) Islet cells of Langerhans (c) IC with spindle-shaped and round-oval nuclei | 3/3 (100%) | n.i. | |
| 3/3 (100%) | n.a. | ||
| 1/3 (33%) | n.a. | ||
| 2/3 (66%) | n.a. | ||
| Stomach/Pars non-glandularis (epithelial) | 2/2 (100%) | 0/1 (0%) | |
| Stomach/Pars glandularis (IC with lancet to spindle-shaped and round-oval nuclei) | 2/2 (100%) | 0/1 (0%) | |
| Duodenum | IC with spindle-shaped nuclei | 3/3 (100%) | 0/1 (0%) |
| Caecum | 1/1 (100%) | n.i. | |
| Colon ascendens | 1/1 (100%) | n.i. | |
| Colon descendens | 2/3 (66%) | 0/1 | |
| Kidney (a) Glomerulum cells * (b) IC with spindle-shaped nuclei (cortex) | 3/3 (100%) | 1/1 (100%) | |
| 3/3 (100%) | 1/1 (100%) | ||
| 2/3 (66%) | 0/1 (0%) | ||
| Heart (kardiomyocytes) | 2/3 (66%) | 1/1 (100%) | |
| Adrenal gland (endocrine cells, IC with lancet to plump, spindle-shaped nuclei (cortex and medulla)) | 1/1 (100%) | n.i. | |
| Brown adipose tissue (interstitium) ** | 1/1 (100%) | n.i. | |
| RT-PCR Pos ELISA Pos (Persistent Infection) | RT-PCR Pos ELISA Neg (Acute Infection) | RT-PCR Neg ELISA Neg | |
|---|---|---|---|
| Total | 14/15 M, 1/15 F | 2/3 M, 1/3 F | 2/2 F |
| Kidney (a) Mild chronic non-suppurative interstitial nephritis (b) Few interstitial mononuclear cells (c) Few plasma cells in renal pelvis (d) Few plasma cells in perirenal adipose tissue | |||
| 2/29 (7%) | 0/3 (0%) | 0/2 (0%) | |
| 2/29 (7%) | 0/3 (0%) | 0/2 (0%) | |
| 1/29 (3%) | 0/3 (0%) | 0/2 (0%) | |
| 0/29 (0%) | 1/3 (33%) | 0/2 (0%) | |
| Urinary bladder (a) Few interstitial plasma cells | |||
| 1/17 (6%) | 0/1 (0%) | 0/2 (0%) | |
| Glandula mandibularis (a) Few interstitial lymphocytes (b) Few interstitial lymphocytes and plasma cells | |||
| 2/16 (13%) | 1/1 (0%) | 0/2 (0%) | |
| 1/16 (6%) | 0/1 (0%) | 0/2 (0%) | |
| Brown adipose tissue (a) Few monocytes (b) Few interstitial lymphocytes and plasma cells | |||
| 0/16 (0%) | 1/1 (100%) | 0/2 (0%) | |
| 0/16 (0%) | 0/1 (0%) | 1/2 (50%) | |
| Heart (a) Few interstitial lymphocytes | |||
| 1/29 (3%) | 0/1 (0%) | 0/2 (0%) |
| Hepatozoon spp. | Sarcocystis spp. | Hepatozoon spp. + Sarcocystis spp. | |
|---|---|---|---|
| RT-PCR pos, ELISA pos (persistent infection) | 7/21 (33%) 5/7 M, 2/7 F | 2/21 (10%) 1/2 M, 1/2 F | 12/21 (57%) 6/12 M, 6/12 F |
| RT-PCR pos, ELISA neg (acute infection) | 1/2 (50%) 1/1 M | 0/2 (0%) | 1/2 (50%) 1/1 M |
| RT-PCR neg, ELISA pos | 2/3 (67%) 2/2 M | 0/3 (0%) | 0/3 (0%) |
| RT-PCR neg, ELISA neg | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) |
| RT-PCR Pos ELISA Pos (Persistent Infection) | RT-PCR Pos ELISA Neg (Acute Infection) | RT-PCR Neg ELISA Pos | Pathogen | |
|---|---|---|---|---|
| Total | 23/29 (79%) 12/23 M, 11/23 F | 3/3 (100%) 2/3 M, 1/3 F | 3/4 (75%) 3/3 M | Hepatozoon spp. |
| 1/29 (3%) 1/1 F | 0/3 (0%) | 0/4 (0%) | E. crescens | |
| 4/29 (14%) 2/4 M, 2/4 F | 0/3 (0%) | 0/4 (0%) | Hepatozoon spp. and E. crescens | |
| Desquamated alveolar macrophages | 6/23 (26%) | 1/3 (33) | 1/4 (25%) | Hepatozoon spp. |
| Interstitial infiltrates: mononuclear cells * | 1/23 (4%) | 0/3 (0%) | 1/4 (25%) | |
| Interstitial infiltrates: mononuclear cells, BALT-hyperplasia * | 1/23 (4%) | 0/3 (0%) | 0/4 (0%) | |
| Interstitial infiltrates: mononuclear cells, neutrophils * | 10/23 (44%) | 0/3 (0%) | 0/4 (0%) | |
| Interstitial infiltrates: mononuclear cells, neutrophils, syncytia * | 0/23 (0%) | 1/3 (33) | 0/4 (0%) | |
| Interstitial infiltrates: mononuclear cells, neutrophils, eosinophils * | 1/23 (4%) | 1/3 (33) | 0/4 (0%) | |
| Interstitial infiltrates: mononuclear cells, neutrophils, eosinophils, syncytia * | 1/23 (4%) | 0/3 (0%) | 0/4(0%) | |
| Interstitial pneumonia, neutrophils, syncytia * | 1/23 (4%) | 0/3 (0%) | 1/4 (25%) | |
| Desquamated alveolar macrophages | 1/28 (4%) | 0/3 (0%) | 0/3 (0%) | E. crescens |
| Desquamated alveolar macrophages | 1/4 (25%) | 0/3 (0%) | 0/3 (0%) | Hepatozoon spp. and E. crescens |
| Interstitial infiltrates: mononuclear cells, neutrophils | 1/4 (25%) | 0/3 (0%) | 0/3 (0%) | |
| Interstitial infiltrates: mononuclear cells, neutrophils, eosinophils * | 1/4 (25%) | 0/3 (0%) | 0/3 (0%) |
| RT-PCR Pos ELISA Pos (Persistent Infection) | |
|---|---|
| Total | 12 |
| Lung (a) Desquamated alveolar macrophages (b) Desquamated alveolar macrophages, BALT-hyperplasia (c) Few interstitial lymphocytes, macrophages and neutrophils | |
| 8/12 (67%) | |
| 1/12 (8%) | |
| 1/12 (8%) | |
| Glandula mandibularis (a) Few interstitial lymphocytes (b) Few interstitial lymphocytes and plasma cells | |
| 1/10 (10%) | |
| 1/10 (10%) | |
| Heart (a) Few interstitial lymphocytes (b) Few interstitial lymphocytes, macrophages and plasma cells | |
| 1/12 (8%) | |
| 1/12 (8%) |
| Yellow-Necked Mice | Wood Mice | ||
|---|---|---|---|
| RT-PCR Neg ELISA Pos | RT-PCR Neg ELISA Neg | RT-PCR Neg ELISA Pos | |
| Total | 3 2 M, 1 F | 1 1 M | 2 1 M, 1 F |
| Lung (a) Desquamated alveolar macrophages, BALT-hyperplasia, Emmonsia crescens (b) Few interstitial mononuclear cells | |||
| 0/3 (0%) | 1/1 (100%) | 0/2 (0%) | |
| 0/3 (0%) | 0/1 (0%) | 1/2 (50%) | |
| Kidney (a) Mild chronic non-suppurative interstitial nephritis (b) Few mononuclear cells in interstitium and renal pelvis (c) Few mononuclear cells in interstitium and renal pelvis, moderate glomerulosclerosis, interstitial fibrosis, multifocal mineralization, intratubular protein casts | |||
| 1/3 (33%) | 0/1 (0%) | 0/2 (0%) | |
| 1/3 (33%) | 0/1 (0%) | 1/2 (50%) | |
| 1/3 (33%) | 0/1 (0%) | 0/2 (0%) | |
| Adrenal gland (a) Few interstitial lymphocytes (b) Few interstitial lymphocytes, macrophages and plasma cells | |||
| 1/2 (50%) | 0/1 (0%) | 0/1 (0%) | |
| 0/2 (0%) | 1/1 (100%) | 0/1 (0%) | |
| Glandula mandibularis (a) Few interstitial lymphocytes, macrophages and plasma cells | |||
| 0/1 (0%) | 1/1 (100%) | n.i. | |
| Heart (a) Few interstitial lymphocytes and plasma cells | |||
| 0/3 (0%) | 1/1 (100%) | 0/2 (0%) | |
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laenen, L.; Vergote, V.; Calisher, C.H.; Klempa, B.; Klingström, J.; Kuhn, J.H.; Maes, P. Hantaviridae: Current Classification and Future Perspectives. Viruses 2019, 11, 788. [Google Scholar] [CrossRef]
- Klingström, J.; Heyman, P.; Escutenaire, S.; Sjölander, K.B.; De Jaegere, F.; Henttonen, H.; Lundkvist, Å. Rodent host specificity of European hantaviruses: Evidence of Puumala virus interspecific spillover. J. Med. Virol. 2002, 68, 581–588. [Google Scholar] [CrossRef]
- Henttonen, H.; Vapalahti, O.; Vaheri, A. How many kinds of hantaviruses? Trends Ecol. Evol. 1996, 11, 7–8. [Google Scholar] [CrossRef]
- Essbauer, S.; Schmidt, J.; Conraths, F.J.; Friedrich, R.; Koch, J.; Hautmann, W.; Pfeffer, M.; Wölfel, R.; Finke, J.; Dobler, G.; et al. A new Puumala hantavirus subtype in rodents associated with an outbreak of Nephropathia epidemica in South-East Germany in 2004. Epidemiol. Infect 2006, 134, 1333–1344. [Google Scholar] [CrossRef]
- Binder, F.; Ryll, R.; Drewes, S.; Jagdmann, S.; Reil, D.; Hiltbrunner, M.; Rosenfeld, U.M.; Imholt, C.; Jacob, J.; Heckel, G.; et al. Spatial and Temporal Evolutionary Patterns in Puumala Orthohantavirus (PUUV) S Segment. Pathogens 2020, 9, 548. [Google Scholar] [CrossRef]
- Kallio, E.R.; Klingström, J.; Gustafsson, E.; Manni, T.; Vaheri, A.; Henttonen, H.; Vapalahti, O.; Lundkvist, Å. Prolonged survival of Puumala hantavirus outside the host: Evidence for indirect transmission via the environment. J. Gen. Virol. 2006, 87 Pt 8, 2127–2134. [Google Scholar] [CrossRef]
- Schönrich, G.; Rang, A.; Lütteke, N.; Raftery, M.J.; Charbonnel, N.; Ulrich, R.G. Hantavirus-induced immunity in rodent reservoirs and humans. Immunol. Rev. 2008, 225, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Settergren, B.; Juto, P.; Trollfors, B.; Wadell, G.; Norrby, S.R. Clinical characteristics of nephropathia epidemica in Sweden: Prospective study of 74 cases. Rev. Infect Dis. 1989, 11, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Golovljova, I.; Vasilenko, V.; Mittženkov, V.; Prükk, T.; Seppet, E.; Vene, S.; Settergren, B.; Plyusnin, A.; Lundkvist, Å. Characterization of hemorrhagic fever with renal syndrome caused by hantaviruses, Estonia. Emerg. Infect Dis. 2007, 13, 1773–1776. [Google Scholar] [CrossRef]
- Plyusnin, A.; Vapalahti, O.; Vaheri, A. Hantaviruses: Genome structure, expression and evolution. J. Gen. Virol. 1996, 77 Pt 11, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Plyusnin, A.; Morzunov, S.P. Virus evolution and genetic diversity of hantaviruses and their rodent hosts. Hantaviruses 2001, 256, 47–75. [Google Scholar] [CrossRef]
- Bernshtein, A.D.; Apekina, N.S.; Mikhailova, T.V.; Myasnikov, Y.A.; Khlyap, L.A.; Korotkov, Y.S.; Gavrilovskaya, I.N. Dynamics of Puumala hantavirus infection in naturally infected bank voles (Clethrinomys glareolus). Arch. Virol. 1999, 144, 2415–2428. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, J.D.; Klein, S.L. Immunological mechanisms mediating hantavirus persistence in rodent reservoirs. PLoS Pathog. 2008, 4, e1000172. [Google Scholar] [CrossRef] [PubMed]
- Noack, D.; Goeijenbier, M.; Reusken, C.; Koopmans, M.P.G.; Rockx, B.H.G. Orthohantavirus pathogenesis and cell tropism. Front. Cell. Infect. Microbiol. 2020, 10, 399. [Google Scholar] [CrossRef]
- Elliott, R.M. Molecular biology of the Bunyaviridae. J. Gen. Virol. 1990, 71 Pt 3, 501–522. [Google Scholar] [CrossRef]
- Schmaljohn, C.S.; Jennings, G.B.; Hay, J.; Dalrymple, J.M. Coding strategy of the S genome segment of Hantaan virus. Virology 1986, 155, 633–643. [Google Scholar] [CrossRef]
- Jääskeläinen, K.M.; Kaukinen, P.; Minskaya, E.S.; Plyusnina, A.; Vapalahti, O.; Elliott, R.M.; Weber, F.; Vaheri, A.; Plyusnin, A. Tula and Puumala hantavirus NSs ORFs are functional and the products inhibit activation of the interferon-beta promoter. J. Med. Virol. 2007, 79, 1527–1536. [Google Scholar] [CrossRef]
- Binder, F.; Gallo, G.; Bendl, E.; Eckerle, I.; Ermonval, M.; Luttermann, C.; Ulrich, R.G. Inhibition of interferon I induction by non-structural protein NSs of Puumala virus and other vole-associated orthohantaviruses: Phenotypic plasticity of the protein and potential functional domains. Arch. Virol. 2021, 166, 2999–3012. [Google Scholar] [CrossRef]
- Löber, C.; Anheier, B.; Lindow, S.; Klenk, H.D.; Feldmann, H. The Hantaan virus glycoprotein precursor is cleaved at the conserved pentapeptide WAASA. Virology 2001, 289, 224–229. [Google Scholar] [CrossRef]
- Kukkonen, S.K.; Vaheri, A.; Plyusnin, A. L protein, the RNA-dependent RNA polymerase of hantaviruses. Arch. Virol. 2005, 150, 533–556. [Google Scholar] [CrossRef]
- Mertens, M.; Kindler, E.; Emmerich, P.; Esser, J.; Wagner-Wiening, C.; Wölfel, R.; Petraityte-Burneikiene, R.; Schmidt-Chanasit, J.; Zvirbliene, A.; Groschup, M.H.; et al. Phylogenetic analysis of Puumala virus subtype Bavaria, characterization and diagnostic use of its recombinant nucleocapsid protein. Virus Genes 2011, 43, 177–191. [Google Scholar] [CrossRef]
- Razzauti, M.; Plyusnina, A.; Henttonen, H.; Plyusnin, A. Microevolution of Puumala hantavirus during a complete population cycle of its host, the bank vole (Myodes glareolus). PLoS ONE 2013, 8, e64447. [Google Scholar] [CrossRef] [PubMed]
- Escutenaire, S.; Chalon, P.; Heyman, P.; Van der Auwera, G.; van der Groen, G.; Verhagen, R.; Thomas, I.; Karelle-Bui, L.; Vaheri, A.; Pastoret, P.P.; et al. Genetic characterization of Puumala hantavirus strains from Belgium: Evidence for a distinct phylogenetic lineage. Virus Res. 2001, 74, 1–15. [Google Scholar] [CrossRef]
- Klempa, B.; Fichet-Calvet, E.; Lecompte, E.; Auste, B.; Aniskin, V.; Meisel, H.; Denys, C.; Koivogui, L.; ter Meulen, J.; Krüger, D.H. Hantavirus in African wood mouse, Guinea. Emerg. Infect. Dis. 2006, 12, 838–840. [Google Scholar] [CrossRef]
- Weber de Melo, V.; Sheikh Ali, H.; Freise, J.; Kühnert, D.; Essbauer, S.; Mertens, M.; Wanka, K.M.; Drewes, S.; Ulrich, R.G.; Heckel, G. Spatiotemporal dynamics of Puumala hantavirus associated with its rodent host, Myodes glareolus. Evol. Appl. 2015, 8, 545–559. [Google Scholar] [CrossRef]
- Ettinger, J.; Hofmann, J.; Enders, M.; Tewald, F.; Oehme, R.M.; Rosenfeld, U.M.; Ali, H.S.; Schlegel, M.; Essbauer, S.; Osterberg, A.; et al. Multiple synchronous outbreaks of Puumala virus, Germany, 2010. Emerg. Infect. Dis. 2012, 18, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Castel, G.; Couteaudier, M.; Sauvage, F.; Pons, J.B.; Murri, S.; Plyusnina, A.; Pontier, D.; Cosson, J.F.; Plyusnin, A.; Marianneau, P.; et al. Complete genome and phylogeny of Puumala hantavirus isolates circulating in France. Viruses 2015, 7, 5476–5488. [Google Scholar] [CrossRef]
- Drewes, S.; Ali, H.S.; Saxenhofer, M.; Rosenfeld, U.M.; Binder, F.; Cuypers, F.; Schlegel, M.; Röhrs, S.; Heckel, G.; Ulrich, R.G. Host-associated absence of human Puumala Virus infections in northern and eastern Germany. Emerg. Infect. Dis. 2017, 23, 83–86. [Google Scholar] [CrossRef]
- Wójcik, J.M.; Kawałko, A.; Marková, S.; Searle, J.B.; Kotlík, P. Phylogeographic signatures of northward post-glacial colonization from high-latitude refugia: A case study of bank voles using museum specimens. J. Zool. 2010, 281, 249–262. [Google Scholar] [CrossRef]
- Filipi, K.; Marková, S.; Searle, J.B.; Kotlík, P. Mitogenomic phylogenetics of the bank vole Clethrionomys glareolus, a model system for studying end-glacial colonization of Europe. Mol. Phylogenet Evol. 2015, 82 Pt A, 245–257. [Google Scholar] [CrossRef]
- Binder, F.; Reiche, S.; Roman-Sosa, G.; Saathoff, M.; Ryll, R.; Trimpert, J.; Kunec, D.; Höper, D.; Ulrich, R.G. Isolation and characterization of new Puumala orthohantavirus strains from Germany. Virus Genes 2020, 56, 448–460. [Google Scholar] [CrossRef]
- Ali, H.S.; Drewes, S.; Sadowska, E.T.; Mikowska, M.; Groschup, M.H.; Heckel, G.; Koteja, P.; Ulrich, R.G. First molecular evidence for Puumala hantavirus in Poland. Viruses 2014, 6, 340–353. [Google Scholar] [CrossRef]
- Rosenfeld, U.M.; Drewes, S.; Ali, H.S.; Sadowska, E.T.; Mikowska, M.; Heckel, G.; Koteja, P.; Ulrich, R.G. A highly divergent Puumala virus lineage in southern Poland. Arch. Virol. 2017, 162, 1177–1185. [Google Scholar] [CrossRef]
- Madrières, S.; Castel, G.; Murri, S.; Vulin, J.; Marianneau, P.; Charbonnel, N. The needs for developing experiments on reservoirs in hantavirus research: Accomplishments, challenges and promises for the future. Viruses 2019, 11, 664. [Google Scholar] [CrossRef]
- Guivier, E.; Galan, M.; Henttonen, H.; Cosson, J.F.; Charbonnel, N. Landscape features and helminth co-infection shape bank vole immunoheterogeneity, with consequences for Puumala virus epidemiology. Heredity 2014, 112, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, E.T.; Stawski, C.; Rudolf, A.; Dheyongera, G.; Chrzascik, K.M.; Baliga-Klimczyk, K.; Koteja, P. Evolution of basal metabolic rate in bank voles from a multidirectional selection experiment. Proc. Biol. Sci. 2015, 282, 20150025. [Google Scholar] [CrossRef]
- Mikowska, M.; Gaura, A.; Sadowska, E.; Koteja, P.; Świergosz-Kowalewska, R. Genetic variation in bank vole populations in natural and metal-contaminated areas. Arch. Environ. Contam. Toxicol. 2014, 67, 535–546. [Google Scholar] [CrossRef]
- Bajer, A.; Bednarska, M.; Pawelczyk, A.; Behnke, J.M.; Gilbert, F.S.; Sinski, E. Prevalence and abundance of Cryptosporidium parvum and Giardia spp. in wild rural rodents from the Mazury Lake District region of Poland. Parasitology 2002, 125 Pt 1, 21–34. [Google Scholar] [CrossRef]
- Kučinskaitė-Kodzė, I.; Petraitytė-Burneikienė, R.; Žvirblienė, A.; Hjelle, B.; Medina, R.A.; Gedvilaitė, A.; Ražanskienė, A.; Schmidt-Chanasit, J.; Mertens, M.; Padula, P.; et al. Characterization of monoclonal antibodies against hantavirus nucleocapsid protein and their use for immunohistochemistry on rodent and human samples. Arch. Virol. 2011, 156, 443–456. [Google Scholar] [CrossRef]
- Žvirblienė, A.; Samonskyte, L.; Gedvilaitė, A.; Voronkova, T.; Ulrich, R.; Sasnauskas, K. Generation of monoclonal antibodies of desired specificity using chimeric polyomavirus-derived virus-like particles. J. Immunol. Methods 2006, 311, 57–70. [Google Scholar] [CrossRef]
- Mertens, M.; Wölfel, R.; Ullrich, K.; Yoshimatsu, K.; Blumhardt, J.; Römer, I.; Esser, J.; Schmidt-Chanasit, J.; Groschup, M.H.; Dobler, G.; et al. Seroepidemiological study in a Puumala virus outbreak area in South-East Germany. Med. Microbiol. Immunol. 2009, 198, 83–91. [Google Scholar] [CrossRef]
- Schmidt, S.; Saxenhofer, M.; Drewes, S.; Schlegel, M.; Wanka, K.M.; Frank, R.; Klimpel, S.; von Blanckenhagen, F.; Maaz, D.; Herden, C.; et al. High genetic structuring of Tula hantavirus. Arch. Virol. 2016, 161, 1135–1149. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In 2010 Gateway Computing Environments Workshop (GCE); IEEE: New Orleans, LA, USA, 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Raftery, M.J.; Lalwani, P.; Lütteke, N.; Kobak, L.; Giese, T.; Ulrich, R.G.; Radosa, L.; Krüger, D.H.; Schönrich, G. Replication in the mononuclear phagocyte system (MPS) as a determinant of hantavirus pathogenicity. Front. Cell. Infect. Microbiol. 2020, 10, 281. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.T.; Ma, X.J.; Luo, Y. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef]
- Mugridge, N.B.; Morrison, D.A.; Johnson, A.M.; Luton, K.; Dubey, J.P.; Votypka, J.; Tenter, A.M. Phylogenetic relationships of the genus Frenkelia: A review of its history and new knowledge gained from comparison of large subunit ribosomal ribonucleic acid gene sequences. Int. J. Parasitol. 1999, 29, 957–972. [Google Scholar] [CrossRef]
- Gardiner, C.H.; Fayer, R.; Dubey, J.P. An atlas of protozoan parasites in animal tissues. In Armed Forces Institute of Pathology, 2nd ed.; American Registry of Pathology: Washington, DC, USA, 1985; ISBN 1-881041-48-4. [Google Scholar]
- Deter, J.; Chaval, Y.; Galan, M.; Gauffre, B.; Morand, S.; Henttonen, H.; Laakkonen, J.; Voutilainen, L.; Charbonnel, N.; Cosson, J.F. Kinship, dispersal and hantavirus transmission in bank and common voles. Arch. Virol. 2008, 153, 435–444. [Google Scholar] [CrossRef]
- Sauvage, F.Y. La Synergie Entre la Dynamique Démographique des Populations Réservoirs de Campagnols Roussâtres et L’excrétion du Hantavirus Puumala: Mise en Évidence du Mécanisme D’émergence de la Néphropathie Épidémique Humaine; Universite’ Claude Bernard-Lyon 1: Villeurbanne, France, 2004. [Google Scholar]
- Olsson, G.E.; White, N.; Ahlm, C.; Elgh, F.; Verlemyr, A.C.; Juto, P.; Palo, R.T. Demographic factors associated with hantavirus infection in bank voles (Clethrionomys glareolus). Emerg. Infect. Dis. 2002, 8, 924–929. [Google Scholar] [CrossRef]
- Tersago, K.; Verhagen, R.; Leirs, H. Temporal variation in individual factors associated with hantavirus infection in bank voles during an epizootic: Implications for Puumala virus transmission dynamics. Vector-Borne Zoonotic Dis. 2011, 11, 715–721. [Google Scholar] [CrossRef]
- Reil, D.; Rosenfeld, U.M.; Imholt, C.; Schmidt, S.; Ulrich, R.G.; Eccard, J.A.; Jacob, J. Puumala hantavirus infections in bank vole populations: Host and virus dynamics in Central Europe. BMC Ecol. 2017, 17, 9. [Google Scholar] [CrossRef]
- Kallio, E.R.; Poikonen, A.; Vaheri, A.; Vapalahti, O.; Henttonen, H.; Koskela, E.; Mappes, T. Maternal antibodies postpone hantavirus infection and enhance individual breeding success. Proc. R Soc. B 2006, 273, 2771–2776. [Google Scholar] [CrossRef]
- Voutilainen, L.; Sironen, T.; Tonteri, E.; Bäck, A.T.; Razzauti, M.; Karlsson, M.; Wahlström, M.; Niemimaa, J.; Henttonen, H.; Lundkvist, Å. Life-long shedding of Puumala hantavirus in wild bank voles (Myodes glareolus). J. Gen. Virol. 2015, 96 Pt 6, 1238–1247. [Google Scholar] [CrossRef]
- Strandin, T.; Smura, T.; Ahola, P.; Aaltonen, K.; Sironen, T.; Hepojoki, J.; Eckerle, I.; Ulrich, R.G.; Vapalahti, O.; Kipar, A.; et al. Orthohantavirus isolated in reservoir host cells displays minimal genetic changes and retains wild-type infection properties. Viruses 2020, 12, 457. [Google Scholar] [CrossRef]
- Yanagihara, R.; Amyx, H.L.; Gajdusek, D.C. Experimental infection with Puumala virus, the etiologic agent of nephropathia epidemica, in bank voles (Clethrionomys glareolus). J. Virol. 1985, 55, 34–38. [Google Scholar] [CrossRef]
- Brummer-Korvenkontio, M.; Vaheri, A.; Hovi, T.; von Bonsdorff, C.H.; Vuorimies, J.; Manni, T.; Penttinen, K.; Oker-Blom, N.; Lähdevirta, J. Nephropathia epidemica: Detection of antigen in bank voles and serologic diagnosis of human infection. J. Infect. Dis. 1980, 141, 131–134. [Google Scholar] [CrossRef]
- Mittler, E.; Dieterle, M.E.; Kleinfelter, L.M.; Slough, M.M.; Chandran, K.; Jangra, R.K. Hantavirus entry: Perspectives and recent advances. Adv. Virus Res. 2019, 104, 185–224. [Google Scholar] [CrossRef]
- Gavrilovskaya, I.N.; Shepley, M.; Shaw, R.; Ginsberg, M.H.; Mackow, E.R. beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. USA 1998, 95, 7074–7079. [Google Scholar] [CrossRef]
- Gavrilovskaya, I.N.; Brown, E.J.; Ginsberg, M.H.; Mackow, E.R. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. J. Virol. 1999, 73, 3951–3959. [Google Scholar] [CrossRef]
- Krautkrämer, E.; Zeier, M. Hantavirus causing hemorrhagic fever with renal syndrome enters from the apical surface and requires decay-accelerating factor (DAF/CD55). J. Virol. 2008, 82, 4257–4264. [Google Scholar] [CrossRef]
- Choi, Y.; Kwon, Y.C.; Kim, S.I.; Park, J.M.; Lee, K.H.; Ahn, B.Y. A hantavirus causing hemorrhagic fever with renal syndrome requires gC1qR/p32 for efficient cell binding and infection. Virology 2008, 381, 178–183. [Google Scholar] [CrossRef]
- Jangra, R.K.; Herbert, A.S.; Li, R.; Jae, L.T.; Kleinfelter, L.M.; Slough, M.M.; Barker, S.L.; Guardado-Calvo, P.; Roman-Sosa, G.; Dieterle, M.E.; et al. Protocadherin-1 is essential for cell entry by New World hantaviruses. Nature 2018, 563, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, M.E.; Sola-Riera, C.; Ye, C.; Goodfellow, S.M.; Mittler, E.; Kasikci, E.; Bradfute, S.B.; Klingstrom, J.; Jangra, R.K.; Chandran, K. Genetic depletion studies inform receptor usage by virulent hantaviruses in human endothelial cells. Elife 2021, 10, e69708. [Google Scholar] [CrossRef]
- Dubois, A.; Castel, G.; Murri, S.; Pulido, C.; Pons, J.B.; Benoit, L.; Loiseau, A.; Lakhdar, L.; Galan, M.; Charbonnel, N.; et al. Experimental infections of wild bank voles (Myodes glareolus) from nephropatia epidemica endemic and non-endemic regions revealed slight differences in Puumala virological course and immunological responses. Virus Res. 2017, 235, 67–72. [Google Scholar] [CrossRef]
- Yamanouchi, T.; Domae, K.; Tanishita, O.; Takahashi, Y.; Yamanishi, K.; Takahashi, M.; Kurata, T. Experimental infection in newborn mice and rats by hemorrhagic fever with renal syndrome (HFRS) virus. Microbiol. Immunol. 1984, 28, 1345–1353. [Google Scholar] [CrossRef]
- Botten, J.; Mirowsky, K.; Kusewitt, D.; Ye, C.; Gottlieb, K.; Prescott, J.; Hjelle, B. Persistent Sin Nombre virus infection in the deer mouse (Peromyscus maniculatus) model: Sites of replication and strand-specific expression. J. Virol. 2003, 77, 1540–1550. [Google Scholar] [CrossRef]
- Hardestam, J.; Karlsson, M.; Falk, K.I.; Olsson, G.; Klingström, J.; Lundkvist, Å. Puumala hantavirus excretion kinetics in bank voles (Myodes glareolus). Emerg. Infect. Dis. 2008, 14, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, P.T.; Perley, C.C.; Brocato, R.L.; Hooper, J.W.; Jürgensen, C.; Schulzke, J.D.; Krüger, D.H.; Bücker, R. Gastrointestinal tract as entry route for hantavirus infection. Front. Microbiol. 2017, 8, 1721. [Google Scholar] [CrossRef]
- Barthold, S.W.; Griffey, S.M.; Percy, D.H. Pathology of Laboratory Rodents and Rabbits, 4th ed.; John Wiley & Sons: Singapore, 2016; ISBN 978-1-118-82424-5. [Google Scholar]
- Cianciolo, R.E.; Mohr, F.C. Urinary System. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals, 6th ed.; Elsevier: St. Louis, MO, USA, 2016; Volume 2, pp. 376–464. [Google Scholar] [CrossRef]
- Mayer-Scholl, A.; Teifke, J.P.; Huber, N.; Luge, E.; Bier, N.S.; Nöckler, K.; Ulrich, R.G. Leptospira spp. in rodents and shrews from Afghanistan. J. Wildl. Dis. 2019, 55, 477–481. [Google Scholar] [CrossRef]
- Krautkrämer, E.; Grouls, S.; Stein, N.; Reiser, J.; Zeier, M. Pathogenic old world hantaviruses infect renal glomerular and tubular cells and induce disassembling of cell-to-cell contacts. J. Virol. 2011, 85, 9811–9823. [Google Scholar] [CrossRef]
- Rasmuson, J.; Andersson, C.; Norrman, E.; Haney, M.; Evander, M.; Ahlm, C. Time to revise the paradigm of hantavirus syndromes? Hantavirus pulmonary syndrome caused by European hantavirus. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, J.; Helin, H.; Pietilä, K.; Brummer-Korvenkontio, M.; Hedman, K.; Vaheri, A.; Pasternack, A. Renal biopsy findings and clinicopathologic correlations in nephropathia epidemica. Clin. Nephrol. 1994, 41, 121–126. [Google Scholar]
- Laakkonen, J.; Sukura, A.; Oksanen, A.; Henttonen, H.; Soveri, T. Haemogregarines of the genus Hepatozoon (Apicomplexa: Adeleina) in rodents from northern Europe. Folia Parasitol. 2001, 48, 263–267. [Google Scholar] [CrossRef]
- Fichet-Calvet, E.; Kia, E.B.; Giraudoux, P.; Quéré, J.P.; Delattre, P.; Ashford, R.W. Frenkelia parasites in a small mammal community. Dynamics of infection and effect on the host. Parasite 2004, 11, 301–310. [Google Scholar] [CrossRef]
- Hubálek, Z. Emmonsiosis of wild rodents and insectivores in Czechland. J. Wildl. Dis. 1999, 35, 243–249. [Google Scholar] [CrossRef]
- Zlatanov, Z.; Genov, T. Isolation of Emmonsia crescens Emmons et Jellison 1960 from small mammals in Bulgaria. Mycopathologia 1975, 56, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Simpson, V.R.; Palmer, M.D.; Linton, C.J.; Johnson, E.M. Adiaspiromycosis due to Emmonsia crescens is widespread in native British mammals. Mycopathologia 2009, 168, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, J.; Takashima, I.; Hashimoto, N. Cell fusion by haemorrhagic fever with renal syndrome (HFRS) viruses and its application for titration of virus infectivity and neutralizing antibody. Arch. Virol. 1985, 86, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, D.R.; Cooke, C.; Prabakaran, I.; Boland, J.; Nathanson, N.; Gonzalez-Scarano, F. Expression of the La Crosse M segment proteins in a recombinant vaccinia expression system mediates pH-dependent cellular fusion. Virology 1993, 193, 993–996. [Google Scholar] [CrossRef]
- McCaughey, C.; Shi, X.; Elliot, R.M.; Wyatt, D.E.; O’Neill, H.J.; Coyle, P.V. Low pH-induced cytopathic effect—A survey of seven hantavirus strains. J. Virol. Methods 1999, 81, 193–197. [Google Scholar] [CrossRef]
- Shi, X.; Kohl, A.; Li, P.; Elliott, R.M. Role of the cytoplasmic tail domains of Bunyamwera orthobunyavirus glycoproteins Gn and Gc in virus assembly and morphogenesis. J. Virol. 2007, 81, 10151–10160. [Google Scholar] [CrossRef]
- Baltrūnaitė, L.; Kitrytė, N.; Križanauskienė, A. Blood parasites (Babesia, Hepatozoon and Trypanosoma) of rodents, Lithuania: Part I. Molecular and traditional microscopy approach. Parasitol. Res. 2020, 119, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Criado-Fornelio, A.; Ruas, J.L.; Casado, N.; Farias, N.A.; Soares, M.P.; Müller, G.; Brumt, J.G.; Berne, M.E.; Buling-Saraña, A.; Barba-Carretero, J.C. New molecular data on mammalian Hepatozoon species (Apicomplexa: Adeleorina) from Brazil and Spain. J. Parasitol. 2006, 92, 93–99. [Google Scholar] [CrossRef]
- Rigó, K.; Majoros, G.; Szekeres, S.; Molnár, I.; Jablonszky, M.; Majláthová, V.; Majláth, I.; Földvári, G. Identification of Hepatozoon erhardovae Krampitz, 1964 from bank voles (Myodes glareolus) and fleas in Southern Hungary. Parasitol. Res. 2016, 115, 2409–2413. [Google Scholar] [CrossRef] [PubMed]
- Salvador, A.R.; Guivier, E.; Xuéreb, A.; Chaval, Y.; Cadet, P.; Poulle, M.L.; Sironen, T.; Voutilainen, L.; Henttonen, H.; Cosson, J.F.; et al. Concomitant influence of helminth infection and landscape on the distribution of Puumala hantavirus in its reservoir, Myodes glareolus. BMC Microbiol. 2011, 11, 30. [Google Scholar] [CrossRef]
- Lõhmus, M.; Verner-Carlsson, J.; Borg, O.; Albihn, A.; Lundkvist, Å. Hantavirus in new geographic regions, Sweden. Infect. Ecol. Epidemiol. 2016, 6, 31465. [Google Scholar] [CrossRef]
| No. of Positive/Total Number of Investigated Voles | |||
| RT-PCR Pos ELISA Pos (Persistent Infection) | RT-PCR Pos ELISA Neg (Acute Infection) | ||
| OS | BF | OS | |
| 36/189 | 12/13 | 3/189 | |
| Detection of N-Protein by IHC | |||
| Total | 19/29 (66%) 10/19 M, 9/19 F | 9/12 (75%) 9/9 F | 3/3 (100%) 2/3 M, 1/3 F |
| Cerebrum | 7/21 (33%) | 2/12 (17%) | 0/2 (0%) |
| (a) Neuron | 2/7 (29%) | 2/2 (100%) | n.a. |
| (b) Glia cells | 4/7 (57%) | 2/2 (100%) | n.a. |
| (c) Endothelial cells | 3/7 (43%) | 0/2 (0%) | n.a. |
| Cerebellum | 1/19 (5%) | 2/12 (17%) | 0/2 (0%) |
| (a) Stratum moleculare | 0/1 (0%) | 2/2 (100%) | n.a. |
| (b) Stratum ganglionare | 0/1 (0%) | 2/2 (100%) | n.a. |
| (c) Stratum granulosum | 1/1 (100%) | 2/2 (100%) | n.a. |
| Lung | 1/28 (4%) | 2/12 (17%) | 1/3 (33%) |
| (a) Bronchiolar epithelial cells | 1/1 (100%) | 2/2 (100%) | 0/1 (0%) |
| (b) Pneumocytes type I | 0/1 (0%) | 0/2 (0%) | 1/1 (100%) |
| (c) IC with spindle-shaped and round-oval nuclei | 1/1 (100%) | 2/2 (100%) | 1/1 (100%) |
| Glandula mandibularis (acini, IC with spindle-shaped nuclei) | 1/16 (6%) | 0/10 (0%) | 0/1 (0%) |
| Glandula parotidea (acini) | 0/16 (0%) | 2/9 (22%) | 0/1 (0%) |
| Tongue (IC with lancet to spindle-shaped nuclei) | 2/14 (14%) | 0/12 (0%) | 1/1 (100%) |
| Liver | 3/26 (12%) | 0/12 (0%) | 2/3 (67%) |
| (a) Kupffer cells | 3/3 (100%) | n.a. | 2/2 (100%) |
| (b) Cells with spindle-shaped nuclei at sinusoid periphery | 0/3 (0%) | n.a. | 1/2 (50%) |
| Pancreas | 1/12 | 3/8 | 0/2 (0%) |
| (a) Acini | 0/1 (0%) | 3/3 (100%) | n.a. |
| (b) Islet cells of Langerhans | 1/1 (100%) | 1/3 (33%) | n.a. |
| (c) IC with spindle-shaped and round-oval nuclei | 1/1 (100%) | 2/3 (67%) | n.a. |
| Stomach/Pars non-glandularis (epithelial cells) | 1/17 (6%) | 2/12 (17%) | 0/1 (0%) |
| Stomach/Pars glandularis (IC with lancet to spindle-shaped nuclei) | 5/13 (38%) | 8/11 (73%) | n.i. |
| Kidney (glomerulum cells *) | 11/28 (39%) | 4/12 (33%) | 3/3 (100%) |
| Testis (sperm precursor cell) | 1/8 (13%) | n.a. | 0/1 (0%) |
| Heart (IC with lancet-shaped nuclei) | 1/28 (4%) | 0/11 (0%) | 0/3 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlohsarczyk, E.K.; Drewes, S.; Koteja, P.; Röhrs, S.; Ulrich, R.G.; Teifke, J.P.; Herden, C. Tropism of Puumala orthohantavirus and Endoparasite Coinfection in the Bank Vole Reservoir. Viruses 2023, 15, 612. https://doi.org/10.3390/v15030612
Schlohsarczyk EK, Drewes S, Koteja P, Röhrs S, Ulrich RG, Teifke JP, Herden C. Tropism of Puumala orthohantavirus and Endoparasite Coinfection in the Bank Vole Reservoir. Viruses. 2023; 15(3):612. https://doi.org/10.3390/v15030612
Chicago/Turabian StyleSchlohsarczyk, Elfi K., Stephan Drewes, Paweł Koteja, Susanne Röhrs, Rainer G. Ulrich, Jens P. Teifke, and Christiane Herden. 2023. "Tropism of Puumala orthohantavirus and Endoparasite Coinfection in the Bank Vole Reservoir" Viruses 15, no. 3: 612. https://doi.org/10.3390/v15030612
APA StyleSchlohsarczyk, E. K., Drewes, S., Koteja, P., Röhrs, S., Ulrich, R. G., Teifke, J. P., & Herden, C. (2023). Tropism of Puumala orthohantavirus and Endoparasite Coinfection in the Bank Vole Reservoir. Viruses, 15(3), 612. https://doi.org/10.3390/v15030612





