Definition of a New HLA B*52-Restricted Rev CTL Epitope Targeted by an HIV-1-Infected Controller
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Peptides
2.3. Isolation of PBMCs
2.4. Generation of B-Lymphoblastoid Cell Lines (B-LCL)
2.5. Generation of HIV-1-Specific T-Cell Lines
2.6. Detection of HIV-1-Specific T-Cells
2.7. HLA Typing
2.8. HLA-Restriction Analysis
2.9. Statistical Analysis
3. Results
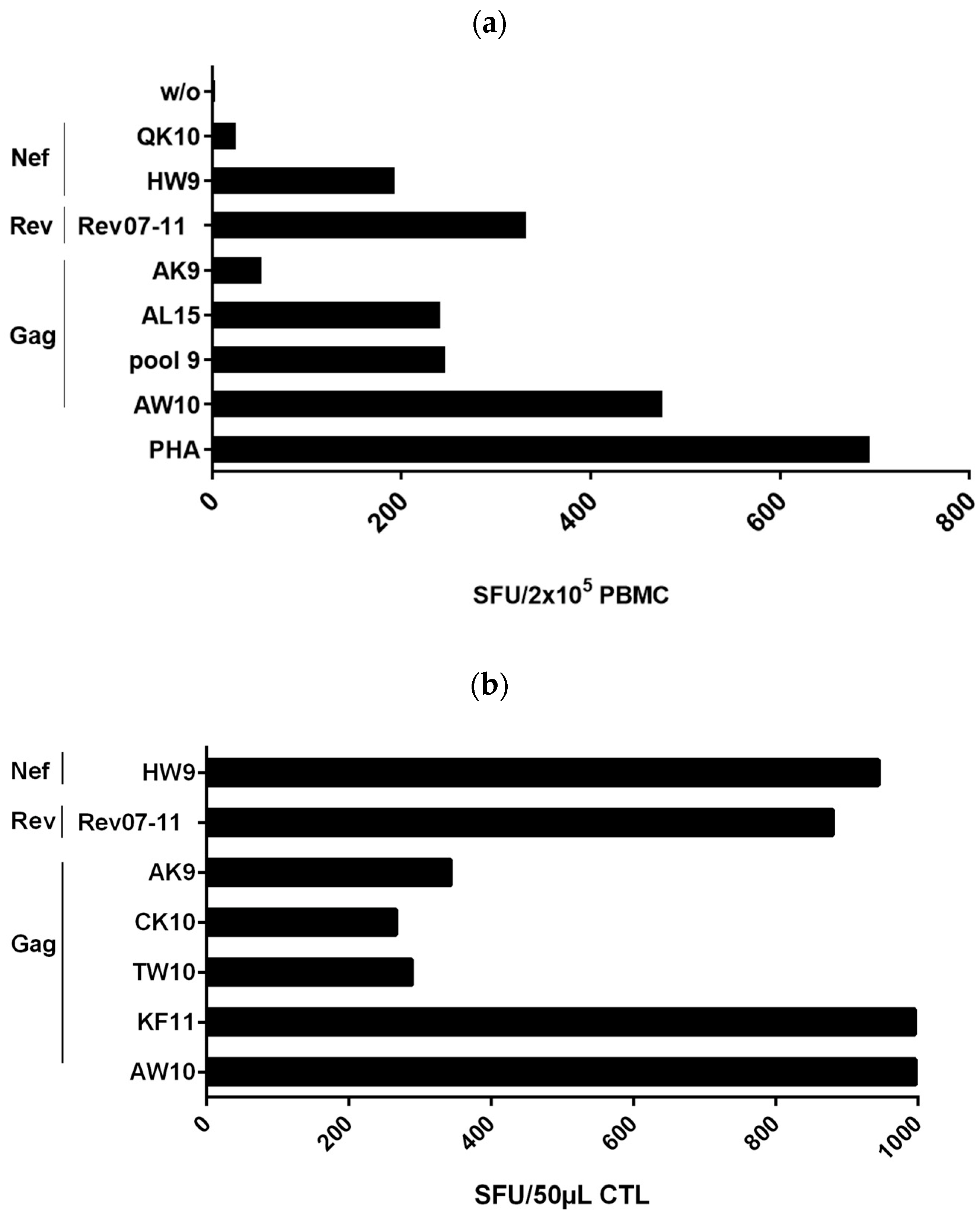
3.1. Detection of a Strong Polyclonal T-Cell Response in Controller #1
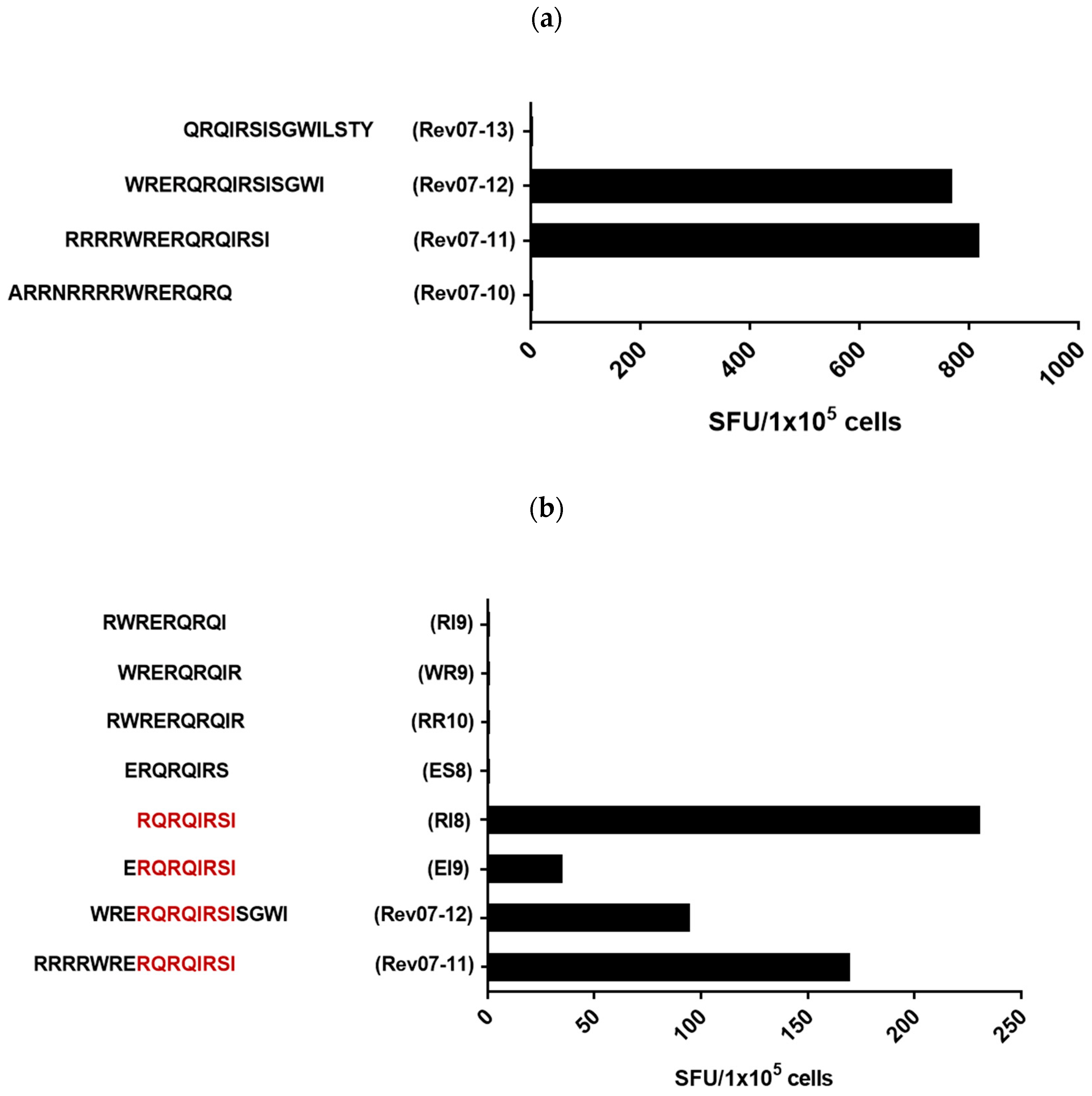
3.2. Definition of the Rev-RI8 Peptide as a New Epitope in Rev
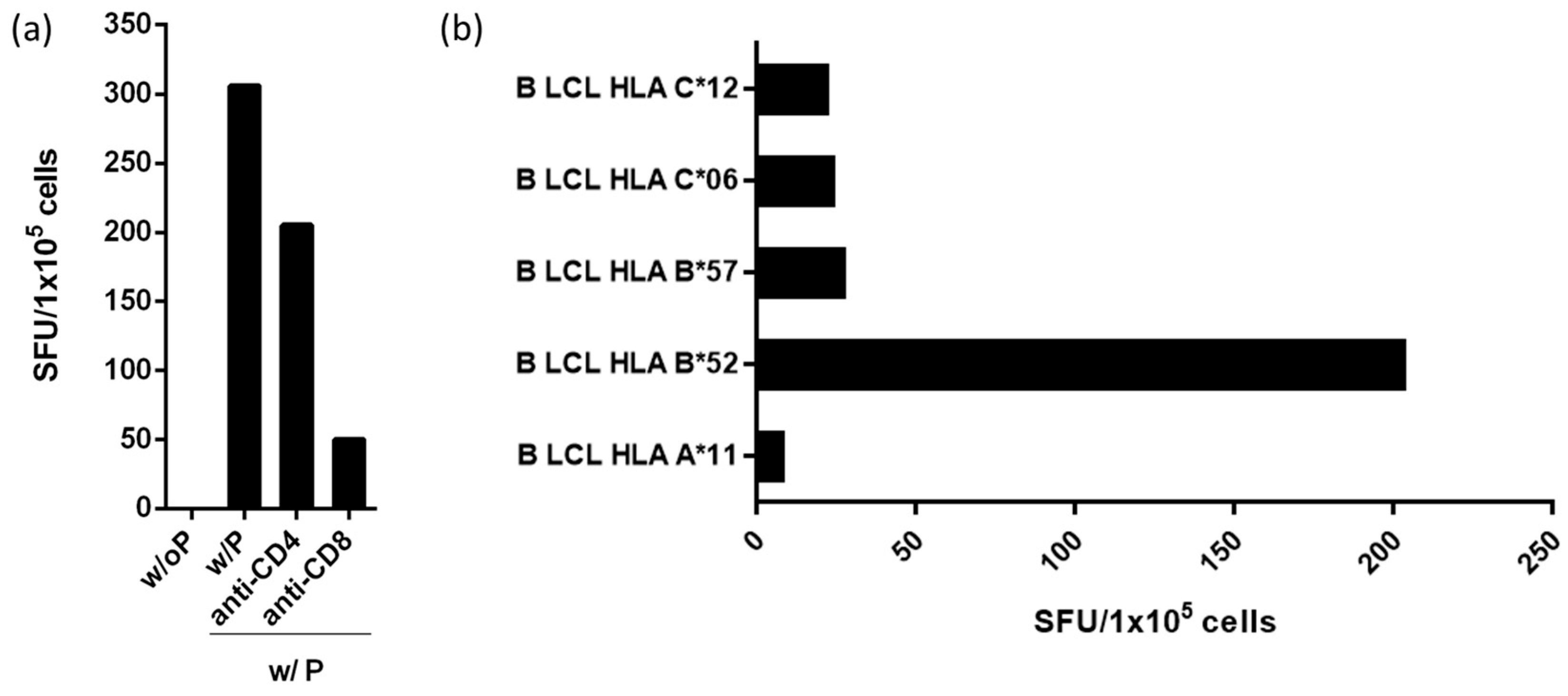
3.3. Peptide Rev-RI8 Is Presented by HLA B*52
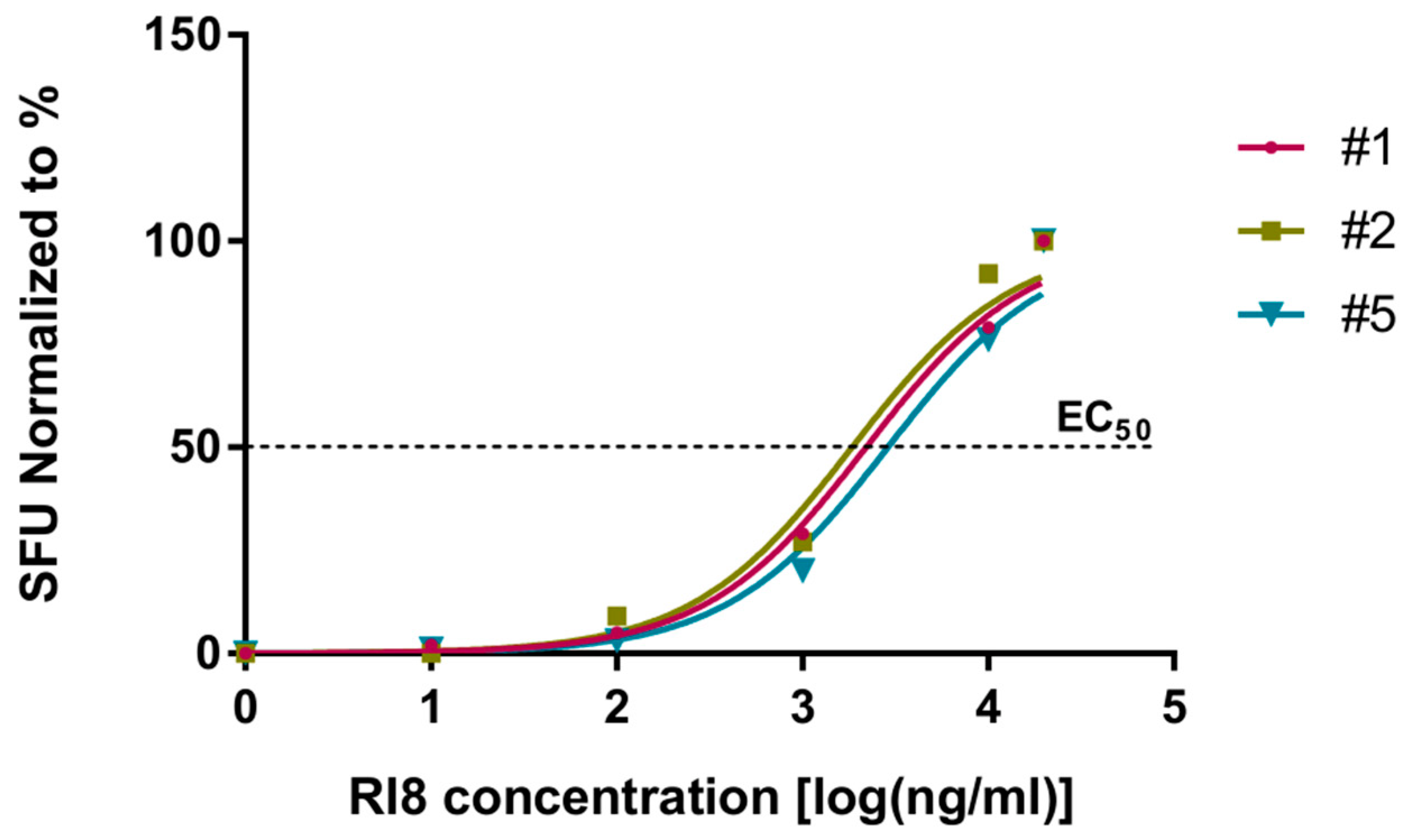
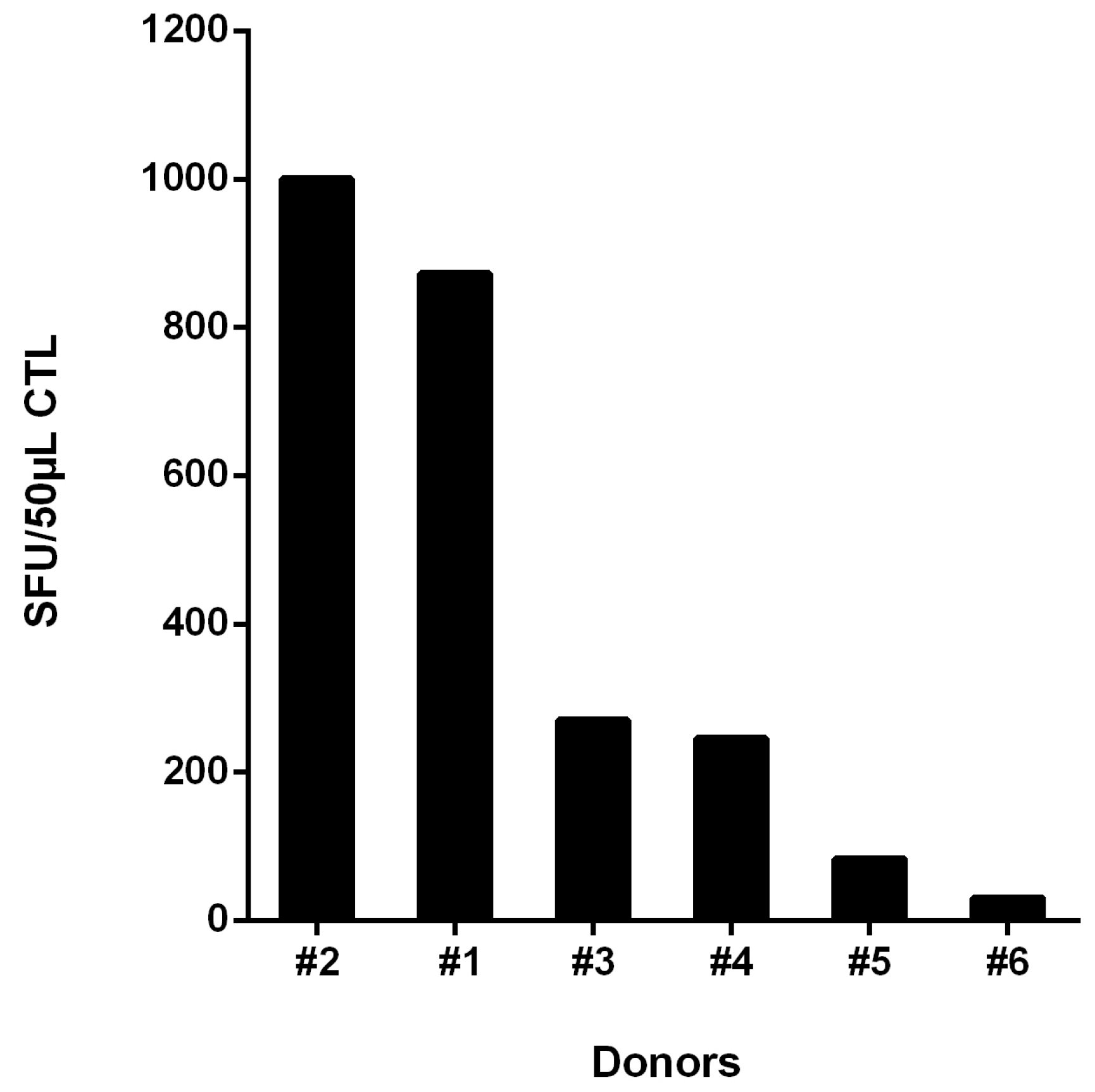
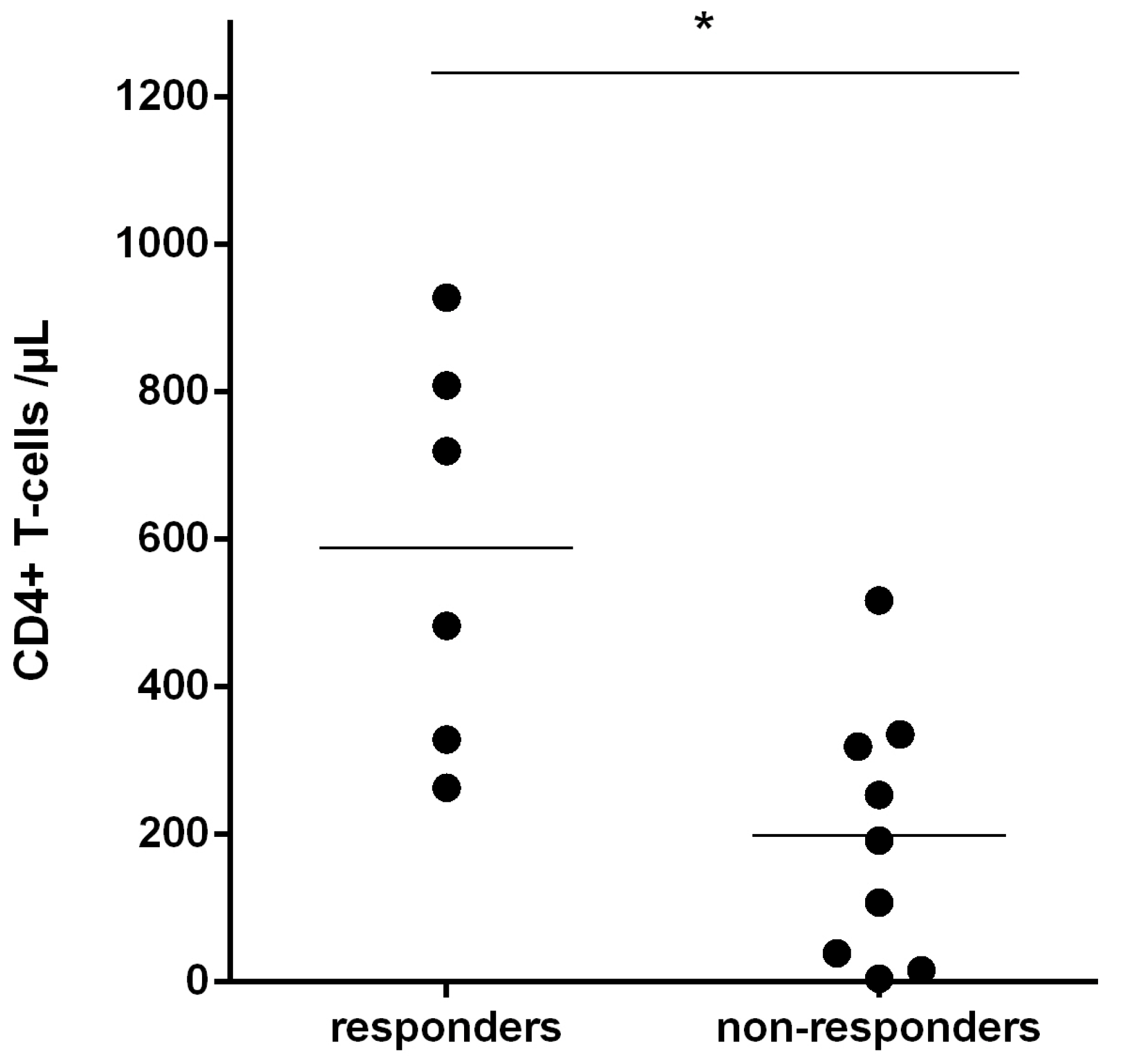
3.4. Recognition of Rev-RI8 in a Cohort of HLA-B*52-Positive HIV-1-Infected Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koup, R.A.; Safrit, J.T.; Cao, Y.; Andrews, C.A.; McLeod, G.; Borkowsky, W.; Farthing, C.; Ho, D.D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 1994, 68, 4650–4655. [Google Scholar] [CrossRef]
- Ogg, G.S.; Jin, X.; Bonhoeffer, S.; Dunbar, P.R.; Nowak, M.A.; Monard, S.; Segal, J.P.; Cao, Y.; Rowland-Jones, S.L.; Cerundolo, V.; et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 1998, 279, 2103–2106. [Google Scholar] [CrossRef]
- Capa, L.; Ayala-Suárez, R.; De La Torre Tarazona, H.E.; González-García, J.; del Romero, J.; Alcamí, J.; Díez-Fuertes, F. Elite controllers long-term non progressors present improved survival and slower disease progression. Sci. Rep. 2022, 12, 16356. [Google Scholar] [CrossRef]
- Altfeld, M.; Addo, M.M.; Rosenberg, E.S.; Hecht, F.M.; Lee, P.K.; Vogel, M.; Yu, X.G.; Draenert, R.; Johnston, M.N.; Strick, D.; et al. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS 2003, 17, 2581–2591. [Google Scholar] [CrossRef]
- International, H.I.V.C.S.; Pereyra, F.; Jia, X.; McLaren, P.J.; Telenti, A.; de Bakker, P.I.; Walker, B.D.; Ripke, S.; Brumme, C.J.; Pulit, S.L.; et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2010, 330, 1551–1557. [Google Scholar]
- Migueles, S.A.; Sabbaghian, M.S.; Shupert, W.L.; Bettinotti, M.P.; Marincola, F.M.; Martino, L.; Hallahan, C.W.; Selig, S.M.; Schwartz, D.; Sullivan, J.; et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 2000, 97, 2709–2714. [Google Scholar] [CrossRef]
- Schneidewind, A.; Brockman, M.A.; Sidney, J.; Wang, Y.E.; Chen, H.; Suscovich, T.J.; Li, B.; Adam, R.I.; Allgaier, R.L.; Mothe, B.R.; et al. Structural and functional constraints limit options for cytotoxic T-lymphocyte escape in the immunodominant HLA-B27-restricted epitope in human immunodeficiency virus type 1 capsid. J. Virol. 2008, 82, 5594–5605. [Google Scholar] [CrossRef]
- Gaiha, G.D.; Rossin, E.J.; Urbach, J.; Landeros, C.; Collins, D.R.; Nwonu, C.; Muzhingi, I.; Anahtar, M.N.; Waring, O.M.; Piechocka-Trocha, A.; et al. Structural topology defines protective CD8(+) T cell epitopes in the HIV proteome. Science 2019, 364, 480–484. [Google Scholar] [CrossRef]
- Wagner, R.; Leschonsky, B.; Harrer, E.; Paulus, C.; Weber, C.; Walker, B.D.; Buchbinder, S.; Wolf, H.; Kalden, J.R.; Harrer, T. Molecular and functional analysis of a conserved CTL epitope in HIV-1 p24 recognized from a long-term nonprogressor: Constraints on immune escape associated with targeting a sequence essential for viral replication. J. Immunol. 1999, 162, 3727–3734. [Google Scholar] [CrossRef]
- Carlson, J.M.; Listgarten, J.; Pfeifer, N.; Tan, V.; Kadie, C.; Walker, B.D.; Ndung’u, T.; Shapiro, R.; Frater, J.; Brumme, Z.L.; et al. Widespread impact of HLA restriction on immune control and escape pathways of HIV-1. J. Virol. 2012, 86, 5230–5243. [Google Scholar] [CrossRef]
- Kelleher, A.D.; Long, C.; Holmes, E.C.; Allen, R.L.; Wilson, J.; Conlon, C.; Workman, C.; Shaunak, S.; Olson, K.; Goulder, P.; et al. Clustered mutations in HIV-1 gag are consistently required for escape from HLA-B27-restricted cytotoxic T lymphocyte responses. J. Exp. Med. 2001, 193, 375–386. [Google Scholar] [CrossRef]
- Carlson, J.M.; Brumme, C.J.; Martin, E.; Listgarten, J.; Brockman, M.A.; Le, A.Q.; Chui, C.K.; Cotton, L.A.; Knapp, D.J.; Riddler, S.A.; et al. Correlates of protective cellular immunity revealed by analysis of population-level immune escape pathways in HIV-1. J. Virol. 2012, 86, 13202–13216. [Google Scholar] [CrossRef]
- Gorin, A.M.; Du, Y.; Liu, F.Y.; Zhang, T.H.; Ng, H.L.; Hofmann, C.; Cumberland, W.G.; Sun, R.; Yang, O.O. HIV-1 epitopes presented by MHC class I types associated with superior immune containment of viremia have highly constrained fitness landscapes. PLoS Pathog. 2017, 13, e1006541. [Google Scholar] [CrossRef]
- David-Fung, E.-S.; Korber, B.T.M.; Brander, C.; Barouch, D.; de Boer, R.; Haynes, B.F.; Koup, R.; Moore, J.P.; Walker, B.D.; Watkins, D.I. HIV Molecular Immunology 2021; Los Alamos National Laboratory, Theoretical Biology and Biophysics: Los Alamos, NM, USA, 2021; Volume LA-UR-21-32446. [Google Scholar]
- Harrer, T.; Harrer, E.; Kalams, S.A.; Elbeik, T.; Staprans, S.I.; Feinberg, M.B.; Cao, Y.; Ho, D.D.; Yilma, T.; Caliendo, A.M.; et al. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res. Hum. Retrovir. 1996, 12, 585–592. [Google Scholar] [CrossRef]
- Antoni, G.; Guergnon, J.; Meaudre, C.; Samri, A.; Boufassa, F.; Goujard, C.; Lambotte, O.; Autran, B.; Rouzioux, C.; Costagliola, D.; et al. MHC-driven HIV-1 control on the long run is not systematically determined at early times post-HIV-1 infection. AIDS 2013, 27, 1707–1716. [Google Scholar] [CrossRef]
- Fellay, J.; Shianna, K.V.; Ge, D.; Colombo, S.; Ledergerber, B.; Weale, M.; Zhang, K.; Gumbs, C.; Castagna, A.; Cossarizza, A.; et al. A whole-genome association study of major determinants for host control of HIV-1. Science 2007, 317, 944–947. [Google Scholar] [CrossRef]
- Falk, K.; Rötzschke, O.; Takiguchi, M.; Gnau, V.; Stevanović, S.; Jung, G.; Rammensee, H.G. Peptide motifs of HLA-B*51, -B*52 and -B78 molecules, and implications for Behćet’s disease. Int. Immunol. 1995, 7, 223–228. [Google Scholar] [CrossRef]
- Apetrei, C.H.B.; Rambaut, A.; Wolinsky, B.J.R.; Keele, B.; Faser, C. (Eds.) HIV Sequence Compendium 2019, LA-UR 23-20934 ed.; Theoretical Biology and Biophysics Group, Los Alamos National Laboratory: Los Alamos, NM, USA, 2019. [Google Scholar]
- Fabio, G.; Scorza, R.; Lazzarin, A.; Marchini, M.; Zarantonello, M.; D’Arminio, A.; Marchisio, P.; Plebani, A.; Luzzati, R.; Costigliola, P. HLA-associated susceptibility to HIV-1 infection. Clin. Exp. Immunol. 1992, 87, 20–23. [Google Scholar] [CrossRef]
- Teixeira, S.L.; de Sa, N.B.; Campos, D.P.; Coelho, A.B.; Guimaraes, M.L.; Leite, T.C.; Veloso, V.G.; Morgado, M.G. Association of the HLA-B*52 allele with non-progression to AIDS in Brazilian HIV-1-infected individuals. Genes Immun. 2014, 15, 256–262. [Google Scholar] [CrossRef]
- Tsai, M.C.; Singh, S.; Adland, E.; Goulder, P. Impact of HLA-B*52:01-Driven Escape Mutations on Viral Replicative Capacity. J. Virol. 2020, 94, e02025-19. [Google Scholar] [CrossRef]
- Murakoshi, H.; Akahoshi, T.; Koyanagi, M.; Chikata, T.; Naruto, T.; Maruyama, R.; Tamura, Y.; Ishizuka, N.; Gatanaga, H.; Oka, S.; et al. Clinical Control of HIV-1 by Cytotoxic T Cells Specific for Multiple Conserved Epitopes. J. Virol. 2015, 89, 5330–5339. [Google Scholar] [CrossRef]
- Naruto, T.; Gatanaga, H.; Nelson, G.; Sakai, K.; Carrington, M.; Oka, S.; Takiguchi, M. HLA class I-mediated control of HIV-1 in the Japanese population, in which the protective HLA-B*57 and HLA-B*27 alleles are absent. J. Virol. 2012, 86, 10870–10872. [Google Scholar] [CrossRef]
- Ramirez de Arellano, E.; Diez-Fuertes, F.; Aguilar, F.; de la Torre Tarazona, H.E.; Sanchez-Lara, S.; Lao, Y.; Vicario, J.L.; Garcia, F.; Gonzalez-Garcia, J.; Pulido, F.; et al. Novel association of five HLA alleles with HIV-1 progression in Spanish long-term non progressor patients. PLoS ONE 2019, 14, e0220459. [Google Scholar] [CrossRef]
- Murakoshi, H.; Koyanagi, M.; Chikata, T.; Rahman, M.A.; Kuse, N.; Sakai, K.; Gatanaga, H.; Oka, S.; Takiguchi, M. Accumulation of Pol Mutations Selected by HLA-B*52:01-C*12:02 Protective Haplotype-Restricted Cytotoxic T Lymphocytes Causes Low Plasma Viral Load Due to Low Viral Fitness of Mutant Viruses. J. Virol. 2017, 91, e02082-16. [Google Scholar] [CrossRef]
- Sloan, E.A.; Kearney, M.F.; Gray, L.R.; Anastos, K.; Daar, E.S.; Margolick, J.; Maldarelli, F.; Hammarskjold, M.L.; Rekosh, D. Limited nucleotide changes in the Rev response element (RRE) during HIV-1 infection alter overall Rev-RRE activity and Rev multimerization. J. Virol. 2013, 87, 11173–11186. [Google Scholar] [CrossRef]
- Addo, M.M.; Altfeld, M.; Rosenberg, E.S.; Eldridge, R.L.; Philips, M.N.; Habeeb, K.; Khatri, A.; Brander, C.; Robbins, G.K.; Mazzara, G.P.; et al. The HIV-1 regulatory proteins Tat and Rev are frequently targeted by cytotoxic T lymphocytes derived from HIV-1-infected individuals. Proc. Natl. Acad. Sci. USA 2001, 98, 1781–1786. [Google Scholar] [CrossRef]
- Jia, M.M.; Hong, K.X.; Chen, J.P.; Liu, H.W.; Liu, S.; Zhang, X.Q.; Zhao, H.J.; Shao, Y.M. CTL responses to regulatory proteins Tat and Rev in HIV-1 B’/C virus-infected individuals. Biomed. Environ. Sci. 2008, 21, 314–318. [Google Scholar] [CrossRef]
- Henderson, G.J.; Lee, S.K.; Irlbeck, D.M.; Harris, J.; Kline, M.; Pollom, E.; Parkin, N.; Swanstrom, R. Interplay between single resistance-associated mutations in the HIV-1 protease and viral infectivity, protease activity, and inhibitor sensitivity. Antimicrob. Agents Chemother. 2012, 56, 623–633. [Google Scholar] [CrossRef]
- Harrigan, P.R.; Kinghorn, I.; Bloor, S.; Kemp, S.D.; Nájera, I.; Kohli, A.; Larder, B.A. Significance of amino acid variation at human immunodeficiency virus type 1 reverse transcriptase residue 210 for zidovudine susceptibility. J. Virol. 1996, 70, 5930–5934. [Google Scholar] [CrossRef]
- Miura, T.; Brumme, Z.L.; Brockman, M.A.; Rosato, P.; Sela, J.; Brumme, C.J.; Pereyra, F.; Kaufmann, D.E.; Trocha, A.; Block, B.L.; et al. Impaired replication capacity of acute/early viruses in persons who become HIV controllers. J. Virol. 2010, 84, 7581–7591. [Google Scholar] [CrossRef]
- Zuñiga, R.; Lucchetti, A.; Galvan, P.; Sanchez, S.; Sanchez, C.; Hernandez, A.; Sanchez, H.; Frahm, N.; Linde, C.H.; Hewitt, H.S.; et al. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J. Virol. 2006, 80, 3122–3125. [Google Scholar] [CrossRef]
- Edwards, B.H.; Bansal, A.; Sabbaj, S.; Bakari, J.; Mulligan, M.J.; Goepfert, P.A. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 2002, 76, 2298–2305. [Google Scholar] [CrossRef]
- Tsomides, T.J.; Aldovini, A.; Johnson, R.P.; Walker, B.D.; Young, R.A.; Eisen, H.N. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J. Exp. Med. 1994, 180, 1283–1293. [Google Scholar] [CrossRef]
- Cohen, W.M.; Bianco, A.; Connan, F.; Camoin, L.; Dalod, M.; Lauvau, G.; Ferries, E.; Culmann-Penciolelli, B.; van Endert, P.M.; Briand, J.P.; et al. Study of antigen-processing steps reveals preferences explaining differential biological outcomes of two HLA-A2-restricted immunodominant epitopes from human immunodeficiency virus type 1. J. Virol. 2002, 76, 10219–10225. [Google Scholar] [CrossRef]
- Sacha, J.B.; Chung, C.; Rakasz, E.G.; Spencer, S.P.; Jonas, A.K.; Bean, A.T.; Lee, W.; Burwitz, B.J.; Stephany, J.J.; Loffredo, J.T.; et al. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J. Immunol. 2007, 178, 2746–2754. [Google Scholar] [CrossRef]
- Miura, T.; Brockman, M.A.; Schneidewind, A.; Lobritz, M.; Pereyra, F.; Rathod, A.; Block, B.L.; Brumme, Z.L.; Brumme, C.J.; Baker, B.; et al. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte [corrected] recognition. J. Virol. 2009, 83, 2743–2755. [Google Scholar] [CrossRef]
- Boutwell, C.L.; Rowley, C.F.; Essex, M. Reduced viral replication capacity of human immunodeficiency virus type 1 subtype C caused by cytotoxic-T-lymphocyte escape mutations in HLA-B57 epitopes of capsid protein. J. Virol. 2009, 83, 2460–2468. [Google Scholar] [CrossRef]
- Gijsbers, E.F.; Feenstra, K.A.; van Nuenen, A.C.; Navis, M.; Heringa, J.; Schuitemaker, H.; Kootstra, N.A. HIV-1 replication fitness of HLA-B*57/58:01 CTL escape variants is restored by the accumulation of compensatory mutations in gag. PLoS ONE 2013, 8, e81235. [Google Scholar] [CrossRef]
- Brockman, M.A.; Schneidewind, A.; Lahaie, M.; Schmidt, A.; Miura, T.; Desouza, I.; Ryvkin, F.; Derdeyn, C.A.; Allen, S.; Hunter, E.; et al. Escape and compensation from early HLA-B57-mediated cytotoxic T-lymphocyte pressure on human immunodeficiency virus type 1 Gag alter capsid interactions with cyclophilin A. J. Virol. 2007, 81, 12608–12618. [Google Scholar] [CrossRef]
- Ganusov, V.V.; Goonetilleke, N.; Liu, M.K.; Ferrari, G.; Shaw, G.M.; McMichael, A.J.; Borrow, P.; Korber, B.T.; Perelson, A.S. Fitness costs and diversity of the cytotoxic T lymphocyte (CTL) response determine the rate of CTL escape during acute and chronic phases of HIV infection. J. Virol. 2011, 85, 10518–10528. [Google Scholar] [CrossRef]
| Days after Diagnosis | CD4/µL | CD8/µL | Viral Load in Copies/mL |
|---|---|---|---|
| 0 | 1004 | 466 | 40 |
| 4 | n.d. 1 | n.d. 1 | <20 |
| 14 | 857 | 529 | 20 |
| 125 | 977 | 553 | 20 |
| 204 | 1074 | 547 | 20 |
| 293 | 863 | 325 | 20 |
| 383 | 928 | 439 | 250 |
| 453 | 1184 | 592 | <20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Kenz, B.; Schmidt, K.G.; Ogungbemi-Alt, V.K.; Bergmann, S.; Steininger, P.; Korn, K.; Spriewald, B.; Harrer, E.G.; Nganou-Makamdop, K.; Harrer, T. Definition of a New HLA B*52-Restricted Rev CTL Epitope Targeted by an HIV-1-Infected Controller. Viruses 2023, 15, 567. https://doi.org/10.3390/v15020567
El Kenz B, Schmidt KG, Ogungbemi-Alt VK, Bergmann S, Steininger P, Korn K, Spriewald B, Harrer EG, Nganou-Makamdop K, Harrer T. Definition of a New HLA B*52-Restricted Rev CTL Epitope Targeted by an HIV-1-Infected Controller. Viruses. 2023; 15(2):567. https://doi.org/10.3390/v15020567
Chicago/Turabian StyleEl Kenz, Boutaina, Katja G. Schmidt, Victoria K. Ogungbemi-Alt, Silke Bergmann, Philipp Steininger, Klaus Korn, Bernd Spriewald, Ellen G. Harrer, Krystelle Nganou-Makamdop, and Thomas Harrer. 2023. "Definition of a New HLA B*52-Restricted Rev CTL Epitope Targeted by an HIV-1-Infected Controller" Viruses 15, no. 2: 567. https://doi.org/10.3390/v15020567
APA StyleEl Kenz, B., Schmidt, K. G., Ogungbemi-Alt, V. K., Bergmann, S., Steininger, P., Korn, K., Spriewald, B., Harrer, E. G., Nganou-Makamdop, K., & Harrer, T. (2023). Definition of a New HLA B*52-Restricted Rev CTL Epitope Targeted by an HIV-1-Infected Controller. Viruses, 15(2), 567. https://doi.org/10.3390/v15020567






