Abstract
Hemorrhagic Fever with Renal Syndrome (HFRS) is the most frequently diagnosed zoonosis in Asia. This zoonotic infection is the result of exposure to the virus-contaminated aerosols. Orthohantavirus infection may cause Hemorrhagic Fever with Renal Syndrome (HRFS), a disease that is characterized by acute kidney injury and increased vascular permeability. Several species of orthohantaviruses were identified as causing infection, where Hantaan, Puumala, and Seoul viruses are most common. Orthohantaviruses are endemic to several Asian countries, such as China, South Korea, and Japan. Along with those countries, HFRS tops the list of zoonotic infections in the Far Eastern Federal District of Russia. Recently, orthohantavirus circulation was demonstrated in small mammals in Thailand and India, where orthohantavirus was not believed to be endemic. In this review, we summarized the current data on orthohantaviruses in Asia. We gave the synopsis of the history and diversity of orthohantaviruses in Asia. We also described the clinical presentation and current understanding of the pathogenesis of orthohantavirus infection. Additionally, conventional and novel approaches for preventing and treating orthohantavirus infection are discussed.
1. Introduction
Orthohantaviruses, are zoonotic pathogens belonging to the genus Orthohantavirus family Hantaviridae [1,2]. Multiple members of the genus Orthohantavirus are human pathogens: Hantaan virus (HNTV), Seoul virus (SEOV), Puumala virus (PUUV), and Dobrava-Belgrade virus (DOBV). Presently, 38 orthohantavirus species have been identified [1], out of which at least 24 are capable of causing infectious diseases in humans [3]. Orthohantaviruses can cause two acute febrile diseases: Hemorrhagic Fever with Renal Syndrome (HFRS) and Hantavirus Cardiopulmonary Syndrome (HCPS) [4,5]. HFRS and HCPS are confined to the geographic distribution of natural small mammal hosts [4]. The group of orthohantaviruses which cause HFRS to circulate primarily in small rodents inhabiting the European and Asian regions [6,7,8].
Orthohantaviruses have a negative sense single-stranded RNA genome, which is organized into three segments: small (S), medium (M), and large (L) (Figure 1). These RNAs encode nucleocapsid (N) protein, glycoprotein precursor (GPC), which is processed into two glycoproteins (Gn and Gc), and the viral RNA-dependent RNA polymerase (RdRp) protein, respectively [9].
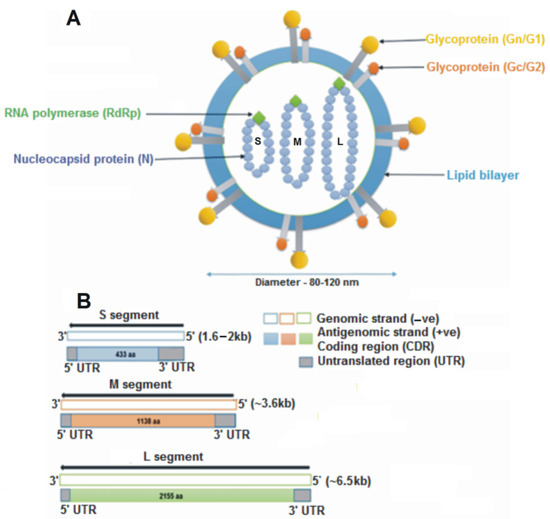
Figure 1.
Schematic representation of an orthohantavirus virion. (A) The structure is spherical, having a diameter of 8–120 nm, enveloped with a lipid bilayer containing spikes of glycoproteins (Gn and Gc). Inside the virion are three segments of single-stranded RNA: small (S), medium (M) and large (L). (B) The S, M, and L genomic segments encode for the nucleocapsid protein (433 aa), glycoprotein precursor (1138 aa) and the RNA-dependent RNA polymerase (2155 aa), respectively.
Orthohantaviruses are endemic in many Asian countries, with the largest number of cases reported in China, South Korea, and the Far Eastern Federal District of Russia [9]. This review provides a history of the orthohantaviruses and the disease they cause in Asia, with particular emphasis on the clinical manifestation and pathogenesis. We will also address challenges in the prevention, treatment, and diagnosis of orthohantaviruses in Asia.
2. History of Orthohantaviruses and the Disease They Cause in Asia
In China, febrile disease with renal dysfunction was documented as early as 1931 [4]. Since then, HFRS has been acknowledged as an endemic disease in this country, where 40–50% of worldwide HFRS cases are diagnosed [9]. China remains the most active HFRS endemic region, where cases increased from 10,378 reported in 1931–1949 [10] to 1,557,622 diagnosed between 1950 and 2007 [11]. In recent decades, the incidence of HFRS in China has decreased. During the period 2004–2019, a total of 209,209 cases of the disease were registered, of which 1855 were fatal [12]. Epidemiological analysis indicated several “hot spots” in China with a high risk of orthohantavirus infection: Shandon, Heilongjiang, Hunan, Jiangxi, and Zhejiang provinces [4].
Still, HFRS is endemic in the Far Eastern Federal District of Russia, where 3145 cases were reported in 15 of the 29 regions of Asian Russia between 1978 and 1995 [4]. The endemic orthohantavirus has a long history in this region, with the first HFRS cases reported in 1934 in Khabarovsk, Primorsky Krai, and the Amur Region. Since then, cases of HFRS have been reported in other regions of Russia [13]. It was suggested that in Asian Russia the disease was only limited to the Far East region of Russia [14,15,16,17].
Symptoms like HFRS were also described in approximately 1700 US soldiers during the Korean War (1950–1953), with less than 5% resulting in deaths [18]. Later, symptoms similar to HFRS from the Far East region were noticed in patients from Scandinavia [15]. At that time, knowledge of the infectious agent causing HFRS remained limited [19,20]. Human infection was usually associated with exposure to excreta from small rodents [21].
The identity of an infectious agent was revealed several years after, following a retrospective study of clinical cases. In 1978, Dr. Lee isolated the etiologic agent of hemorrhagic fever from the Apodemus agrarius lung tissue, which reacted with convalescent serum from HFRS patients [22]. The morphology of a new virus, named the Hantaan virus, was identified as a member of the family Bunyaviridae [23].
In 1980, antibodies to the causative agent of epidemic nephropathy (NE) were detected in the bank vole [24]. Later a causative agent was identified as hantavirus and named Puumala (PUUV) after the Puumala municipality in Finland. Two years later, Lee et al. discovered a variant of the Hantaan virus, now known as SEOV, which caused severe HFRS in Seoul city residents [8]. Since patients had no history of traveling outside the city, urban rats were suggested as the natural reservoir of this virus in South Korea. The retrospective research showed that 15% of the rats in Seoul and 28% in Tokyo carried the newly identified virus [25].
The first cases of suspected orthohantavirus infection in India were reported in 1964 in Vellore [26]. Investigation of these outbreaks led to identifying a novel member of the genus Orthohantavirus named Thottapalayam virus (TPMV) [26,27]. Initially, this virus was isolated from a non-rodent host, the house musk shrew (Suncus murinus) [26]. Although TPMV had morphological and genetic similarities with members of the genus Orthohantavirus of the family Hantaviridae, later, TPMV was shown to form a phylogenetically distinct genus [1]. Therefore, it was assigned a new name, a Thottapalayam thottimvirus, which is a proto-type shrew-borne orthohantavirus that belongs to the genus Thottimvirus. In addition to Vellore, a high rate of orthohantavirus seropositivity was reported in healthy individuals from the Cochin and Chennai regions of India [28]. Analysis of serum samples revealed the cross-reactivity with SEOV in 12% and PUUV in 5% of individuals, respectively [28]. In another study of 152 serum samples, 23 reacted with HTNV, PUUV, or SEOV [29]. These results provide strong evidence that orthohantaviruses are endemic in India.
Hantavirus-reactive antibodies were found in serum samples from 5461 small mammals belonging to 16 different species in Taiwan [30]. It was demonstrated that Rattus norvegicus was the most common rodent species captured in that region, and these rodents were major contributors to orthohantavirus circulation in small mammals in the region. Using reverse transcriptase polymerase chain reaction (RT-PCR), SEOV was detected in Rattus norvegicus in lung tissues. As a result of further analyses, it was discovered that almost all orthohantavirus infections in Taiwan were caused by SEOV [30].
Also, 3.8% of rodents captured in the Nakhon Pathom region were seropositive for orthohantavirus antigens [31]. All the sera-positive rodents were Bandicoot indica species. It was shown that these rodents were carrying the Hantaan-like virus [31]. Antibodies to the Hantaan-like virus were found in humans living near locations of infected rodents. The positive titer of antibodies to Hantavirus varied from 5–7% to 31–33 [31]. Later this virus was named Thailand orthohantavirus (THAIV).
For the first time after the detection of hantaviruses, the prevailing opinion was that their carriers were rodents; however, later studies have identified hantavirus antigen (virus) or/and specific antibodies in domestic animals, such as cats, rabbits, and dogs [32]. A case of human infection with hantavirus from a domestic rat has also been reported [33]. The geographic distribution of orthohantaviruses endemic to Asia and their small mammal hosts are summarized in Table 1.

Table 1.
Geographic distribution of orthohantaviruses and their host reservoirs.
3. Clinical Presentation of Orthohantavirus Disease in Asia
More than 38 orthohantavirus species are known to cause human disease worldwide. While 20 are associated with the disease in Asia, the spectrum ranges from acute febrile illness with or without renal impairment, fever with shock, and multi-organ failure to hemorrhagic illness such as HFRS. Hemorrhage and acute renal injury (AKI) are common clinical manifestations in severe HFRS cases [51].
Clinical presentation of HFRS may range from subclinical, mild, and moderate to severe. HTNV, DOBV, and AMRV are more often cause severe HFRS, while the moderate form is frequently diagnosed in SEOV causes. Also, Nephropathia epidemica (NE), a mild form of HFRS linked to PUUV infection, is diagnosed in Russia [52].
The incubation period of HFRS generally ranges between two and four weeks; however, in some cases, it could be as long as six weeks [52]. Clinically, HFRS progresses through five phases: febrile, hypotensive, oliguric, polyuric, and convalescent. These phases are demarcated in severe HFRS but may overlap or be absent in mild and moderate forms of the disease [52,53].
The febrile phase usually lasts three to seven days and is characterized by high fever, chills, headache, backache, abdominal pains, nausea, and vomiting. Symptoms are non-specific, making orthohantavirus infection diagnosis challenging during this phase. By day three to four post-onset, hemorrhagic manifestations appear in the forms of diffuse petechiae on the conjunctiva and the palate. AKI symptoms of hematuria and proteinuria become evident by day seven of the disease [54].
Shock is typical for the severe form of HFRS. Approximately 11–40% of febrile patients develop hypotension, and one-third have a shock [52]. The hypotensive phase could last from several hours to a couple of days. Thrombocytopenia and leukocytosis are commonly documented during this phase. Also, symptoms of an AKI, such as acute tubulointerstitial nephritis, necrotizing glomerulonephritis, and IgA nephropathy, are characteristic of this phase.
The oliguric phase lasts three to seven days. Patients are at risk of hypotension, severe pulmonary edema, and AKI, as symptoms of oliguria, anuria, proteinuria, hematuria, and azotemia are commonly described. The severe form of HFRS could require hemodialysis at this phase. This is the critical phase, as half of the total fatalities occur during this phase. Typical laboratory findings are elevated serum creatinine and urea [9,55]. The polyuric phase, which could last for days or weeks, is characterized by increased urinary output. The renal function restores, and symptoms of AKI dissolve.
Convalescence is usually extended and could last up to six months. Recovery is complete, though sequelae of chronic renal failure and hypertension have been reported [56,57]. The fatality rate of 5–15% in HTNV/DOBV-related HFRS is likely due to complications, such as renal insufficiency, edema, hemorrhages, encephalopathy, and shock. SEO infection causes a moderate form of HFRS with a clinical presentation similar to HFRS caused by HTNV [58]. The SEOV infection fatality rate is 1% [59]. Even lower than 1.0% is the mortality rate of PUUV infection [60].
4. Pathogenesis of Orthohantavirus Infections
It is believed that orthohantavirus infection results from inhaling virus-contaminated aerosol or other forms of contact with the virus [61,62]. The initial site of orthohantavirus replication appears to be the respiratory tract. The susceptibility of human epithelial cells derived from bronchi, bronchioles, and alveoli to PUUV infection was recently in vitro [63]. The most intriguing finding was the substantial donor-specific variation in the efficacy of virus replication in respiratory epithelial cells. These data provide the basis for an individual-specific susceptibility to orthohantavirus infection.
Infection of respiratory epithelial cells and dissemination of orthohantavirus happens early before clinical symptoms can be identified (Figure 2). Several studies have demonstrated orthohantavirus RNA in the serum of patients up to three weeks before the onset of the disease [64,65,66]. These data indicate that the virus replicates in the respiratory tissue; however, the body’s reaction to infection is delayed. The non-cytopathic nature of orthohantavirus replication could explain this delay in clinical symptoms. The lack of infected cell death was demonstrated in vitro [67,68]. Also, virus replication-explained damage was not found in tissues collected from orthohantavirus-infected patients [69,70,71]. In contrast, HNTV RNA was detected in the plasma of patients at an early stage of HFRS and the high viral load led to the correlation with the severity of the disease [72]. Also, DOBV RNA level in HFRS patients’ serum correlated with disease severity [73].
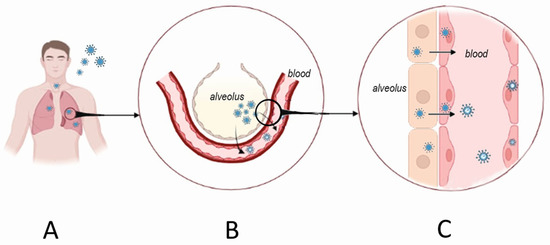
Figure 2.
Orthohantavirus infection portal of entry and initial replication sites. Orthohantaviruses use the respiratory tract as one of the portals of entry (A). The initial replication site is believed to be in alveolar pneumocytes. From there, the virus crosses the respiratory membrane infecting nearby endothelial cells of the alveolar capillaries (B). These steps occur before the patient presents with HFRS symptoms. Once orthohantavirus infects endothelial cells, it becomes released into the blood leading to viremia (C). The immune system’s reaction to viremia could cause tissue damage, while increased endothelial permeability and blood coagulation could result from infected endothelial cell activation.
Slow virus replication capacity could contribute to delayed symptom development after the beginning of viremia [74].
Infection of endothelial cells is the next crucial step in orthohantavirus pathogenesis. Orthohantavirus most likely reaches the endothelial cells of the respiratory tract at the gas exchange membrane site first. Then, viremia follows, making endothelial cells of the small vessels the main target of infection [75]. Endothelial cells are commonly found positive for orthohantavirus antigens in biopsies, and postmortem collected tissues [63,75,76]. Endothelial cells were also susceptible to orthohantavirus infection and support virus replication in vitro [77]. For cell entry, orthohantaviruses use integrin receptors [77], which are expressed in endothelial cells [78].
Infection and replication in endothelial cells appear essential for the pathogenesis of orthohantavirus disease. There are three main consequences of orthohantavirus infection of endothelial cells: (a) loss of the endothelium barrier integrity, (b) activation of coagulation, and (c) release of cytokines and activation of the immune response. The loss of the blood tissue barrier function will increase vascular permeability leading to edema and hemorrhages [53]. Orthohantavirus infection activates endothelial cells to initiate thrombocyte aggregation and blood coagulation. Also, infection of endothelial cells could initiate the release of various cytokines with the potential to induce inflammation, activate the immune response, and sustain the impaired permeability of the endothelium.
Loss of endothelium barrier function: Orthohantaviruses endemic in Asia use beta integrin receptors to adhere to and penetrate endothelial cells [79]. Virus replication is demonstrated in vitro and in vivo without cytopathic effect [67,68,80]. Therefore, the loss of the barrier function is not essential for virus replication but rather the result of the endothelial cells’ response to infection (Figure 3). This assumption is supported by an in vitro study by Gorbunova et al., which demonstrated vascular endothelial (VE)-cadherin internalization in HNTV-infected endothelial cells [81]. In another study, Wang et al. demonstrated the formation of a functional complex between β3 integrin and VEGFR2 in HNTV-infected cells [82]. This complex could initiate signaling leading to cytoskeleton reorganization and subsequently, hyperpermeability. These authors also confirmed the role of VEGF in the disruption of junctions between endothelial cells. Decreased expression of Claudin-1, a tight junction (TJ) component [83], was found in HNTV-infected endothelial cells [84]. Another molecule part of the TJ zona occludens 1 (ZO-1) was altered in HFRS kidney biopsies [85]. Krautkramer et al. reported decreased expression of ZO-1 in the kidney tubular epithelium of orthohantavirus-infected patients [85]. Recently, the role of protocadherin one as an orthohantavirus receptor was demonstrated by Dieterle et al. [86] and Jangra et al. [87]. Its involvement in virus entry supports the hypothesis of the profoundly disturbing endothelial monolayer integrity in orthohantavirus-infected patients. Together, these data provide strong evidence for the substantial destruction of the structures maintaining endothelium integrity.
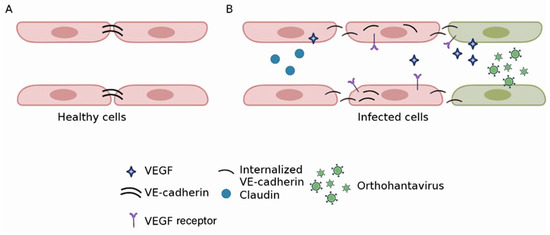
Figure 3.
Orthohantavirus effect on endothelium barrier integrity. In uninfected endothelial cells, VE-cadherin is expressed in junctions between adjacent cells (A). In orthohantavirus-infected cells, a profound reorganization of VE-cadherin was described (B). It was shown that VE-cadherin becomes internalized in orthohantavirus-infected endothelial cells. This change in molecule expression appears to be a response to the binding of infected cells to VEGF. As a result, the integrity of adherence junctions becomes compromised, leading to increased endothelium permeability. Expression of claudin, a TJ molecule, decreased in orthohantavirus-infected cells. This would also contribute to the disintegration of cell adhesion and vascular leakage.
Data in vivo supports increased endothelial permeability in orthohantavirus-infected patients. These include finding a decreased serum level of the glycocalyx in HFRS [88]. Also, a syndecan-1 level was found to decrease HFRS. Additionally, a lower serum level of pro-angiogenic angiopoietin 1 was demonstrated in HFRS [89].
Activation of coagulation: Decreased expression of von Willebrand (VW) factor in HNTV-infected endothelial cells was demonstrated by Cho et al. [90]. Also, we demonstrated the inhibition of thrombospondin 1 in HNTV-infected endothelial cells in vitro [91]. Our data corroborate Lain et al. findings of decreased thrombospondin 1 level in PUUV-infected patients [61]. Thrombospondin 1, secreted by endothelial cells [92], can directly affect fibrin degradation [93] and cleave vWF [94]. In the absence of thrombospondin 1, ultra-large vWF complexes could cause a spontaneous aggregation of platelets and thrombosis [94].
There is evidence of disturbed hemostasis in orthohantavirus-infected patients. Thrombocytopenia is an early and most consistent sign of HFRS, indicating disturbed hemostasis [95,96]. It appears that thrombocytopenia contributes to the pathogenesis of HFRS. Nadir thrombocyte counts were explained by platelet consumption and decreased survival time [97,98]. Wang et al. found a correlation between low thrombocyte counts and AKI [57]. A similar conclusion was made by Rasche, et al., in 15 PUUV convalescent patients [99]. In contrast, only a correlation between thrombocytopenia, the severity of inflammation, and capillary leakage was found by Outinen et al. [95], while the severity of AKI was independent of nadir thrombocyte counts.
Coagulation is activated in the acute phase of the infection [88,100]. An increased level of circulating prothrombin fragments 1 + 2, the fibrin D-dimers as well as consumption of the anticoagulant antithrombin was also demonstrated in acute orthohantavirus-infected patients [97,101,102]. An elevated serum level of these factors could indicate a risk of thrombosis [101]. This assumption is supported by Connolly-Andersen et al., who demonstrated a high risk of thromboembolism in post-HFRS patients [103]. Analysis of coagulopathy in HFRS demonstrated prolonged prothrombin time and activated partial thromboplastin [98]. Also, decreased levels of coagulation factors II, V, VIII, IX, and X and an increased serum level of fibrinogen were shown to correlate negatively with thrombocytopenia [61,100].
Blood coagulation is a process where dynamic and complex interactions between platelets and endothelial cells lead to the formation of the initial platelet plug [104,105] (Figure 4). Typically, the vascular endothelium has anti-thrombotic properties due to the expression of heparin-like glycosaminoglycans, secretion of platelet inhibitors, coagulation inhibitors, and fibrinolysis activators [106]. However, when endothelial cells are injured or activated, they can express the Tissue Factor (TF) [107,108]. TF initiates the extrinsic pathways of blood coagulation. This pathway appears to be commenced in orthohantavirus-infected patients as increased expression of TF was demonstrated in orthohantavirus-infected endothelial cells [109].
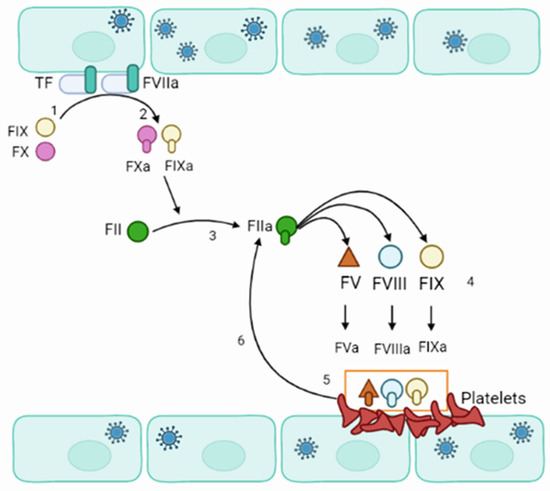
Figure 4.
Schematics of orthohantavirus effect on blood coagulation. (1) Orthohantavirus-infected endothelial cell express TF. (2) TF interacts with active FVII (FVIIa) and calcium to convert FIX and FX to active IXa and Xa, respectively. FXa binds to factor II to form the thrombin (FIIa). (3) Thrombin interacts with FV, FVIII, and FXI. (4) Thrombin activates FV, FVIII, and FXI, forming FVa, FVIIIa, and FXIa. (5,6) FVIIIa forms a complex with FVa and FXa, which acts as a prothrombinase and accelerates the formation of thrombin (FIIa).
Additionally, an increased activity level of the circulating extracellular vesicle tissue factor was shown in HFRS [110]. More pro-coagulation of endothelial cells in orthohantavirus-infected patients is supported by finding glycocalyx degradation in HFRS [88]. Exposed TF could bind to factor VIIa and calcium to support the conversion of factors IX and X to active IXa and Xa, respectively [111]. Factor Xa binds to factor II to form the thrombin (factor IIa) [112]. Thrombin signals platelet activation and aggregation. Thrombin activates factors V, VIII, and XI on the platelet’s surface. The active VIIa forms a complex with active Va and Xa, which acts as a prothrombinase and accelerates the formation of Xa and thrombin. That will generate a large amount of thrombin and cleaves fibrinogen to fibrin monomers. Fibrin monomers start polymerizing and forming fibrin, the coagulation cascade’s final product.
Release of cytokines and activation of the immune response: Human endothelial cells are mainly targeted by orthohantaviruses. Infections of these cells appear to be essential for compromised endothelium barrier function. Endothelial cells were also shown to produce and release cytokines, which was demonstrated in vitro [113,114]. After the initial replication in the lung epithelium, orthohantaviruses could infect the alveolar macrophages in proximity [115] (Figure 5). In addition, they could infect other cells, especially those initiating and propagating the immune response: macrophages and dendritic cells [116,117]. These leukocytes could disseminate the virus to other sites, deliver virus antigens to the lymph nodes, and contribute to the systemic cytokine release and “cytokine storm”. It should be noted that the systemic release of cytokines could further affect the endothelial barrier permeability and facilitate leukocyte adhesion and extravasation [118].
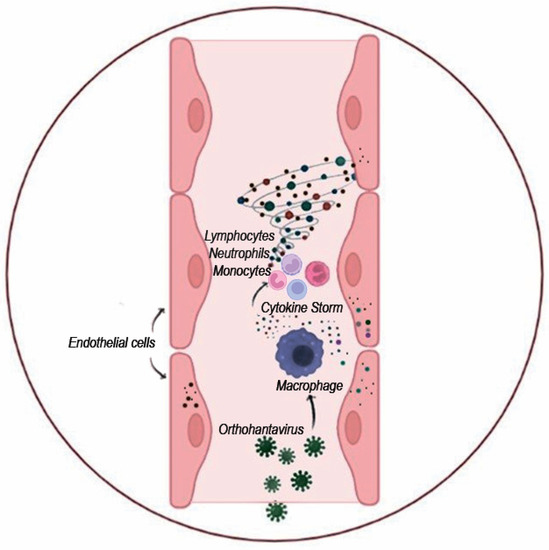
Figure 5.
Schematics of “cytokine storm” activation upon orthohantavirus infection. Orthohantavirus infection activates endothelial cells and leukocytes to produce various cytokines. These cytokines could activate leukocytes and attract them to the site of infection. Massive infiltration of leukocytes producing proinflammatory cytokines and chemoattractants could induce severe local inflammation inflicting tissue destruction.
Early activation of cytokines in HFRS was evident, as their high serum level is commonly detected [119,120,121]. An increased serum level of IL-1β, IL-6, and TNF-α [122], pleiotropic cytokines was demonstrated in patients [123]. Each of these cytokines contributes to the pathophysiology of inflammation, while together, they can potentiate each other’s effect. This synergistic effect of IL-1β, IL-6, and TNF-α is evident in their pyrogenic effect. All three are endogenous pyrogens [124], where IL-6 is required for TNF-α-induced fever [125]. Overall, IL-1β and TNF-α have multiple overlapping functions. TNF-α and IL-1β could increase endothelium permeability [115,126,127]. These combined effects of TNF-α and IL-1β could establish and support the leaky endothelium [126]. IL-1β produces rapid upregulation of TF expression [126], which could contribute to coagulopathy in orthohantavirus-infected patients.
Interestingly, TF expression is also supported by IL-6 [128], indicating a synergistic effect with TNF-α and IL-1β. IL-6 also contributes to inflammation by rapid induction of acute phase proteins produced in the liver [129]. Increased serum levels of C-reactive protein (CRP) [130] and fibrinogen [131], acute phase response proteins, are commonly found in orthohantavirus-infected patients.
In addition to the activation of inflammation, each cytokine has a distinct function. IL-1β is a product of activated inflammasome [132] contributing to developing Th1 and Th17 immune responses. IL-1β supports T cells priming [133] to release IFN-γ. This combination of IL-1β and IFN-γ promotes Th1 immune response [134]. In contrast, when IL-1β is combined with IL-6, the immune response is skewed towards the Th17 type [135,136]. IL-6 also has specific functions, such as stimulation of B cell proliferation [137] and thrombopoiesis [138]. Together, increased serum level of these cytokines suggests activation of Th1 and Th2 type immune responses and thrombocyte production. Clinical laboratory findings support these conclusions: detection of circulating CD8+ lymphocytes [81], increased serum level of anti-orthohantavirus antibodies [139], and activated thrombopoiesis [140].
Chemokines attract leukocytes to the site of infection and can direct the recruitment of specific populations, thus influencing the development of the specific immune response. Activation of the subset of cytokines functionally identified as chemokines is demonstrated in HFRS and NE [122,141,142]. These chemokines, such as CCL4, CCL5, CXCL9, CXCL10, and CXCL12, regulate leukocyte recruitment across the endothelium into the tissue [143]. Chemokines commonly found activated in HFRS and NE attract mononuclear cells (CCL2 and CCL5) with a preference for Th1 lymphocytes, natural killer (NK) cells, and CD8+ lymphocytes (CXCL9 and CXCL10) [144,145,146]. These Th1 lymphocytes and NK cells are essential for protection against virus infection [147,148], especially against orthohantaviruses, which are non-cytopathic [149]. Studies have shown that CD8+ cells from convalescent HFRS could identify and eliminate orthohantavirus-infected endothelial cells [150,151]. The role of these cells in the pathogenesis of the disease is supported by the demonstration of the association between CD8+ lymphocyte count and disease severity [152]. Additionally, the potential contribution of these cells to the pathogenesis of kidney damage was shown by Temonen, et al. [153]. However, the role of CD8+ T lymphocytes in the pathogenesis of the severe form of HFRS could be more complicated, as Wang et al. has demonstrated a higher frequency of IFN-γ producing T cells in patients with a mild and moderate form of HFRS [154]. This data implies that orthohantavirus-specific CD8+ lymphocytes could play a protective role, as was also shown by Tang et al. [155]. In another study, the decline in circulating CD8+ lymphocyte counts correlated with lower virus titer, suggesting their protective role in the pathogenesis of orthohantavirus infection [156].
5. Vaccines and Prevention of Hantavirus Infections
Treatment of orthohantavirus infection is supportive and not specific [62,157]. Therefore, the main form of orthohantavirus control is to prevent infection. Prevention measures include minimizing exposure to rodents and utilizing vaccination.
Minimizing human exposure: Inhaling virus-contaminated aerosol or direct contact with rodent excreta are routes of orthohantavirus infection [158]. In order to minimize exposure, measures should be taken to prevent small rodent entry into buildings. [55,159,160]. Also, rodent control inside and around the home and workplaces will reduce contact with infected small mammals. Rodent urine and droppings should be removed regularly [161]. Clean-up starts with ventilation of the space for at least 30 min [52], followed by spraying and soaking urine and droppings with disinfectant before removal.
Monitoring the rodent population by local authorities is essential for predicting and preventing orthohantavirus outbreaks. There are multiple small mammals identified in Asia that could carry HNTV, SEOV, and THAIV [162]. These carrier mammals include Apodemus agrarius, the primary reservoir host for HTNV [163], and Rattus norvegicus, the carrier for SEOV [164]. Also, Bandicota indica, Rattus rattus, and Eliurus major rat species should be included in the monitoring as they are shown to be reservoirs for THAIV [31,165,166]. Studies have shown that the density of the population could affect the horizontal transmission of orthohantavirus, increasing the number of infected small rodents [167,168]. It was also demonstrated that the threshold density of the rodent population is required to maintain orthohantavirus [169,170,171]. A higher number of infected small mammals could potentially increase the chance of human contact and exposure to orthohantavirus. Therefore, the efficacy of zoonotic carrier population control remains a necessary measure to prevent orthohantavirus infection.
Hantavirus vaccine: There is no World Health Organization (WHO) approved vaccine to prevent orthohantavirus infection. Likewise, local authorities do not approve the orthohantavirus vaccine in endemic areas such as Europe and North and South America [172]. However, inactivated orthohantavirus vaccines are licensed in China and Korea [173]. Inactivated rodent brain or cell culture-derived HFRS vaccines are used in China [11,174]. In 1993, the first inactivated orthohantavirus vaccine was approved in China [11]. Since then, four inactivated HTNV, and SEOV-based vaccines have been used in China and demonstrated the safety and efficacy of protection [175]. Started in 1990, an inactivated HFRS vaccine has also been used in Korea [176]. Since the beginning of vaccination, the number of HFRS cases has reduced significantly [176]. The vaccine’s effectiveness was also reported by Park et al.; however, the authors state that using a large cohort and a long monitoring period is required to make conclusions regarding vaccine efficacy [177].
During the 30 years of the HFRS vaccine development, novel approaches were developed to improve the delivery, orthohantavirus antigens expression, the efficacy of the immune system activation, and reducing side effects.
Inactivated vaccines: These were the first type of vaccines containing inactivated orthohantavirus virion [176]. HNTV was propagated in the brains of suckling mice, followed by chemical inactivation before being tested for immunogenicity [178]. In 1990, this vaccine, under the commercial name Hantavax, was tested in a clinical trial where safety and seroconversion of over 90% was demonstrated [179,180]. Neutralizing antibodies were also demonstrated in 75% of vaccinated one month after the booster [179]. Lesser antibody prevalence, 23 and 41%, as demonstrated in several studies [180,181]. Studies have demonstrated anti-orthohantavirus antibodies several months after immunization [181,182]. Also, the immune response was detected one year after immunization [180]. The protective efficacy of the Hantavax vaccine was suggested to explain the decline in the number of HFRS cases in South Korea between 1991 and 1997 [176]. Later studies aimed to analyze the effect of the Hantavax vaccine to affect the disease progression and demonstrated the reduction of stage 3 acute kidney injury and the requirement for dialysis in the vaccinated cohort [183]. In another study, the efficacy of the anti-orthohantavirus vaccine was demonstrated in an immunized cohort from Yugoslavia [184]. Also, the developers of Hantavax, in collaboration with the Yugoslavia research team, demonstrated the vaccine’s protective efficacy [177].
Inactivated hantavirus vaccines in South Korea demonstrated controversy regarding their effectiveness [185]. In contrast, studies of vaccines in China showed that the absorbance value of HFRS-IgG was four times higher in vaccinated persons than those nonimmunized in the epidemic areas [186]. Also, later, the protective effectiveness of the three-doses regimen of the inactivated HFRS vaccine compared to the two-doses regimen was demonstrated [187].
Bivalent, HNTV, and SEOV inactivated orthohantavirus vaccines were manufactured in China [188]. The persistence of anti-orthohantavirus antibodies was demonstrated after a three-dose series in China [187]. In another study, the bivalent inactivated vaccine induced anti-orthohantavirus antibodies one month after immunization [189]. Antibodies were detectable 33 months after vaccination. Recently, pre-clinical studies of inactivated polyvalent HFRS vaccine demonstrated the activation of a balanced immune response to PUUV, HNTV, and DOBV [190].
Virus-like particle (VLP) vaccine: Inactivated vaccines effectively activate the humoral immune response. However, these vaccines had substantial limitations, such as failure to induce long-term antibody response, multiple immunizations, and potential side effects. Therefore, there was still an interest in developing vaccines that could address these obstacles. VLP vaccine could provide a solution to some of these limitations. Several VLP is nano-sized self-assembly competent structures made by viral proteins [191]. They have spike proteins, which could bind to the host receptor [192,193]. Therefore, VLP host cell entry resembles a natural infection. However, VLPs lack nucleic acid, rendering them incapable of replication [191]. As a result, VLPs could deliver the viral antigens to the host cells without virus replication and, subsequently, disease symptoms. This feature of VLP made them an attractive tool for developing second-generation vaccines.
The efficacy of the VLP containing HNTV N and Gn/Gc proteins was tested in a mouse model [194]. To enhance the immunogenicity, HNTV chimeric VLPs containing glycosylphosphatidylinositol (GPI)-anchored granulocyte-macrophage colony-stimulating factor (GM-CSF) or CD40 ligand (CD40L) were generated. These chimeric VLPs induce humoral and cellular immune responses, which are more potent than HNTV VLP or commercially available inactivated vaccines. It should be noted that chimeric VLPs also protected mice from the HTNV challenge. Cheng et al. obtained similar results using GM-CSF-CD40L chimeric VLP [194]. Additionally, authors have demonstrated that incorporating GM-CSF-CD40L stimulated macrophages and dendritic cells. In another study by Dong et al., chimeric VLPs were shown to induce long-term immune responses with neutralizing antibodies circulating six months after immunization [195].
Production of anti-orthohantavirus antibodies was evidenced after immunization with chimeric hepatitis B virus (HBV) particles containing PUUV N protein polypeptide [196]. These chimeric HBVs expressing PUUV N protein polypeptide induced a protective immune response in bank voles, the natural reservoir of the PUUV [197]. In another study, the immunogenic efficacy of HBV core particles carrying the N protein polypeptide of the DOBV, HNTV, or PUUV in a mice model was demonstrated [198]. These chimeric particles induced a high titer of cross-reactive antibodies [199].
DNA vaccine: DNA vaccines have multiple advantages as compared to inactivated vaccines. DNA vaccines are safe, as they are replication defective. They are also non-virulent and fail to produce clinical symptoms of the disease. As early as 1992, the HNTV DNA vaccine expressing N and G proteins was developed using the vaccinia virus as a vector [200]. This vaccine elicited a protective immune response in a hamster model. The same vaccine was later proved protective against HNTV, SEOV, and PUUV [201].
Interestingly, anti-HNTV neutralizing antibodies were detected in immunized animals. In contrast, there were no antibodies to the SEOV virus. In clinical trials phase I, neutralizing antibodies was demonstrated in immunized individuals. However, previous exposure to the vaccinia virus appears to interfere with the efficacy of developing neutralizing antibodies in volunteers [198].
DNA vaccines appear to be immunogenic and induce neutralizing antibody responses. For example, two DNA vaccines, HTNV and PUUV, were tested in phase I clinical trials [202]. Both vaccines elicited neutralizing antibodies; however, only about half vaccinated were seropositive. DNA vaccine against SEOV induced antibody response in Syrian hamsters and protected against infection [203]. Rhesus monkey immunization with another DNA vaccine, coding for HNTV and ANDV M genes, was shown to induce neutralizing antibodies [204]. Neutralizing antibodies can bind to envelop proteins and prevent viral entry [205,206]. This reduces virus infectivity and prevents dissemination. The ability to elicit neutralizing antibodies is an established benchmark for assessing vaccine efficacy.
Subunit vaccines: Orthohantavirus Gn/Gc and N proteins could elicit a strong humoral immune response [207,208,209]. Gn/Gc proteins could induce neutralizing antibody response [210], while N protein activates non-neutralizing antibody [211]. Additionally, the N protein stimulates the T cell immune response [212,213]. N protein was shown to induce the cross-reacting immune response [150,214]. Similarly, recombinant DOBV N protein was shown to induce antibodies cross-reacting with PUUV and HNTV in mice [215]. In another study, immunization with SEOV recombinant N protein induced high-titer antibody [216]. Recently, the efficacy of eliciting humoral and T cell immune response by delivery of PUUV Gn/Gc and N protein using microvesicles was demonstrated [217]. However, the efficacy of these recombinant proteins’ protection against lethal infection remains to be determined [218].
6. Treatment of Orthohantavirus Infection
There are no specific post-exposure therapeutics for HFRS. Therefore, treatment may differ between healthcare facilities adapting to patient management protocols based on local regulatory authority’s recommendations. Therapeutics to treat HFRS could be classified as targeting viruses, immune response, and supportive therapy.
Therapeutics targeting viruses could block virus entry and replication. Neutralizing antibodies can bind to surface glycoproteins, preventing binding to integrin receptors. Transfer of orthohantavirus convalescent serum containing neutralizing antibodies was shown to have a protective effect in the non-randomized multi-center trial [219]. Also, the protection efficacy of convalescent serum was confirmed in vivo experiments [220,221]. Several monoclonal antibodies with neutralizing activity were generated against HNTV [203,221]. Phase I and II clinical studies have demonstrated the therapeutic efficacy of these neutralizing antibodies in the early stage of HFRS [221,222].
Novel therapeutic approaches targeting orthohantavirus entry are still in the development stage. One is based on the peptides binding to αvβ3 integrin receptors [223]. Orthohantaviruses bind to this integrin receptor for cell entry [79]. Therefore, the initial interaction between orthohantavirus and the integrin receptor is crucial in the virus replication process. By preventing this interaction, virus entry could be abrogated, protecting cells from infection. A study by Song et al. supported this hypothesis, where monoclonal antibodies to β3 integrin protected mice from HNTV infection [224]. This concept was further developed by Hall et al., where peptides binding to integrin receptors were shown to block orthohantavirus entry [225]. A follow-up study demonstrated the efficacy of neutralizing SNV, ANDV, and HTNV by selected cyclic nonapeptides [223]. In vivo studies could evaluate the therapeutic efficacy of these peptides against orthohantaviruses.
Another approach targets virus replication. One of the earliest drugs tested for its therapeutic efficacy against the orthohantavirus infection is Ribavirin. Ribavirin is a nucleotide analog (1-beta-D-ribofuranosyl1,2,4-triazole-3-carboxamide) used to treat HFRS [226,227]. The primary mechanism of antiviral activity of this drug is the induction of mutation into the viral RNA leading to fatal errors [228]. Clinical trials demonstrated reduced morbidity and mortality in HFRS when treatment was initiated early after HNTV Jameson exposure [226]. However, later initiation of treatment was less effective. A lack of Ribavirin therapeutic efficacy was reported when treating PUUV-infected patients [227]. Another drug used is Favipiravir, an antiviral drug that selectively inhibits the RdRp of negative strand segmented viruses [229]. The efficacy of Favipiravir was demonstrated against SNV and ANDV in vitro and in vivo [230]. Similar to Ribavirin, the protective effect of this drug was demonstrated only when used at the early stage of infection. Late administration failed to reduce virus load and protect hamsters from lethal ANDV infection.
Targeting the immune response: Activation of the kinin–kallikrein system and liberation of bradykinin were shown in endothelial cells infected by HNTV and ANDV [231]. This bradykinin was suggested to contribute to increased vascular permeability in orthohantavirus-infected patients. Inhibition of bradykinin was introduced as a novel approach for treating orthohantavirus infection. Icatibant, a blocker of bradykinin binding to its receptor, was demonstrated effective in some case reports. Antonen et al. have shown the efficacy of icatibant in PUUV infection cases, where a single dose of the drug stabilizes the patient’s condition and complete recovery [232]. Similar to this case study, Laine et al. reported a successful outcome using icatibant [233] in another PUUV infection case.
Corticosteroids are most commonly used for treating orthohantavirus infection for their anti-inflammatory effect [234]. Also, decreased corticosteroids were demonstrated in orthohantavirus-infected rats [235], suggesting the role of these hormones in the pathogenesis of this infection. Data from HNTV and PUUV-infected patients supported the hypothesis of corticosteroids’ contribution to the pathogenesis of HFRS. Damage to hypophysis and the adrenal gland could lower cortisol levels and potentially contribute to inflammation [236,237]. This data supported the inclusion of corticosteroids in the HFRS treatment protocol. Rapid recovery was demonstrated in two severe PUUV cases after administering corticosteroids [238]. Also, thrombocyte counts were restored in NE patients after prednisolone treatment was initiated [239,240]. However, the lack of corticosteroid therapeutic effect in ANDV-infected patients was reported by Vial et al. in a double-blind, randomized controlled clinical trial [241].
Supportive therapy: Supportive therapy is fundamental for HFRS management. Fluid and electrolyte replacement therapy maintains blood pressure [242]. Thrombocyte transfusion is used in severe thrombocytopenia; however, caution should be exercised to prevent thrombosis [243]. Additionally, continuous renal replacement therapy was effective in patients with multiple organ dysfunction syndromes [244].
7. Challenges of Orthohantavirus Diagnostics and Treatment
Orthohantavirus diagnosis is based on clinical presentation, epidemiological data, and detection of serum antibodies. The diagnosis in endemic areas is often based on clinical symptoms and the presence of IgM. However, the expertise of a healthcare provider is essential for early diagnosis and appropriate treatment. Kim and Han reported that 54% of HFRS patients were misdiagnosed at admission [245] due to unusual symptoms. These led to more extended hospitalization than patients with HFRS diagnosed early after admission. HFRS could be misdiagnosed as an acute abdomen [246,247]. It was suggested that this diagnosis could lead to unnecessary surgery and potentially life-threatening complications. In another study, an analysis of 1250 HFRS cases revealed that 13.2% were diagnosed with acute abdomen [248]. Authors suggest that fibro gastroduodenoscopy and diagnostic laparoscopy are optimal for differential diagnosis.
Another challenge could be HFRS diagnosis in children. This is mainly because the disease is commonly mild at this age [249]. Zhang et al. demonstrated two cases of HFRS in children with atypical symptoms [250]. The initial differential diagnosis of systemic lupus erythematosus was made in these patients. Also, differential diagnosis with leptospirosis, rickettsiosis, and heart failure is suggested [251,252]. Leptospirosis is one of the most considered differential diagnoses with HFRS. This is explained by the pathology of leptospirosis, where tubule-interstitial nephritis and thrombocytopenia are common [253,254]. Damage to kidney tissue and decreased thrombocyte counts are also commonly found in HFRS, making clinical symptoms of these diseases reasonably similar. That could be the reason for the misdiagnosis of HFRS, especially in low HFRS endemic regions.
8. Conclusions
Orthohantavirus infection is an endemic zoonosis in Asia. Several serotypes, such as HNTV, PUUV, and SEOV, were identified as causing HFRS in China, South Korea, and Japan. China remains the most active HFRS epidemic region, where 90% of all orthohantavirus infections diagnosed worldwide are documented. HFRS clinically is characterized by AKI and increased vascular permeability. There are limited specific treatment options. Therefore, the most effective approach for HFRS management is to prevent infection. Recently, novel orthohantavirus serotypes have been isolated from small animals habituating in India and Thailand. These data provide evidence for the emergence of new genotypes of orthohantaviruses in Asia.
HFRS management remains challenging due to limited therapeutic options specifically targeting orthohantavirus entry and replication. Therefore, the treatment protocol is mainly symptomatic. In this view, the measures to prevent orthohantavirus infection appear essential to control outbreaks. Currently, the only vaccine approved for the prevention of orthohantavirus infection is Hantavax, which could protect against HNTV and SEOV, the orthohantaviruses endemic in Asia. These vaccines could induce long-term immune response with antibodies detected 33 months post-immunization.
Among the novel approaches in treating orthohantavirus infection is using monoclonal antibodies with neutralizing activity against HNTV. These antibodies demonstrated therapeutic efficacy in the I and II phases of clinical studies in the early stage of HFRS. Another novel therapeutic approach is targeting orthohantavirus via αvβ3 integrin receptors.
The emergence of orthohantavirus infection could impact the healthcare system in many Asian countries that have limited experience in diagnosing and treating emerging infections. Therefore, awareness of the circulation of orthohantaviruses and associated healthcare burdens is essential for developing prevention measures. One approach could be integrating screening small mammals for orthohantavirus antibodies and antigens using ELISA or PCR methods into epidemiological surveys. Also, serological testing of febrile patients for anti-orthohantavirus IgM could facilitate early diagnosis of orthohantavirus infection in non-endemic countries. Additionally, analysis of the seroprevalence in the human population could provide a better understanding of orthohantavirus infection prevalence, especially in non-endemic regions.
Author Contributions
Conceptualization—Y.D., S.K., S.C., A.S. and M.B.; literature analysis—Y.D., E.K., E.M., A.S., S.M. and K.S.; formal analysis— S.K., S.C. and M.B.; visualization—S.C., E.M. and E.K.; funding acquisition—S.K. and A.R.; supervision—S.K., Y.D. and A.R.; writing—original draft—A.S., S.M., S.C., E.M., S.K. and M.B.; writing—review and editing—Y.D., E.K., E.M., S.K., K.S., S.C., A.S., A.R., S.M. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by RSF-DST 22-44-02007 grant. Also, the Kazan Federal University Strategic Academic Leadership Program (PRIORITY-2030) supported this research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Same as funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- ICTV Current ICTV Taxonomy Release. Available online: https://ictv.global/taxonomy (accessed on 8 August 2022).
- Laenen, L.; Vergote, V.; Calisher, C.H.; Klempa, B.; Klingström, J.; Kuhn, J.H.; Maes, P. Hantaviridae: Current Classification and Future Perspectives. Viruses 2019, 11, 788. [Google Scholar] [CrossRef]
- Noack, D.; Goeijenbier, M.; Reusken, C.B.E.M.; Koopmans, M.P.G.; Rockx, B.H.G. Orthohantavirus Pathogenesis and Cell Tropism. Front. Cell. Infect. Microbiol. 2020, 10, 399. [Google Scholar] [CrossRef]
- Kariwa, H.; Yoshimatsu, K.; Arikawa, J. Hantavirus Infection in East Asia. Comp. Immunol. Microbiol. Infect. Dis. 2007, 30, 341–356. [Google Scholar] [CrossRef]
- Chandy, S.; Abraham, P.; Sridharan, G. Hantaviruses: An Emerging Public Health Threat in India? A Review. J. Biosci. 2008, 33, 495–504. [Google Scholar] [CrossRef]
- Brummer-Korvenkontio, M.; Manni, T.; Ukkonen, S.; Vaheri, A. Detection of Hemagglutination-Inhibiting Antibodies in Patients with Nephropathia Epidemica and Korean Hemorrhagic Fever by Using Puumala Virus Cell Culture Antigen. J. Infect. Dis. 1986, 153, 997–998. [Google Scholar] [CrossRef]
- Taller, A.; Xiao, S.; Godec, M.; Gligic, A. Belgrade Virus, a Cause of Hemorrhagic Fever with Renal Syndrome in the Balkans, Is Closely Related to Dobrava Virus of Field Mice. J. Infect. 1993, 3, 123–145. [Google Scholar]
- Lee, H.W.; Baek, L.J.; Johnson, K.M. Isolation of Hantaan Virus, the Etiologic Agent of Korean Hemorrhagic Fever, from Wild Urban Rats. J. Infect. Dis. 1982, 146, 638–644. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, X.; Wang, L.; Du, H.; Wang, P.; Bai, X. Hantavirus Infection: A Global Zoonotic Challenge. Virol. Sin. 2017, 32, 32–43. [Google Scholar] [CrossRef]
- Chen, H.X.; Qiu, F.X. Epidemiologic Surveillance on the Hemorrhagic Fever with Renal Syndrome in China. Chin. Med. J. (Engl.) 1993, 106, 857–863. [Google Scholar]
- Zhang, Y.Z.; Zou, Y.; Fu, Z.F.; Plyusnin, A. Hantavirus Infections in Humans and Animals, China. Emerg. Infect. Dis. 2010, 16, 1195. [Google Scholar] [CrossRef]
- Zou, L.-X.; Sun, L. Analysis of Hemorrhagic Fever with Renal Syndrome Using Wavelet Tools in Mainland China, 2004–2019. Front. Public Health 2020, 8, 571984. [Google Scholar] [CrossRef]
- Tkachenko, E.A.; Ishmukhametov, A.A.; Dzagurova, T.K.; Bernshtein, A.D.; Morozov, V.G.; Siniugina, A.A.; Kurashova, S.S.; Balkina, A.S.; Tkachenko, P.E.; Kruger, D.H.; et al. Hemorrhagic Fever with Renal Syndrome, Russia. Emerg Infect. Dis. 2019, 25, 2325–2328. [Google Scholar] [CrossRef]
- Tkachenko, E.A.; Dzagurova, T.K.; Tkachenko, P.E. Current Status of Hantavirus Vaccines Development. Nov. Technol. Vaccine Dev. 2014, 1, 113–151. [Google Scholar] [CrossRef]
- Casals, J.; Henderson, B.E.; Hoogstraal, H.; Johnson, K.M.; Shelokov, A. A Review of Soviet Viral Hemorrhagic Fevers, 1969. J. Infect. Dis. 1970, 122, 437–453. [Google Scholar] [CrossRef]
- Povalishina, T.P. On the Influence of the Human Activity upon the Existence and Detection of Natural Foci of Haemorrhagic Fever with a Renal Syndrome; Rasicky, B., Heyberger, K., Eds.; Theoretical Questions of Natural Foci of Diseases; Czechoslovak Academy of Sciences: Prague, Czech Republic, 1965. [Google Scholar]
- Johnson, K.M. Hantaviruses: History and Overview. Curr. Top. Microbiol. Immunol. 2001, 256, 1–14. [Google Scholar]
- Smadel, J.E. Epidemic Hemorrhagic Fever. Am. J. Public Health Nations Health 1953, 43, 1327–1330. [Google Scholar] [CrossRef]
- Myhrman, G. Nephropathia Epidemica a New Infectious Disease in Northern Scandinavia. Acta Med. Scand. 1951, 140, 52–56. [Google Scholar] [CrossRef]
- Smorodintsev, A.A.; Chudakov, V.G.; Churilov, A.V. Haemorrhagic Nephroso-Nephritis; Pergamon Press Ltd.: London, UK; New York, NY, USA; Paris, France; Los Angeles, CA, USA, 1959. [Google Scholar]
- Lokugamage, K.; Kariwa, H.; Hayasaka, D.; Cui, B.Z.; Iwasaki, T.; Lokugamage, N.; Ivanov, L.I.; Volkov, V.I.; Demenev, V.A.; Slonova, R.; et al. Genetic Characterization of Hantaviruses Transmitted by the Korean Field Mouse (Apodemus peninsulae), Far East Russia. Emerg. Infect. Dis. 2002, 8, 768–776. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, P.W.; Johnson, K.M. Isolation of the Etiologic Agent of Korean Hemorrhagic Fever. J. Infect. Dis. 1978, 137, 298–308. [Google Scholar] [CrossRef]
- McCormick, J.B.; Palmer, E.L.; Sasso, D.R.; Kiley, M.P. Morphological identification of the agent of korean haemorrhagic fever (Hantaan virus) as a member of the bunyaviridae. Lancet 1982, 319, 765–768. [Google Scholar] [CrossRef]
- Brummer-Korvenkontio, M.; Vaheri, A.; Hovi, T.; von Bonsdorff, C.-H.; Vuorimies, J.; Manni, T.; Penttinen, K.; Oker-Blom, N.; Lahdevirta, J. Nephropathia Epidemica: Detection of Antigen in Bank Voles and Serologic Diagnosis of Human Infection. J. Infect. Dis. 1980, 141, 131–134. [Google Scholar] [CrossRef]
- Plyusnin, A.; Elliott Richard, M. Bunyaviridae: Molecular and Cellular Biology; Caister Academic Press: Norwich, UK, 2011; ISBN 978-1-912530-52-6. [Google Scholar]
- Song, J.W.; Baek, L.J.; Schmaljohn, C.S.; Yanagihara, R. Thottapalayam Virus, a Prototype Shrewborne Hantavirus. Emerg. Infect. Dis. 2007, 13, 980. [Google Scholar] [CrossRef]
- Carey, D.E.; Reuben, R.; Panicker, K.N.; Shope, R.E.; Myers, R.M. Thottapalayam Virus: A Presumptive Arbovirus Isolated from a Shrew in India. Indian J. Med. Res. 1971, 59, 1758–1760. [Google Scholar]
- Clement, J.; Maes, P.; Muthusethupathi, M.; Nainan, G.; van Ranst, M. First Evidence of Fatal Hantavirus Nephropathy in India, Mimicking Leptospirosis. Nephrol. Dial. Transplant. 2006, 21, 826–827. [Google Scholar] [CrossRef]
- Chandy, S.; Mitra, S.; Sathish, N.; Vijayakumar, T.S.; Abraham, O.C.; Jesudason, M.V.; Abraham, P.; Yoshimatsu, K.; Arikawa, J.; Sridharan, G. A Pilot Study for Serological Evidence of Hantavirus Infection in Human Population in South India. Indian J. Med. Res. 2005, 122, 211–215. [Google Scholar]
- Chin, C.; Chiueh, T.-S.; Yang, W.-C.; Yang, T.-H.; Shih, C.-M.; Lin, H.-T.; Lin, K.-C.; Lien, J.-C.; Tsai, T.F.; Ruo, S.L.; et al. Hantavirus Infection in Taiwan: The Experience of a Geographically Unique Area. J. Med. Virol. 2000, 60, 237–247. [Google Scholar] [CrossRef]
- Elwell, M.; Ward, G. Serologic Evidence of Hantaan-like Virus in Rodents and Man in Thailand. Southeast Asian J. Trop. 1985, 16, 349–354. [Google Scholar]
- Song G Epidemiological Progresses of Hemorrhagic Fever with Renal Syndrome in China. Chin. Med. J. (Engl.) 1999, 112, 472–477.
- Jameson, L.J.; Taori, S.K.; Atkinson, B.; Levick, P.; Featherstone, C.A.; van der Burgt, G.; McCarthy, N.; Hart, J.; Osborne, J.C.; Walsh, A.L.; et al. Pet Rats as a Source of Hantavirus in England and Wales, 2013. Eurosurveillance 2013, 18, 20415. [Google Scholar] [CrossRef]
- Fang, L.Z.; Zhao, L.; Wen, H.L.; Zhang, Z.T.; Liu, J.W.; He, S.T.; Xue, Z.F.; Ma, D.Q.; Zhang, X.S.; Zhang, Y.; et al. Reservoir Host Expansion of Hantavirus, China. Emerg. Infect. Dis. 2015, 21, 170. [Google Scholar] [CrossRef]
- Wang, H.; Yoshimatsu, K.; Ebihara, H.; Ogino, M.; Araki, K.; Kariwa, H.; Wang, Z.; Luo, Z.; Li, D.; Hang, C.; et al. Genetic Diversity of Hantaviruses Isolated in China and Characterization of Novel Hantaviruses Isolated from Niviventer Confucianus and Rattus Rattus. Virology 2000, 278, 332–345. [Google Scholar] [CrossRef]
- Ge, X.-Y.; Yang, W.-H.; Pan, H.; Zhou, J.-H.; Han, X.; Zhu, G.-J.; Desmond, J.S.; Daszak, P.; Shi, Z.-L.; Zhang, Y.-Z. Fugong Virus, a Novel Hantavirus Harbored by the Small Oriental Vole (Eothenomys eleusis) in China. Virol. J. 2016, 13, 27. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.-B.; Gaowa, H.-S.; Yao, L.-S.; Hu, G.-W.; Li, M.-H.; Chen, H.-X.; Plyusnin, A.; Shao, R.; Zhang, Y.-Z. Isolation and Genetic Characterization of Hantaviruses Carried ByMicrotus Voles in China. J. Med. Virol. 2008, 80, 680–688. [Google Scholar] [CrossRef]
- Lin, X.-D.; Zhou, R.-H.; Fan, F.-N.; Ying, X.-H.; Sun, X.-Y.; Wang, W.; Holmes, E.C.; Zhang, Y.-Z. Biodiversity and Evolution of Imjin Virus and Thottapalayam Virus in Crocidurinae Shrews in Zhejiang Province, China. Virus Res. 2014, 189, 114–120. [Google Scholar] [CrossRef]
- Arai, S.; Gu, S.H.; Baek, L.J.; Tabara, K.; Bennett, S.N.; Oh, H.S.; Takada, N.; Kang, H.J.; Tanaka-Taya, K.; Morikawa, S.; et al. Divergent ancestral lineages of newfound hantaviruses harbored by phylogenetically related crocidurine shrew species in Korea. Virology 2012, 424, 99–105. [Google Scholar] [CrossRef]
- Jiang, J.-F.; Zhang, W.-Y.; Wu, X.-M.; Zhang, P.-H.; Cao, W.-C. Soochong Virus and Amur Virus Might Be the Same Entities of Hantavirus. J. Med. Virol. 2007, 79, 1792–1795. [Google Scholar] [CrossRef]
- Plyusnina, A.; Ibrahim, I.N.; Plyusnin, A. A Newly Recognized Hantavirus in the Asian House Rat (Rattus tanezumi) in Indonesia. J. Gen. Virol. 2009, 90, 205–209. [Google Scholar] [CrossRef]
- Kutanan, W.; Kampuansai, J.; Brunelli, A.; Ghirotto, S.; Pittayaporn, P.; Ruangchai, S.; Schröder, R.; MacHoldt, E.; Srikummool, M.; Kangwanpong, D.; et al. New Insights from Thailand into the Maternal Genetic History of Mainland Southeast Asia. Eur. J. Hum. Genet. 2018, 26, 898–911. [Google Scholar] [CrossRef]
- Garanina, S.B.; Platonov, A.E.; Zhuravlev, V.I.; Murashkina, A.N.; Yakimenko, V.V.; Korneev, A.G.; Shipulin, G.A. Genetic Diversity and Geographic Distribution of Hantaviruses in Russia. Zoonoses Public Health 2009, 56, 297–309. [Google Scholar] [CrossRef]
- Abu Daud, N.H.; Kariwa, H.; Tanikawa, Y.; Nakamura, I.; Seto, T.; Miyashita, D.; Yoshii, K.; Nakauchi, M.; Yoshimatsu, K.; Arikawa, J.; et al. Mode of Infection of Hokkaido Virus (Genus hantavirus) among Grey Red-Backed Voles, Myodes Rufocanus, in Hokkaido, Japan. Microbiol. Immunol. 2007, 51, 1081–1090. [Google Scholar] [CrossRef]
- Song, K.J.; Baek, L.J.; Moon, S.; Ha, S.J.; Kim, S.H.; Park, K.S.; Klein, T.A.; Sames, W.; Kim, H.C.; Lee, J.S.; et al. Muju Virus, a Novel Hantavirus Harboured by the Arvicolid Rodent Myodes Regulus in Korea. J. Gen. Virol. 2007, 88, 3121. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, J.; Yang, X.; Zhou, J.; Yang, W.; Peng, C.; Zhang, H.-L.; Shi, Z. A Novel Hantavirus Detected in Yunnan Red-Backed Vole (Eothenomys miletus) in China. J. Gen. Virol. 2011, 92, 1454–1457. [Google Scholar] [CrossRef]
- Song, J.-W.; Kang, H.J.; Song, K.-J.; Truong, T.T.; Bennett, S.N.; Arai, S.; Truong, N.U.; Yanagihara, R. Newfound Hantavirus in Chinese Mole Shrew, Vietnam. Emerg. Infect. Dis. 2007, 13, 1784–1787. [Google Scholar] [CrossRef]
- Guo, W.P.; Lin, X.D.; Wang, W.; Tian, J.H.; Cong, M.L.; Zhang, H.L.; Wang, M.R.; Zhou, R.H.; Wang, J.B.; Li, M.H.; et al. Phylogeny and Origins of Hantaviruses Harbored by Bats, Insectivores, and Rodents. PLoS Pathog. 2013, 9, e1003159. [Google Scholar] [CrossRef]
- Arai, S.; Ohdachi, S.D.; Asakawa, M.; Kang, H.J.; Mocz, G.; Arikawa, J.; Okabe, N.; Yanagihara, R. Molecular Phylogeny of a Newfound Hantavirus in the Japanese Shrew Mole (Urotrichus talpoides). Proc. Natl. Acad. Sci. USA 2008, 105, 16296–16301. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Gao, J.-H.; Li, M.; Guo, W.-P.; Lu, M.-Q.; Wang, W.; Hu, M.-X.; Li, M.-H.; Yang, J.; Liang, H.-J.; et al. Co-Circulation of Hantaan, Kenkeme, and Khabarovsk Hantaviruses in Bolshoy Ussuriysky Island, China. Virus Res. 2014, 191, 51–58. [Google Scholar] [CrossRef]
- Krautkrämer, E.; Zeier, M. Old World Hantaviruses: Aspects of Pathogenesis and Clinical Course of Acute Renal Failure. Virus Res. 2014, 187, 59–64. [Google Scholar] [CrossRef]
- Jonsson, C.B.; Figueiredo, L.T.M.; Vapalahti, O. A Global Perspective on Hantavirus Ecology, Epidemiology, and Disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef]
- Jiang, H.; Du, H.; Wang, L.M.; Wang, P.Z.; Bai, X.F. Hemorrhagic Fever with Renal Syndrome: Pathogenesis and Clinical Picture. Front. Cell. Infect. Microbiol. 2016, 6, 1. [Google Scholar] [CrossRef]
- Guang, M.Y.; Liu, G.Z.; Cosgriff, T.M. Hemorrhage in Hemorrhagic Fever with Renal Syndrome in China. Clin. Infect. Dis. 1989, 11, S884–S890. [Google Scholar] [CrossRef]
- Krüger, D.H.; Ulrich, R.; Lundkvist, Å. Hantavirus Infections and Their Prevention. Microbes Infect. 2001, 3, 1129–1144. [Google Scholar] [CrossRef]
- Vaheri, A.; Henttonen, H.; Voutilainen, L.; Mustonen, J.; Sironen, T.; Vapalahti, O. Hantavirus Infections in Europe and Their Impact on Public Health. Rev. Med. Virol. 2012, 23, 35–49. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Wang, T.; Li, J.; Hui, L.; Ha, X. Thrombocytopenia as a Predictor of Severe Acute Kidney Injury in Patients with Hantaan Virus Infections. PLoS ONE 2013, 8, e53236. [Google Scholar] [CrossRef]
- Linderholm, M.; Elgh, F. Clinical Characteristics of Hantavirus Infections on the Eurasian Continent. Curr. Top. Microbiol. Immunol. 2000, 256, 135–151. [Google Scholar] [CrossRef]
- Shastri, B.; Kofman, A.; Hennenfent, A.; Klena, J.D.; Nicol, S.; Graziano, J.C.; Morales-Betoulle, M.; Cannon, D.; Maradiaga, A.; Tran, A.; et al. Domestically Acquired Seoul Virus Causing Hemophagocytic Lymphohistiocytosis—Washington, DC, 2018. In Open Forum Infectious Diseases 2019; Oxford University Press: New York, NY, USA, 2019; Volume 6. [Google Scholar] [CrossRef]
- Bergstedt Oscarsson, K.; Brorstad, A.; Baudin, M.; Lindberg, A.; Forssén, A.; Evander, M.; Eriksson, M.; Ahlm, C. Human Puumala Hantavirus Infection in Northern Sweden; Increased Seroprevalence and Association to Risk and Health Factors. BMC Infect. Dis. 2016, 16, 566. [Google Scholar] [CrossRef]
- Laine, O.; Mäkelä, S.; Mustonen, J.; Helminen, M.; Vaheri, A.; Lassila, R.; Joutsi-Korhonen, L. Platelet Ligands and ADAMTS13 during Puumala Hantavirus Infection and Associated Thrombocytopenia. Blood Coagul. Fibrinolysis 2011, 22, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Dheerasekara, K.; Sumathipala, S.; Muthugala, R. Hantavirus Infections—Treatment and Prevention. Curr. Treat. Options Infect. Dis. 2020, 12, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Hägele, S.; Nusshag, C.; Müller, A.; Baumann, A.; Zeier, M.; Krautkrämer, E. Cells of the Human Respiratory Tract Support the Replication of Pathogenic Old World Orthohantavirus Puumala. Virol. J. 2021, 18, 169. [Google Scholar] [CrossRef] [PubMed]
- Galeno, H.; Mora, J.; Villagra, E.; Fernandez, J.; Hernandez, J.; Mertz, G.J.; Ramirez, E. First Human Isolate of Hantavirus (Andes virus) in the Americas. Emerg. Infect. Dis. 2002, 8, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Rasmuson, J.; Andersson, C.; Norrman, E.; Haney, M.; Evander, M.; Ahlm, C. Time to Revise the Paradigm of Hantavirus Syndromes? Hantavirus Pulmonary Syndrome Caused by European Hantavirus. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 685–690. [Google Scholar] [CrossRef]
- Ferrés, M.; Vial, P.; Marco, C.; Yañez, L.; Godoy, P.; Castillo, C.; Hjelle, B.; Delgado, I.; Lee, S.J.; Mertz, G.J. Prospective Evaluation of Household Contacts of Persons with Hantavirus Cardiopulmonary Syndrome in Chile. J. Infect. Dis. 2007, 195, 1563–1571. [Google Scholar] [CrossRef]
- Pensiero, M.N.; Sharefkin, J.B.; Dieffenbach, C.W.; Hay’, J. Hantaan Virus Infection of Human Endothelial Cells. J. Virol. 1992, 66, 5929–5936. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, R.; Silverman, D.J. Experimental Infection of Human Vascular Endothelial Cells by Pathogenic and Nonpathogenic Hantaviruses. Arch. Virol. 1990, 111, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Antoine, M.; Langlois, M.E.; Bres, E.; Rabeyrin, M.; Reynes, J.M.; Deeb, A. Imported Haemorrhagic Fever with Renal Syndrome Caused by Dobrava-Belgrade Hantavirus in France. Clin. Kidney J. 2021, 14, 1014–1016. [Google Scholar] [CrossRef] [PubMed]
- Vaheri, A.; Strandin, T.; Hepojoki, J.; Sironen, T.; Henttonen, H.; Mäkelä, S.; Mustonen, J. Uncovering the Mysteries of Hantavirus Infections. Nat. Rev. Microbiol. 2013, 11, 539–550. [Google Scholar] [CrossRef]
- Romero, M.G.; Anjum, F. Hemorrhagic Fever Renal Syndrome; StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560660/ (accessed on 1 January 2022).
- Yi, J.; Xu, Z.; Zhuang, R.; Wang, J.; Zhang, Y.; Ma, Y.; Liu, B.; Zhang, Y.; Zhang, C.; Yan, G.; et al. Hantaan Virus RNA Load in Patients Having Hemorrhagic Fever with Renal Syndrome: Correlation with Disease Severity. J. Infect. Dis. 2013, 207, 1457–1461. [Google Scholar] [CrossRef]
- Saksida, A.; Duh, D.; Korva, M.; Avsic-Zupanc, T. Dobrava Virus RNA Load in Patients Who Have Hemorrhagic Fever with Renal Syndrome. J. Infect. Dis. 2008, 197, 681–685. [Google Scholar] [CrossRef]
- Avšič-Županc, T.; Saksida, A.; Korva, M. Hantavirus Infections. Clin. Microbiol. Infect. 2019, 21, e6–e16. [Google Scholar] [CrossRef] [PubMed]
- Cosgriff, T.M. Mechanisms of Disease in Hantavirus Infection: Pathophysiology of Hemorrhagic Fever with Renal Syndrome. Rev. Infect. Dis. 1990, 13, 97–107. [Google Scholar] [CrossRef]
- Ferluga, D.; Vizjak, A. Hantavirus Nephropathy. J. Am. Soc. Nephrol. 2008, 19, 1653–1658. [Google Scholar] [CrossRef]
- Gavrilovskaya, I.N.; Peresleni, T.; Geimonen, E.; Mackow, E.R. Pathogenic Hantaviruses Selectively Inhibit Β3 Integrin Directed Endothelial Cell Migration. Arch. Virol. 2002, 147, 1913–1931. [Google Scholar] [CrossRef] [PubMed]
- Leavesley, D.I.; Schwartz, M.A.; Rosenfeld, M.; Cheresh, D.A. Integrin Β1- and Β3-Mediated Endothelial Cell Migration Is Triggered through Distinct Signaling Mechanisms. J. Cell Biol. 1993, 121, 163–170. [Google Scholar] [CrossRef]
- Gavrilovskaya, I.N.; Brown, E.J.; Ginsberg, M.H.; Mackow, E.R. Cellular Entry of Hantaviruses Which Cause Hemorrhagic Fever with Renal Syndrome Is Mediated by β 3 Integrins. J. Virol. 1999, 73, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, E.T.; Kim, Y.G.; Han, J.S.; Lee, J.S.; Kim, Y.I.; Hall, W.C.; Dalrymple, J.M.; Peters, C.J. Localization of Hantaan Viral Envelope Glycoproteins by Monoclonal Antibodies in Renal Tissues from Patients with Korean Hemorrhagic Fever H. Am. J. Clin. Pathol. 1993, 100, 398–403. [Google Scholar] [CrossRef]
- Gorbunova, E.; Gavrilovskaya, I.N.; Mackow, E.R. Pathogenic Hantaviruses Andes Virus and Hantaan Virus Induce Adherens Junction Disassembly by Directing Vascular Endothelial Cadherin Internalization in Human Endothelial Cells. J. Virol. 2010, 84, 7405–7411. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Li, Y.; Pan, L.; Bai, L.; Zhuang, Y.; Huang, C.X.; Wang, J.P.; Yu, H.T.; Wei, X.; et al. Dysregulation of the Β3 Integrin-VEGFR2 Complex in Hantaan Virus-Directed Hyperpermeability upon Treatment with VEGF. Arch. Virol. 2012, 157, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel Integral Membrane Proteins Localizing at Tight Junctions with No Sequence Similarity to Occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.; Wang, J.P.; Pan, L.; Zhang, Y.; Yu, H.T.; Jiang, W.; Wang, P.Z.; Bai, X.F. Elevated Vascular Endothelial Growth Factor Levels Induce Hyperpermeability of Endothelial Cells in Hantavirus Infection. J. Int. Med. Res. 2012, 40, 1812–1821. [Google Scholar] [CrossRef]
- Krautkramer, E.; Grouls, S.; Stein, N.; Reiser, J.; Zeier, M. Pathogenic Old World Hantaviruses Infect Renal Glomerular and Tubular Cells and Induce Disassembling of Cell-to-Cell Contacts. J. Virol. 2011, 85, 9811–9823. [Google Scholar] [CrossRef]
- Dieterle, M.E.; Solà-Riera, C.; Ye, C.; Goodfellow, S.M.; Mittler, E.; Kasikci, E.; Bradfute, S.B.; Klingström, J.; Jangra, R.K.; Chandran, K. Genetic Depletion Studies Inform Receptor Usage by Virulent Hantaviruses in Human Endothelial Cells. Elife 2021, 10, e69708. [Google Scholar] [CrossRef]
- Jangra, R.K.; Herbert, A.S.; Li, R.; Jae, L.T.; Kleinfelter, L.M.; Slough, M.M.; Barker, S.L.; Guardado-Calvo, P.; Román-Sosa, G.; Dieterle, M.E.; et al. Protocadherin-1 Is Essential for Cell Entry by New World Hantaviruses. Nature 2018, 563, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Connolly-Andersen, A.M.; Thunberg, T.; Ahlm, C. Endothelial Activation and Repair during Hantavirus Infection: Association with Disease Outcome. In Open Forum Infectious Diseases; Oxford University Press: New York, NY, USA, 2014; Volume 1. [Google Scholar] [CrossRef]
- Nusshag, C.; Osberghaus, A.; Baumann, A.; Schnitzler, P.; Zeier, M.; Krautkrämer, E. Deregulation of Levels of Angiopoietin-1 and Angiopoietin-2 Is Associated with Severe Courses of Hantavirus Infection. J. Clin. Virol. 2017, 94, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.R.; Hwang, J.Y.; Park, H.S. Hantaan Virus Reduces the von Willebrand Factor in Human Umbilical Vein Endothelial Cells. J. Bacteriol. Virol. 2007, 37, 225–230. [Google Scholar] [CrossRef]
- Khaiboullina, S.F.; Morzunov, S.P.; St. Jeor, S.C.; Rizvanov, A.A.; Lombardi, V.C. Hantavirus Infection Suppresses Thrombospondin-1 Expression in Cultured Endothelial Cells in a Strain-Specific Manner. Front. Microbiol. 2016, 7, 1077. [Google Scholar] [CrossRef]
- McPherson, J.; Sage, H.; Bornstein, P. Isolation and Characterization of a Glycoprotein Secreted by Aortic Endothelial Cells in Culture. Apparent Identity with Platelet Thrombospondin. J. Biol. Chem. 1981, 256, 11330–11336. [Google Scholar] [CrossRef]
- Silverstein, R.L.; Harpel, P.C.; Nachman, R.L. Tissue Plasminogen Activator and Urokinase Enhance the Binding of Plasminogen to Thrombospondin. J. Biol. Chem. 1986, 261, 9959–9965. [Google Scholar] [CrossRef] [PubMed]
- Stockschlaeder, M.; Schneppenheim, R.; Budde, U. Update on von Willebrand Factor Multimers: Focus on High-Molecular-Weight Multimers and Their Role in Hemostasis. Blood Coagul. Fibrinolysis 2014, 25, 206. [Google Scholar] [CrossRef] [PubMed]
- Outinen, T.K.; Laine, O.K.; Mäkelä, S.; Pörsti, I.; Huhtala, H.; Vaheri, A.; Mustonen, J. Thrombocytopenia Associates with the Severity of Inflammation and Variables Reflecting Capillary Leakage in Puumala Hantavirus Infection, an Analysis of 546 Finnish Patients. Infect. Dis. 2016, 48, 682–687. [Google Scholar] [CrossRef]
- Koskela, S.; Mäkelä, S.; Strandin, T.; Vaheri, A.; Outinen, T.; Joutsi-Korhonen, L.; Pörsti, I.; Mustonen, J.; Laine, O. Coagulopathy in Acute Puumala Hantavirus Infection. Viruses 2021, 13, 1553. [Google Scholar] [CrossRef]
- Laine, O.; Joutsi-Korhonen, L.; Lassila, R.; Koski, T.; Huhtala, H.; Vaheri, A.; Mäkelä, S.; Mustonen, J. Hantavirus Infection-Induced Thrombocytopenia Triggers Increased Production but Associates with Impaired Aggregation of Platelets except for Collagen. Thromb. Res. 2015, 136, 1126–1132. [Google Scholar] [CrossRef]
- Lee, M.; Kim, B.K.; Suhnggwon; Park, S.; Han, J.Ŝ.; Kim, S.T.; Lee, J.S. Coagulopathy in Hemorrhagic Fever with Renal Syndrome (Korean Hemorrhagic Fever). Rev. Infect. Dis. 1989, 11, S877–S883. [Google Scholar] [CrossRef] [PubMed]
- Rasche, F.M.; Uhel, B.; Ulrich, R.; Krüger, D.H.; Karges, W.; Czock, D.; Hampl, W.; Keller, F.; Meisel, H.; Von Müller, L. Thrombocytopenia and Acute Renal Failure in Puumala Hantavirus Infections. Emerg. Infect. Dis. 2004, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Laine, O.; Mäkelä, S.; Mustonen, J.; Huhtala, H.; Szanto, T.; Vaheri, A.; Lassila, R.; Joutsi-Korhonen, L. Enhanced Thrombin Formation and Fibrinolysis during Acute Puumala Hantavirus Infection. Thromb. Res. 2010, 126, 154–158. [Google Scholar] [CrossRef]
- Sundberg, E.; Hultdin, J.; Nilsson, S.; Ahlm, C. Evidence of Disseminated Intravascular Coagulation in a Hemorrhagic Fever with Renal Syndrome—Scoring Models and Severe Illness. PLoS ONE 2011, 6, e21134. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Wada, H.; Abe, Y.; Yamada, E.; Sakaguchi, A.; Nishioka, J.; Hatada, T.; Ishikura, K.; Yamada, N.; Sudo, A.; et al. Elevated Levels of Prothrombin Fragment 1 + 2 Indicate High Risk of Thrombosis. Clin. Appl. Thromb./Hemost. 2008, 14, 279–285. [Google Scholar] [CrossRef]
- Connolly-Andersen, A.M.; Whitaker, H.; Klingström, J.; Ahlm, C. Risk of Venous Thromboembolism Following Hemorrhagic Fever with Renal Syndrome: A Self-Controlled Case Series Study. Clin. Infect. Dis. 2018, 66, 268–273. [Google Scholar] [CrossRef]
- De Caterina, R.; Husted, S.; Wallentin, L.; Andreotti, F.; Arnesen, H.; Bachmann, F.; Baigent, C.; Huber, K.; Jespersen, J.; Kristensen, S.D.; et al. General Mechanisms of Coagulation and Targets of Anticoagulants (Section I): Position Paper of the ESC Working Group on Thrombosis—Task Force on Anticoagulants in Heart Disease. Thromb. Haemost. 2013, 109, 569–579. [Google Scholar] [CrossRef]
- Kohler, H.P.; Grant, P.J. Plasminogen-Activator Inhibitor Type 1 and Coronary Artery Disease. N. Engl. J. Med. 2000, 342, 1792–1801. [Google Scholar] [CrossRef]
- Van Hinsbergh, V.W.M. Endothelium—Role in Regulation of Coagulation and Inflammation. Semin. Immunopathol. 2012, 34, 93. [Google Scholar] [CrossRef]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial Cell Control of Thrombosis. BMC Cardiovasc. Disord 2015, 15, 130. [Google Scholar] [CrossRef]
- Kaneto, T.; Fujii, S.; Matsumoto, A.; Goto, D.; Makita, N.; Hamada, J.; Moriuchi, T.; Kitabatake, A. Induction of Tissue Factor Expression in Endothelial Cells by Basic Fibroblast Growth Factor and Its Modulation by Fenofibric Acid. Thromb. J. 2003, 1, 6. [Google Scholar] [CrossRef]
- Goeijenbier, M.; Meijers, J.C.M.; Anfasa, F.; Roose, J.M.; van de Weg, C.A.M.; Bakhtiari, K.; Henttonen, H.; Vaheri, A.; Osterhaus, A.D.M.E.; van Gorp, E.C.M.; et al. Effect of Puumala Hantavirus Infection on Human Umbilical Vein Endothelial Cell Hemostatic Function: Platelet Interactions, Increased Tissue Factor Expression and Fibrinolysis Regulator Release. Front. Microbiol. 2015, 6, 220. [Google Scholar] [CrossRef] [PubMed]
- Schmedes, C.M.; Grover, S.P.; Hisada, Y.M.; Goeijenbier, M.; Hultdin, J.; Nilsson, S.; Thunberg, T.; Ahlm, C.; MacKman, N.; Connolly, A.M.F. Circulating Extracellular Vesicle Tissue Factor Activity during Orthohantavirus Infection Is Associated with Intravascular Coagulation. J. Infect. Dis. 2020, 222, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Owens, A.P.; Mackman, N. Tissue Factor and Thrombosis: The Clot Starts Here. Thromb. Haemost. 2010, 104, 432–439. [Google Scholar] [CrossRef]
- Göbel, K.; Eichler, S.; Wiendl, H.; Chavakis, T.; Kleinschnitz, C.; Meuth, S.G. The Coagulation Factors Fibrinogen, Thrombin, and Factor XII in Inflammatory Disorders—A Systematic Review. Front. Immunol. 2018, 9, 1731. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Zhuang, R.; Ma, Y.; Zhang, Y.; Yi, J.; Yang, A.; Jin, B. IL-33/ST2 Correlates with Severity of Haemorrhagic Fever with Renal Syndrome and Regulates the Inflammatory Response in Hantaan Virus-Infected Endothelial Cells. PLoS Negl. Trop. Dis. 2015, 9, e0003514. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, J.B.; McMullan, L.K.; Spiropoulou, C.F.; Hooper, W.C.; Ansari, A.A.; Peters, C.J.; Rollin, P.E. Hantavirus Infection Induces the Expression of RANTES and IP-10 without Causing Increased Permeability in Human Lung Microvascular Endothelial Cells. J. Virol. 2001, 75, 6070–6085. [Google Scholar] [CrossRef]
- Khaiboullina, S.F.; Netski, D.M.; Krumpe, P.; St Jeor, S.C. Effects of Tumor Necrosis Factor Alpha on Sin Nombre Virus Infection In Vitro. J. Virol. 2000, 74, 11966–11971. [Google Scholar] [CrossRef]
- Raftery, M.J.; Kraus, A.A.; Ulrich, R.; Krüger, D.H.; Schönrich, G. Hantavirus Infection of Dendritic Cells. J. Virol. 2002, 76, 10724–10733. [Google Scholar] [CrossRef]
- Nagai, T.; Tanishita, O.; Takahashi, Y.; Yamanouchi, T.; Domae, K.; Kondo, K.; Dantas, J.R.; Yamanishi, K. Isolation of Haemorrhagic Fever with Renal Syndrome Virus from Leukocytes of Rats and Virus Replication in Cultures of Rat and Human Macrophages. J. Gen. Virol. 1985, 66, 1271–1278. [Google Scholar] [CrossRef]
- Nourshargh, S.; Alon, R. Leukocyte Migration into Inflamed Tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Martynova, E.; Davidyuk, Y.; Kabwe, E.; Garanina, E.E.; Shakirova, V.; Pavelkina, V.; Uskova, Y.; Stott, R.J.; Foster, T.L.; Markelova, M.; et al. Cytokine, Chemokine, and Metalloprotease Activation in the Serum of Patients with Nephropathia Epidemica from the Republic of Tatarstan and the Republic of Mordovia, Russia. Pathogens 2021, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Eckerle, I.; Daniel, V.; Burkhardt, U.; Opelz, G.; Schnitzler, P. Cytokine Expression during Early and Late Phase of Acute Puumala Hantavirus Infection. BMC Immunol. 2011, 12, 65. [Google Scholar] [CrossRef]
- Guo, J.; Guo, X.; Wang, Y.; Tian, F.; Luo, W.; Zou, Y. Cytokine Response to Hantaan Virus Infection in Patients with Hemorrhagic Fever with Renal Syndrome. J. Med. Virol. 2017, 89, 1139–1145. [Google Scholar] [CrossRef]
- Khaiboullina, S.F.; Levis, S.; Morzunov, S.P.; Martynova, E.V.; Anokhin, V.A.; Gusev, O.A.; St Jeor, S.C.; Lombardi, V.C.; Rizvanov, A.A. Serum Cytokine Profiles Differentiating Hemorrhagic Fever with Renal Syndrome and Hantavirus Pulmonary Syndrome. Front. Immunol. 2017, 8, 567. [Google Scholar] [CrossRef]
- Brebner, K.; Hayley, S.; Zacharko, R.; Merali, Z.; Anisman, H. Synergistic Effects of Interleukin-1β, Interleukin-6, and Tumor Necrosis Factor-α: Central Monoamine, Corticosterone, and Behavioral Variations. Neuropsychopharmacology 2000, 22, 566–580. [Google Scholar] [CrossRef]
- Netea, M.G.; Kullberg, B.J.; Van der Meer, J.W.M. Circulating Cytokines as Mediators of Fever. Clin. Infect. Dis. 2000, 31, S178–S184. [Google Scholar] [CrossRef]
- Sundgren-Andersson, A.K.; Ostlund, P.; Bartfai, T. IL-6 Is Essential in TNF-α-Induced Fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 275, R2028–R2034. [Google Scholar] [CrossRef] [PubMed]
- Puhlmann, M.; Weinreich, D.M.; Farma, J.M.; Carroll, N.M.; Turner, E.M.; Alexander, H.R. Interleukin-1β Induced Vascular Permeability Is Dependent on Induction of Endothelial Tissue Factor (TF) Activity. J. Transl. Med. 2005, 3, 37. [Google Scholar] [CrossRef]
- Sawant, D.A.; Tharakan, B.; Wilson, R.L.; Stagg, H.W.; Hunter, F.A.; Childs, E.W. Regulation of TNF-α-Induced Microvascular Endothelial Cell Hyperpermeability by Recombinant Bcl-XL. J. Surg. Res. 2013, 184, 628. [Google Scholar] [CrossRef]
- Tang, X.L.; Jiang, Z.Y.; Dong, J.; Liu, X.C.; Cai, S.Y.; Xiao, R.; Lu, Y.R. Expression of Tissue Factor Induced by IL-6 in HUVEC. Sichuan Da Xue Xue Bao Yi Xue Ban 2006, 37, 234–237. [Google Scholar] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Klingström, J.; Plyusnin, A.; Vaheri, A.; Lundkvist, Å. Wild-Type Puumala Hantavirus Infection Induces Cytokines, C-Reactive Protein, Creatinine, and Nitric Oxide in Cynomolgus Macaques. J. Virol. 2002, 76, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Song, C.H.; Choi, S.O. Acute Polyarthritis Associated with Hantavirus Infection. Nephrol. Dial. Transplant. 1999, 14, 2204–2205. [Google Scholar] [CrossRef]
- Martin-Sanchez, F.; Diamond, C.; Zeitler, M.; Gomez, A.I.; Baroja-Mazo, A.; Bagnall, J.; Spiller, D.; White, M.; Daniels, M.J.D.; Mortellaro, A.; et al. Inflammasome-Dependent IL-1β Release Depends upon Membrane Permeabilisation. Cell Death Differ. 2016, 23, 1219–1231. [Google Scholar] [CrossRef]
- Hatscher, L.; Lehmann, C.H.K.; Purbojo, A.; Onderka, C.; Liang, C.; Hartmann, A.; Cesnjevar, R.; Bruns, H.; Gross, O.; Nimmerjahn, F.; et al. Select Hyperactivating NLRP3 Ligands Enhance the TH1-and TH17-Inducing Potential of Human Type 2 Conventional Dendritic Cells. Sci. Signal. 2021, 14, eabe1757. [Google Scholar] [CrossRef]
- Tominaga, K.; Yoshimoto, T.; Torigoe, K.; Kurimoto, M.; Matsui, K.; Hada, T.; Okamura, H.; Nakanishi, K. IL-12 Synergizes with IL-18 or IL-1β for IFN-γ Production from Human T Cells. Int. Immunol. 2000, 12, 151–160. [Google Scholar] [CrossRef]
- Zhou, L.; Ivanov, I.I.; Spolski, R.; Min, R.; Shenderov, K.; Egawa, T.; Levy, D.E.; Leonard, W.J.; Littman, D.R. IL-6 Programs TH-17 Cell Differentiation by Promoting Sequential Engagement of the IL-21 and IL-23 Pathways. Nat. Immunol. 2007, 8, 967–974. [Google Scholar] [CrossRef]
- Qin, H.; Wang, L.; Feng, T.; Elson, C.O.; Niyongere, S.A.; Lee, S.J.; Reynolds, S.L.; Weaver, C.T.; Roarty, K.; Serra, R.; et al. TGF-β Promotes Th17 Cell Development through Inhibition of SOCS3. J. Immunol. 2009, 183, 97–105. [Google Scholar] [CrossRef]
- Friederichs, K.; Schmitz, J.; Weissenbach, M.; Heinrich, P.C.; Schaper, F. Interleukin-6-Induced Proliferation of Pre-B Cells Mediated by Receptor Complexes Lacking the SHP2/SOCS3 Recruitment Sites Revisited. Eur. J. Biochem. 2001, 268, 6401–6407. [Google Scholar] [CrossRef]
- Kaser, A.; Brandacher, G.; Steurer, W.; Kaser, S.; Offner, F.A.; Zoller, H.; Theurl, I.; Widder, W.; Molnar, C.; Ludwiczek, O.; et al. Interleukin-6 Stimulates Thrombopoiesis through Thrombopoietin: Role in Inflammatory Thrombocytosis. Blood 2001, 98, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, S.; Zhang, Q.; Li, J.; Zhang, S.; Wu, W.; Qu, J.; Li, C.; Liang, M.; Li, D. Comparison of Serological Assays to Titrate Hantaan and Seoul Hantavirus-Specific Antibodies. Virol. J. 2017, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Connolly-Andersen, A.M.; Sundberg, E.; Ahlm, C.; Hultdin, J.; Baudin, M.; Larsson, J.; Dunne, E.; Kenny, D.; Lindahl, T.L.; Ramstrom, S.; et al. Increased Thrombopoiesis and Platelet Activation in Hantavirus-Infected Patients. J. Infect. Dis. 2015, 212, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Saksida, A.; Wraber, B.; Avšič-Županc, T. Serum Levels of Inflammatory and Regulatory Cytokines in Patients with Hemorrhagic Fever with Renal Syndrome. BMC Infect. Dis. 2011, 11, 142. [Google Scholar] [CrossRef]
- Wang, P.Z.; Li, Z.D.; Yu, H.T.; Zhang, Y.; Wang, W.; Jiang, W.; Bai, X.F. Elevated Serum Concentrations of Inflammatory Cytokines and Chemokines in Patients with Haemorrhagic Fever with Renal Syndrome. J. Int. Med. Res. 2012, 40, 648–656. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef]
- Dos Santos, A.C.; Barsante, M.M.; Esteves Arantes, R.M.; Bernard, C.C.A.; Teixeira, M.M.; Carvalho-Tavares, J. CCL2 and CCL5 Mediate Leukocyte Adhesion in Experimental Autoimmune Encephalomyelitis—An Intravital Microscopy Study. J. Neuroimmunol. 2005, 162, 122–129. [Google Scholar] [CrossRef]
- Groom, J.R.; Richmond, J.; Murooka, T.T.; Sorensen, E.W.; Sung, J.H.; Bankert, K.; von Andrian, U.H.; Moon, J.J.; Mempel, T.R.; Luster, A.D. CXCR3 Chemokine Receptor-Ligand Interactions in the Lymph Node Optimize CD4+ T Helper 1 Cell Differentiation. Immunity 2012, 37, 1091–1103. [Google Scholar] [CrossRef]
- Thapa, M.; Welner, R.S.; Pelayo, R.; Carr, D.J.J. CXCL9 and CXCL10 Expression Are Critical for Control of Genital Herpes Simplex Virus Type 2 Infection through Mobilization of HSV-Specific CTL and NK Cells to the Nervous System. J. Immunol. 2008, 180, 1098–1106. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of Natural Killer Cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Schmitz, J.E.; Kuroda, M.J.; Santra, S.; Sasseville, V.G.; Simon, M.A.; Lifton, M.A.; Racz, P.; Tenner-Racz, K.; Dalesandro, M.; Scallon, B.J.; et al. Control of Viremia in Simian Immunodeficiency Virus Infection by CD8+ Lymphocytes. Science 1999, 283, 857–860. [Google Scholar] [CrossRef]
- Schuch, A.; Hoh, A.; Thimme, R. The Role of Natural Killer Cells and CD8+ T Cells in Hepatitis B Virus Infection. Front. Immunol. 2014, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- van Epps, H.L.; Schmaljohn, C.S.; Ennis, F.A. Human Memory Cytotoxic T-Lymphocyte (CTL) Responses to Hantaan Virus Infection: Identification of Virus-Specific and Cross-Reactive CD8+ CTL Epitopes on Nucleocapsid Protein. J. Virol. 1999, 73, 5301–5308. [Google Scholar] [CrossRef] [PubMed]
- Van Epps, H.L.; Terajima, M.; Mustonen, J.; Arstila, T.P.; Corey, E.A.; Vaheri, A.; Ennis, F.A. Long-Lived Memory T Lymphocyte Responses after Hantavirus Infection. J. Exp. Med. 2002, 196, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Rasmuson, J.; Pourazar, J.; Mohamed, N.; Lejon, K.; Evander, M.; Blomberg, A.; Ahlm, C. Cytotoxic Immune Responses in the Lungs Correlate to Disease Severity in Patients with Hantavirus Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Temonen, M.; Mustonen, J.; Helin, H.; Pasternack, A.; Vaheri, A.; Holthöfer, H. Cytokines, Adhesion Molecules, and Cellular Infiltration in Nephropathia Epidemica Kidneys: An Immunohistochemical Study. Clin. Immunol. Immunopathol. 1996, 78, 47–55. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Zhu, Y.; Xu, Z.; Yang, K.; Yang, A.; Jin, B. Cellular Immune Response to Hantaan Virus Nucleocapsid Protein in the Acute Phase of Hemorrhagic Fever with Renal Syndrome: Correlation with Disease Severity. J. Infect. Dis. 2009, 199, 188–195. [Google Scholar] [CrossRef]
- Tang, K.; Cheng, L.; Zhang, C.; Zhang, Y.; Zheng, X.; Zhang, Y.; Zhuang, R.; Jin, B.; Zhang, F.; Ma, Y. Novel Identified HLA-A*0201-Restricted Hantaan Virus Glycoprotein Cytotoxic T-Cell Epitopes Could Effectively Induce Protective Responses in HLA-A2.1/Kb Transgenic Mice May Associate with the Severity of Hemorrhagic Fever with Renal Syndrome. Front. Immunol. 2017, 8, 1797. [Google Scholar] [CrossRef]
- Lindgren, T.; Ahlm, C.; Mohamed, N.; Evander, M.; Ljunggren, H.-G.; Björkström, N.K. Longitudinal Analysis of the Human T Cell Response during Acute Hantavirus Infection. J. Virol. 2011, 85, 10252–10260. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Technical/Clinical Information: HPS Treatment—Hantavirus. Available online: https://www.cdc.gov/hantavirus/technical/hps/treatment.html (accessed on 12 July 2022).
- Centers for Disease Control and Prevention Facts about Hantaviruses. What You Need to Know to Prevent the Disease Pulmonary Syndrome (HPS). Available online: https://www.cdc.gov/hantavirus/pdf/hps_brochure.pdf (accessed on 23 August 2022).
- Zhang, Y.Z.; Xiao, D.L.; Wang, Y.; Wang, H.X.; Sun, L.; Tao, X.X.; Qu, Y.G. The Epidemic Characteristics and Preventive Measures of Hemorrhagic Fever with Syndromes in China. Zhonghua Liu Xing Bing Xue Za Zhi 2004, 25, 466–469. [Google Scholar]
- PAHO/WHO Hantavirus. Available online: https://www3.paho.org/hq/index.php?option=com_content&view=article&id=14911:hantavirus&Itemid=40721&lang=en#:~:text=Hantavirus%20(HV)%20is%20an%20emerging,of%20respiratory%20distress%20and%20hypotension (accessed on 13 October 2022).
- Centers for Disease Control and Prevention Cleaning Up after Rodents|Rodents|CDC. Available online: https://www.cdc.gov/rodents/prevent_infestations/clean_up.html#:~:text=Clean%20up%20rodent%20urine%20and,or%20droppings%20and%20cleaning%20product (accessed on 15 September 2022).
- Tian, H.; Stenseth, N.C. The Ecological Dynamics of Hantavirus Diseases: From Environmental Variability to Disease Prevention Largely Based on Data from China. PLoS Negl. Trop. Dis. 2019, 13, e0006901. [Google Scholar] [CrossRef]
- Park, K.; Kim, W.K.; Lee, S.H.; Kim, J.; Lee, J.; Cho, S.; Lee, G.Y.; Noid, J.S.; Lee, K.H.; Song, J.W. A Novel Genotype of Hantaan Orthohantavirus Harbored by Apodemus Agrarius Chejuensis as a Potential Etiologic Agent of Hemorrhagic Fever with Renal Syndrome in Republic of Korea. PLoS Negl. Trop. Dis. 2021, 15, e0009400. [Google Scholar] [CrossRef]
- Ling, J.; Verner-Carlsson, J.; Eriksson, P.; Plyusnina, A.; Löhmus, M.; Järhult, J.D.; van de Goot, F.; Plyusnin, A.; Lundkvist, Å.; Sironen, T. Genetic Analyses of Seoul Hantavirus Genome Recovered from Rats (Rattus norvegicus) in the Netherlands Unveils Diverse Routes of Spread into Europe. J. Med. Virol. 2019, 91, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Reynes, J.M.; Razafindralambo, N.K.; Lacoste, V.; Olive, M.M.; Barivelo, T.A.; Soarimalala, V.; Heraud, J.M.; Lavergne, A. Anjozorobe Hantavirus, a New Genetic Variant of Thailand Virus Detected in Rodents from Madagascar. Vector-Borne Zoonotic Dis. 2014, 14, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Raharinosy, V.; Olive, M.M.; Andriamiarimanana, F.M.; Andriamandimby, S.F.; Ravalohery, J.P.; Andriamamonjy, S.; Filippone, C.; Rakoto, D.A.D.; Telfer, S.; Heraud, J.M. Geographical Distribution and Relative Risk of Anjozorobe Virus (Thailand orthohantavirus) Infection in Black Rats (Rattus rattus) in Madagascar. Virol. J. 2018, 15, 83. [Google Scholar] [CrossRef]
- Olsson, G.E.; White, N.; Ahlm, C.; Elgh, F.; Verlemyr, A.C.; Juto, P.; Thomas Palo, R. Demographic Factors Associated with Hantavirus Infection in Bank Voles (Clethrionomys glareolus). Emerg. Infect. Dis. 2002, 8, 924. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.N. Regulation of Rodent-Borne Viruses in the Natural Host: Implications for Human Disease. In Infectious Diseases from Nature: Mechanisms of Viral Emergence and Persistence; Springer: Vienna, Austria, 2005; pp. 45–57. [Google Scholar]
- Tian, H.; Yu, P.; Bjørnstad, O.N.; Cazelles, B.; Yang, J.; Tan, H.; Huang, S.; Cui, Y.; Dong, L.; Ma, C.; et al. Anthropogenically Driven Environmental Changes Shift the Ecological Dynamics of Hemorrhagic Fever with Renal Syndrome. PLoS Pathog. 2017, 13, e1006198. [Google Scholar] [CrossRef] [PubMed]
- Boone, J.D.; Otteson, E.W.; McGWIRE, K.C.; Villard, P.; Rowe, J.E.; St Jeor, S.C. Ecology and Demographics of Hantavirus Infections in Rodent Populations in the Walker River Basin of Nevada and California. Citeseer 1998, 59, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Hörnfeldt, B.; Evander, M.; Magnusson, M.; Olsson, G.; Ecke, F. Dynamics and Drivers of Hantavirus Prevalence in Rodent Populations. Vector-Borne Zoonotic Dis. 2014, 14, 537–551. [Google Scholar] [CrossRef]
- Schmaljohn, C.S. Vaccines for Hantaviruses: Progress and Issues. Expert Rev. Vaccines 2014, 11, 511–513. [Google Scholar] [CrossRef]
- Liu, R.; Ma, H.; Shu, J.; Zhang, Q.; Han, M.; Liu, Z.; Jin, X.; Zhang, F.; Wu, X. Vaccines and Therapeutics Against Hantaviruses. Front. Microbiol. 2020, 10, 2989. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Han, L.; An, Q.; Liu, W.; Kong, Y. Immunization Effect of Purified Bivalent Vaccine to Haemorrhagic Fever with Renal Syndrome Manufactured from Primary Cultured Hamster Kidney Cells. Chin. Med. 2005, 5, 1554–1587. [Google Scholar]
- Chen, H.X.; Luo, Z.Z.; Zhang, J.J. Hantavirus Vaccine Efficacy Evaluation Working Group. Large Scale Field Evaluation on Vaccines of Hemorrhagic Fever with Renal Syndrome in China. Chin. J. Epidemiol. 2002, 23, 145–147. [Google Scholar]
- Cho, H.W.; Howard, C.R.; Lee, H.W. Review of an Inactivated Vaccine against Hantaviruses. Intervirology 2002, 45, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, C.S.; Moon, K.T. Protective Effectiveness of Hantavirus Vaccine. Emerg. Infect. Dis. 2004, 10, 2218. [Google Scholar] [CrossRef]
- Yamanishi, K.; Tanishita, O.; Tamura, M.; Asada, H.; Kondo, K.; Takagi, M.; Yoshida, I.; Konobe, T.; Fukai, K. Development of Inactivated Vaccine against Virus Causing Haemorrhagic Fever with Renal Syndrome. Vaccine 1988, 6, 278–282. [Google Scholar] [CrossRef]
- Cho, H.W.; Howard, C.R. Antibody Responses in Humans to an Inactivated Hantavirus Vaccine (Hantavax®). Vaccine 1999, 17, 2569–2575. [Google Scholar] [CrossRef]
- Song, J.Y.; Woo, H.J.; Cheong, H.J.; Noh, J.Y.; Baek, L.J.; Kim, W.J. Long-Term Immunogenicity and Safety of Inactivated Hantaan Virus Vaccine (HantavaxTM) in Healthy Adults. Vaccine 2016, 34, 1289–1295. [Google Scholar] [CrossRef]
- Sohn, Y.M.; Rho, H.O.; Park, M.S.; Kim, J.S.; Summers, P.L. Primary Humoral Immune Responses to Formalin Inactivated Hemorrhagic Fever with Renal Syndrome Vaccine (Hantavax®): Consideration of Active Immunization in South Korea. Yonsei Med. J. 2009, 42, 278–284. [Google Scholar] [CrossRef]
- Song, J.Y.; Jeong, H.W.; Yun, J.W.; Lee, J.; Woo, H.J.; Bae, J.Y.; Park, M.S.; Choi, W.S.; Park, D.W.; Noh, J.Y.; et al. Immunogenicity and Safety of a Modified Three-Dose Priming and Booster Schedule for the Hantaan Virus Vaccine (Hantavax): A Multi-Center Phase III Clinical Trial in Healthy Adults. Vaccine 2020, 38, 8016–8023. [Google Scholar] [CrossRef]
- Yi, Y.; Park, H.; Jung, J. Effectiveness of Inactivated Hantavirus Vaccine on the Disease Severity of Hemorrhagic Fever with Renal Syndrome. Kidney Res. Clin. Pr. 2018, 37, 366. [Google Scholar] [CrossRef]
- Chu, Y.K.; Gligic, A.; Tomanovic, S.; Bozovjc, B.; Obradovic, M.; Woo, Y.D.; An, C.N.; Kim, H.; Jiang, Y.S.; Park, S.C.; et al. A Field Efficacy Trial of Inactivated Hantaan Virus Vaccine (Hantavax(TM)) Against Hemorrhagic Fever with Renal Syndrome (HFRS) in the Endemic Areas of Yugoslavia from 1996 to 1998. J. Korean Soc. Virol. 1999, 29, 55–64. [Google Scholar]
- Jung, J.; Ko, S.-J.; Oh, H.S.; Moon, S.M.; Song, J.-W.; Huh, K. Protective Effectiveness of Inactivated Hantavirus Vaccine Against Hemorrhagic Fever with Renal Syndrome. J. Infect. Dis. 2018, 217, 1417–1420. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Z.; Wei, J.; Ma, C.; Dong, J.; Lu, X.; Zheng, Y.; Yu, P.; Qu, J.; Dong, L. Long Term Epidemiological Effects of Vaccination on Hemorrhagical Fever with Renal Syndrome (HFRS) in Shaanxi Provincial HFRS Epidemic Areas. Zhonghua Liu Xing Bing Xue Za Zhi 2012, 33, 309–312. [Google Scholar] [PubMed]
- Zheng, Y.; Zhou, B.-Y.; Wei, J.; Xu, Y.; Dong, J.-H.; Guan, L.-Y.; Ma, P.; Yu, P.-B.; Wang, J.-J. Persistence of Immune Responses to Vaccine against Haemorrhagic Fever with Renal Syndrome in Healthy Adults Aged 16–60 Years: Results from an Open-Label2-Year Follow-up Study. Infect. Dis. 2018, 50, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Maes, P.; Clement, J.; van Ranst, M. Recent Approaches in Hantavirus Vaccine Development. Expert Rev. Vaccines 2009, 8, 67–76. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, H.; Wang, Y.; Zhang, Y.; Cheng, L.; Zhang, F.; Lei, Y.; Jin, B.; Ma, Y.; Chen, L. The Assessment of Hantaan Virus-Specific Antibody Responses after the Immunization Program for Hemorrhagic Fever with Renal Syndrome in Northwest China. Hum. Vaccines Immunother. 2017, 13, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Dzagurova, T.K.; Siniugina, A.A.; Ishmukhametov, A.A.; Egorova, M.S.; Kurashova, S.S.; Balovneva, M.V.; Deviatkin, A.A.; Tkachenko, P.E.; Leonovich, O.A.; Tkachenko, E.A. Pre-Clinical Studies of Inactivated Polyvalent HFRS Vaccine. Front. Cell. Infect. Microbiol. 2020, 10, 545372. [Google Scholar] [CrossRef]
- Wagenknecht, H.-A. Book Review. Protein Sci. 2009, 13, 3331–3332. [Google Scholar] [CrossRef]
- Acuna, R.; Cifuentes-Munoz, N.; Marquez, C.L.; Bulling, M.; Klingstrom, J.; Mancini, R.; Lozach, P.-Y.; Tischler, N.D. Hantavirus Gn and Gc Glycoproteins Self-Assemble into Virus-Like Particles. J. Virol. 2014, 88, 2344–2348. [Google Scholar] [CrossRef]
- Fougeroux, C.; Goksøyr, L.; Idorn, M.; Soroka, V.; Myeni, S.K.; Dagil, R.; Janitzek, C.M.; Søgaard, M.; Aves, K.-L.; Horsted, E.W.; et al. Capsid-like Particles Decorated with the SARS-CoV-2 Receptor-Binding Domain Elicit Strong Virus Neutralization Activity. Nat. Commun. 2021, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.F.; Wang, F.; Zhang, L.; Yu, L.; Ye, W.; Liu, Z.Y.; Ying, Q.K.; Wu, X.A.; Xu, Z.K.; Zhang, F.L. Incorporation of GM-CSF or CD40L Enhances the Immunogenicity of Hantaan Virus-like Particles. Front. Cell. Infect. Microbiol. 2016, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ma, T.; Zhang, X.; Ying, Q.; Han, M.; Zhang, M.; Yang, R.; Li, Y.; Wang, F.; Liu, R.; et al. Incorporation of CD40 Ligand or Granulocyte-Macrophage Colony Stimulating Factor into Hantaan Virus (HTNV) Virus-like Particles Significantly Enhances the Long-Term Immunity Potency against HTNV Infection. J. Med. Microbiol. 2019, 68, 480–492. [Google Scholar] [CrossRef]
- Koletzki, D.; Biel, S.S.; Meisel, H.; Nugel, E.; Gelderblom, H.R.; Krüger, D.H.; Ulrich, R. HBV Core Particles Allow the Insertion and Surface Exposure of the Entire Potentially Protective Region of Puumala Hantavirus Nucleocapsid Protein. Biol. Chem. 1999, 380, 325–333. [Google Scholar] [CrossRef]
- Ulrich, R.; Koletzki, D.; Lachmann, S.; Lundkvist, Å.; Zankl, A.; Kazaks, A.; Kurth, A.; Gelderblom, H.R.; Borisova, G.; Meisel, H.; et al. New Chimaeric Hepatitis B Virus Core Particles Carrying Hantavirus (Serotype puumala) Epitopes: Immunogenicity and Protection against Virus Challenge. J. Biotechnol. 1999, 73, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Mcclain, D.J.; Summers, P.L.; Harrison, S.A.; Schmaljohn, A.L.; Schmaljohn, C.S. Clinical Evaluation of a Vaccinia-Vectored Hantaan Virus Vaccine. J. Med. Virol. 2000, 60, 77–85. [Google Scholar] [CrossRef]
- Geldmacher, A.; Skrastina, D.; Petrovskis, I.; Borisova, G.; Berriman, J.A.; Roseman, A.M.; Crowther, R.A.; Fischer, J.; Musema, S.; Gelderblom, H.R.; et al. An Amino-Terminal Segment of Hantavirus Nucleocapsid Protein Presented on Hepatitis B Virus Core Particles Induces a Strong and Highly Cross-Reactive Antibody Response in Mice. Virology 2004, 323, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohn, C.S.; Hasty, S.E.; Dalrymple, J.M. Preparation of Candidate Vaccinia-Vectored Vaccines for Haemorrhagic Fever with Renal Syndrome. Vaccine 1992, 10, 10–13. [Google Scholar] [CrossRef]
- Chu, Y.-K.; Jennings, G.B.; Schmaljohn, C.S. A Vaccinia Virus-Vectored Hantaan Virus Vaccine Protects Hamsters from Challenge with Hantaan and Seoul Viruses but Not Puumala Virus. J. Virol. 1995, 69, 6417–6423. [Google Scholar] [CrossRef]
- Boudreau, E.F.; Josleyn, M.; Ullman, D.; Fisher, D.; Dalrymple, L.; Sellers-Myers, K.; Loudon, P.; Rusnak, J.; Rivard, R.; Schmaljohn, C.; et al. A Phase 1 Clinical Trial of Hantaan Virus and Puumala Virus M-Segment DNA Vaccines for Hemorrhagic Fever with Renal Syndrome. Vaccine 2012, 30, 1951–1958. [Google Scholar] [CrossRef]
- Hooper, J.W.; Custer, D.M.; Thompson, E.; Schmaljohn, C.S. DNA Vaccination with the Hantaan Virus M Gene Protects Hamsters against Three of Four HFRS Hantaviruses and Elicits a High-Titer Neutralizing Antibody Response in Rhesus Monkeys. J. Virol. 2001, 75, 8469–8477. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.W.; Custer, D.M.; Smith, J.; Wahl-Jensen, V. Hantaan/Andes Virus DNA Vaccine Elicits a Broadly Cross-Reactive Neutralizing Antibody Response in Nonhuman Primates. Virology 2006, 347, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Klasse, P.J.; Sattentau, Q.J. Occupancy and Mechanism in Antibody-Mediated Neutralization of Animal Viruses. J. Gen. Virol. 2002, 83, 2091–2108. [Google Scholar] [CrossRef] [PubMed]
- Pantophlet, R.; Burton, D.R. GP120: Target for Neutralizing HIV-1 Antibodies. Immunol. Annu. Rev. 2006, 24, 739–769. [Google Scholar] [CrossRef] [PubMed]
- Dantas, J.R.; Okuno, Y.; Asada, H.; Tamura, M.; Takahashi, M.; Tanishita, O.; Takahashi, Y.; Kurata, T.; Yamanishi, K. Characterization of Glycoproteins of Viruses Causing Hemorrhagic Fever with Renal Syndrome (HFRS) Using Monoclonal Antibodies. Virology 1986, 151, 379–384. [Google Scholar] [CrossRef]
- Pettersson, L.; Thunberg, T.; Rocklöv, J.; Klingström, J.; Evander, M.; Ahlm, C. Viral Load and Humoral Immune Response in Association with Disease Severity in Puumala Hantavirus-Infected Patients-Implications for Treatment. Clin. Microbiol. Infect. 2014, 20, 235–241. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, J. A Soluble and Heat-Resistant Form of Hantavirus Nucleocapsid Protein for the Serodiagnosis of HFRS. J. Virol. Methods 2008, 147, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, I.; Krumm, S.A.; Stass, R.; Whitaker, A.; Voss, J.E.; Bruce, E.A.; Rothenberger, S.; Kunz, S.; Burton, D.R.; Huiskonen, J.T.; et al. Structural Basis for a Neutralizing Antibody Response Elicited by a Recombinant Hantaan Virus Gn Immunogen. mBio 2021, 12, e02531-20. [Google Scholar] [CrossRef]
- Lundkvist, Å.; Niklasson, B. Bank Vole Monoclonal Antibodies against Puumala Virus Envelope Glycoproteins: Identification of Epitopes Involved in Neutralization. Arch. Virol. 1992, 126, 93–105. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, Y.; Wang, J.; Lv, T.; Jin, B. Identification of Three Novel CTL Epitopes within Nucleocapsid Protein of Hantaan Virus. Viral Immunol. 2011, 24, 449–454. [Google Scholar] [CrossRef]
- Elgh, F.; Linderholm, M.; Wadell, G.; Tärnvik, A.; Juto, P. Development of Humoral Cross-Reactivity to the Nucleocapsid Protein of Heterologous Hantaviruses in Nephropathia Epidemica. FEMS Immunol. Med. Microbiol. 1998, 22, 309–315. [Google Scholar] [CrossRef]
- De Carvalho Nicacio, C.; Gonzalez Della Valle, M.; Padula, P.; Björling, E.; Plyusnin, A.; Lundkvist, Å. Cross-Protection against Challenge with Puumala Virus after Immunization with Nucleocapsid Proteins from Different Hantaviruses. J. Virol. 2002, 76, 6669–6677. [Google Scholar] [CrossRef]
- Geldmacher, A.; Schmaler, M.; Krüger, D.H.; Ulrich, R. Yeast-Expressed Hantavirus Dobrava Nucleocapsid Protein Induces a Strong, Long-Lasting, and Highly Cross-Reactive Immune Response in Mice. Viral. Immunol. 2004, 17, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Jandrig, B.; Klempa, B.; Yoshimatsu, K.; Arikawa, J.; Meisel, H.; Niedrig, M.; Pitra, C.; Krüger, D.H.; Ulrich, R. Nucleocapsid Protein of Cell Culture-Adapted Seoul Virus Strain 80-39: Analysis of Its Encoding Sequence, Expression in Yeast and Immuno-Reactivity. Virus Genes 2005, 30, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Shkair, L.; Garanina, E.; Martynova, E.; Kolesnikova, A.I. Immunogenic Properties of MVs Containing Structural Hantaviral Proteins: An Original Study. Pharmaceutics 2022, 1, 93. [Google Scholar] [CrossRef]
- Krüger, D.; Schönrich, G.; Klempa, B. Human Pathogenic Hantaviruses and Prevention of Infection. Hum. Vaccines 2011, 7, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Vial, P.A.; Valdivieso, F.; Calvo, M.; Rioseco, M.L.; Riquelme, R.; Araneda, A.; Tomicic, V.; Graf, J.; Paredes, L.; Florenzano, M.; et al. A Non-Randomized Multicentre Trial of Human Immune Plasma for Treatment of Hantavirus Cardiopulmonary Syndrome Caused by Andes Virus. Antivir. Ther. 2015, 20, 377–386. [Google Scholar] [CrossRef]
- Zhang, X.K.; Takashima, I.; Hashimoto, N. Characteristics of Passive Immunity against Hantavirus Infection in Rats. Arch. Virol. 1989, 105, 235–246. [Google Scholar] [CrossRef]
- Xu, Z.; Wei, L.; Wang, L.; Wang, H.; Jiang, S. The in Vitro and in Vivo Protective Activity of Monoclonal Antibodies Directed against Hantaan Virus: Potential Application for Immunotherapy and Passive. Biochem. Biophys. Res. Commun. 2002, 298, 552–558. [Google Scholar] [CrossRef]
- Rong, X.; Xiao, Y.Y.; Dao, F.Y.; Chang, Y.Z.; Pei, L.G.; Fan, D.Z. Phase I Evaluation of the Safety and Pharmacokinetics of a Single-Dose Intravenous Injection of a Murine Monoclonal Antibody against Hantaan Virus in Healthy Volunteers. Antimicrob. Agents Chemother. 2009, 53, 5055–5059. [Google Scholar] [CrossRef]
- Hall, P.; Leitão, A.; Ye, C.; Kilpatrick, K.; Hjelle, B. Small Molecule Inhibitors of Hantavirus Infection. Bioorg. Med. Chem. Lett. 2010, 20, 7085–7091. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-W.; Song, K.-J.; Baek, L.-J.; Frost, B.; Poncz, M.; Park, K. In Vivo Characterization of the Integrin Β3 as a Receptor for Hantaan Virus Cellular Entry. Exp. Mol. Med. 2005, 37, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.R.; Hjelle, B.; Brown, D.C.; Ye, C.; Bondu-Hawkins, V.; Kilpatrick, K.A.; Larson, R.S. Multivalent Presentation of Antihantavirus Peptides on Nanoparticles Enhances Infection Blockade. Antimicrob. Agents Chemother. 2008, 52, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Huggins, J.W.; Hsiang, C.M.; Cosgriff, T.M.; Guang, M.Y.; Smith, J.I.; Wu, Z.O.; LeDuc, J.W.; Zheng, Z.M.; Meegan, J.M.; Wang, Q.N.; et al. Prospective, Double-Blind, Concurrent, Placebo-Controlled Clinical Trial of Intravenous Ribavirin Therapy of Hemorrhagic Fever with Renal Syndrome. J. Infect. Dis. 1991, 164, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Malinin, O.V.; Platonov, A.E. Insufficient Efficacy and Safety of Intravenous Ribavirin in Treatment of Haemorrhagic Fever with Renal Syndrome Caused by Puumala Virus. Infect. Dis. 2017, 49, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Severson, W.E.; Schmaljohn, C.S.; Javadian, A.; Jonsson, C.B. Ribavirin Causes Error Catastrophe during Hantaan Virus Replication. J. Virol. 2003, 77, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a Broad Spectrum Inhibitor of Viral RNA Polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Mayor, J.; Engler, O.; Rothenberger, S. Antiviral Efficacy of Ribavirin and Favipiravir against Hantaan Virus. Microorganisms 2021, 9, 1306. [Google Scholar] [CrossRef]
- Taylor, S.L.; Wahl-Jensen, V.; Copeland, A.M.; Jahrling, P.B.; Schmaljohn, C.S. Endothelial Cell Permeability during Hantavirus Infection Involves Factor XII-Dependent Increased Activation of the Kallikrein-Kinin System. PLoS Pathog. 2013, 9, e1003470. [Google Scholar] [CrossRef]
- Antonen, J.; Leppänen, I. A Severe Case of Puumala Hantavirus Infection Successfully Treated with Bradykinin Receptor Antagonist Icatibant. J. Infect. 2013, 45, 494–496. [Google Scholar] [CrossRef]
- Laine, O.; Leppänen, I.; Koskela, S.; Antonen, J.; Mäkelä, S.; Sinisalo, M.; Vaheri, A.; Mustonen, J. Severe Puumala Virus Infection in a Patient with a Lymphoproliferative Disease Treated with Icatibant. Infect. Dis. 2015, 47, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory Action of Glucocorticoids—New Mechanisms for Old Drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, J.D.; Klein, S.L. Corticosteroids Modulate Seoul Virus Infection, Regulatory T Cell Responses, and MMP-9 Expression in Male, but Not Female, Norway Rats. J. Gen. Virol. 2008, 89, 2723. [Google Scholar] [CrossRef] [PubMed]
- Steer, A. Pathology of Hemorrhagic Fever: A Comparison of the Findings: 1951 and 1952. Am. J. Pathol. 1955, 3, 201–221. [Google Scholar]
- Hautala, T.; Sironen, T.; Vapalahti, O.; Pääkkö, E.; Särkioja, T.; Salmela, P.I.; Vaheri, A.; Plyusnin, A.; Kauma, H. Hypophyseal Hemorrhage and Panhypopituitarism during Puumala Virus Infection: Magnetic Resonance Imaging and Detection of Viral Antigen in the Hypophysis. Clin. Infect. Dis. 2002, 35, 96–101. [Google Scholar] [CrossRef]
- Seitsonen, E.; Hynninen, M.; Kolho, E.; Kallio-Kokko, H.; Pettilä, V. Corticosteroids Combined with Continuous Veno-Venous Hemodiafiltration for Treatment of Hantavirus Pulmonary Syndrome Caused by Puumala Virus Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 261–266. [Google Scholar] [CrossRef]
- Dunst, R.; Mettang, T.; Kuhlmann, U. Severe Thrombocytopenia and Response to Corticosteroids in a Case of Nephropathia Epidemica. Am. J. Kidney Dis. 1998, 31, 116–120. [Google Scholar] [CrossRef]
- Latus, J.; Kitterer, D.; Segerer, S.; Artunc, F.; Alscher, M.D.; Braun, N. Severe Thrombocytopenia in Hantavirus-Induced Nephropathia Epidemica. Infection 2015, 43, 83–87. [Google Scholar] [CrossRef]
- Vial, P.; Valdivieso, F.; Ferres, M.; Riquelme, R.; Rioseco, M.L.; Calvo, M.; Castillo, C.; Díaz, R.; Scholz, L.; Cuiza, A.; et al. Hantavirus Study Group in Chile. High-Dose Intravenous Methylprednisolone for Hantavirus Cardiopulmonary Syndrome in Chile: A Double-Blind, Randomized Controlled Clinical Trial. Clin. Infect. Dis. 2013, 57, 943–951. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Hemorrhagic Fever with Renal Syndrome (HFRS)—Hantavirus. Available online: https://www.cdc.gov/hantavirus/hfrs/index.html#:~:text=Suggested%20Reading-,What%20is%20hemorrhagic%20fever%20with%20renal%20syndrome%3F,hemorrhagic%20fever%2C%20and%20nephropathia%20epidemica (accessed on 15 September 2022).
- Connolly-Andersen, A.-M.; Rasmuson, J.; Öman, M.; Ahlm, C. Mesenteric Vein Thrombosis Following Platelet Transfusion in a Patient with Hemorrhagic Fever with Renal Syndrome: A Case Report. TH Open 2018, 2, e261–e264. [Google Scholar] [CrossRef]
- Ying, X.; Lai, X.; Jin, X.; Cai, L.; Li, X. Continuous Renal Replacement Therapy Rescues Severe Haemorrhagic Fever with Renal Syndrome in Pregnancy: A Case Report. BMC Infect. Dis 2020, 20, 920. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, S.W. Diagnostic Challenge of Hemorrhagic Fever with Renal Syndrome on Admission before Its Serological Confirmation. Korean J. Nephrol. 2004, 23, 82–91. [Google Scholar]
- Wichmann, D.; Slenczka, W.; Alter, P.; Boehm, S.; Feldmann, H. Hemorrhagic Fever with Renal Syndrome: Diagnostic Problems with a Known Disease. J. Clin. Microbiol. 2001, 39, 3414–3416. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.; Gerding, M.; Jordans, J.G.M.; Clement, J.P.; Osterhaus, A.D.M.E. Class and Subclass Distribution of Hantavirus-Specific Serum Antibodies at Different Times after the Onset of Nephropathia Epidemica. J. Med. Virol. 1994, 43, 39–43. [Google Scholar] [CrossRef]
- Sakhautdinov, V.G.; Galimov, O.V.; Nurtdinov, M.A.; Sibaev, I.M. Differential Diagnosis of Hemorrhagic Fever with Renal Syndrome and Acute Surgical Diseases of Abdominal Organs. Khirurgiia 2001, 3, 23–25. (In Russian) [Google Scholar]
- Dusek, J.; Pejcoch, M.; Kolsky, A.; Seeman, T.; Nemec, V.; Stejskal, J.; Vondrak, K.; Janda, J. Mild Course of Puumala Nephropathy in Children in an Area with Sporadic Occurrence Hantavirus Infection. Pediatr. Nephrol. 2006, 21, 1889–1892. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Q.S.; Zhang, Y.; Sun, B.C.; Zhao, L.Y. Analysis of Misdiagnosed Cases of Hemorrhagic Fever with Renal Syndrome in Children: Two Cases and Literature Review. BMC Nephrol. 2019, 20, 383. [Google Scholar] [CrossRef] [PubMed]
- Mattar, S.; Guzmán, C.; Figueiredo, L.T. Diagnosis of Hantavirus Infection in Humans. Expert. Rev. Anti. Infect. 2015, 13, 939–946. [Google Scholar] [CrossRef]
- Galimzyanov, K.M.; Vasil’kova, V.V.; Cherenov, I.V.; Seidov, K.S.; Akmaeva, L.R.; Zhidovinov, A.A. Differential diagnostic criteria of kidney injury due to leptospirosis. Arch. Euromed. 2017, 7, 41–43. [Google Scholar]
- Yang, C.W. Leptospirosis Renal Disease: Understanding the Initiation by Toll-like Receptors. Kidney Int. 2007, 72, 918–925. [Google Scholar] [CrossRef]
- Ciferska, H.; Honsova, E.; Lodererova, A.; Hruskova, Z.; Neprasova, M.; Vachek, J.; Suchanek, M.; Zima, T.; Coppo, R.; Tesar, V.; et al. Does the Renal Expression of Toll-like Receptors Play a Role in Patients with IgA Nephropathy? J. Nephrol. 2020, 33, 307–316. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).