Recombinant Protein Vaccines Formulated with Enantio-Specific Cationic Lipid R-DOTAP Induce Protective Cellular and Antibody-Mediated Immune Responses in Mice
Abstract
1. Introduction
2. Results
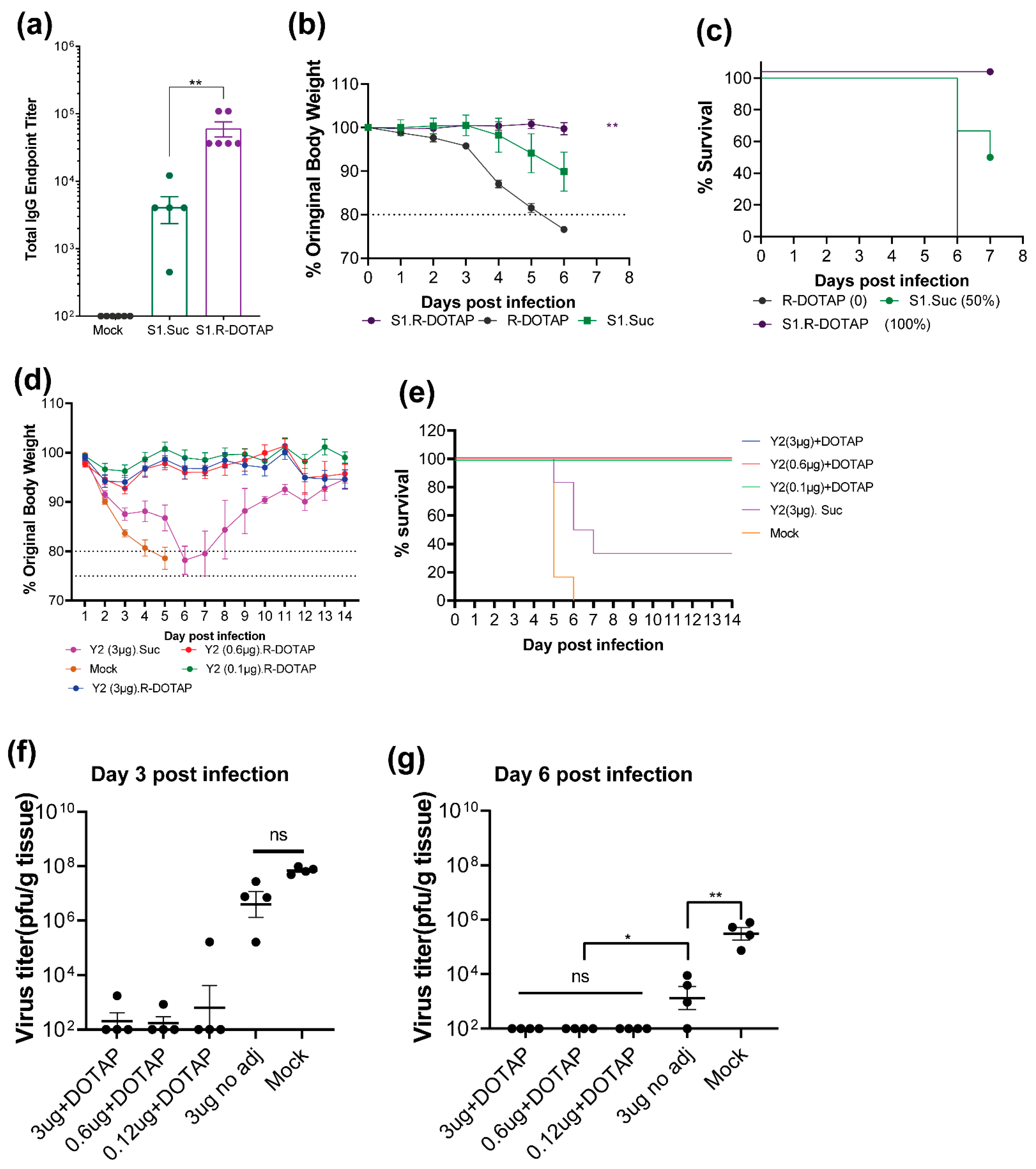
2.1. Immunogenicity of Recombinant SARS-CoV2 Proteins Formulated with R-DOTAP
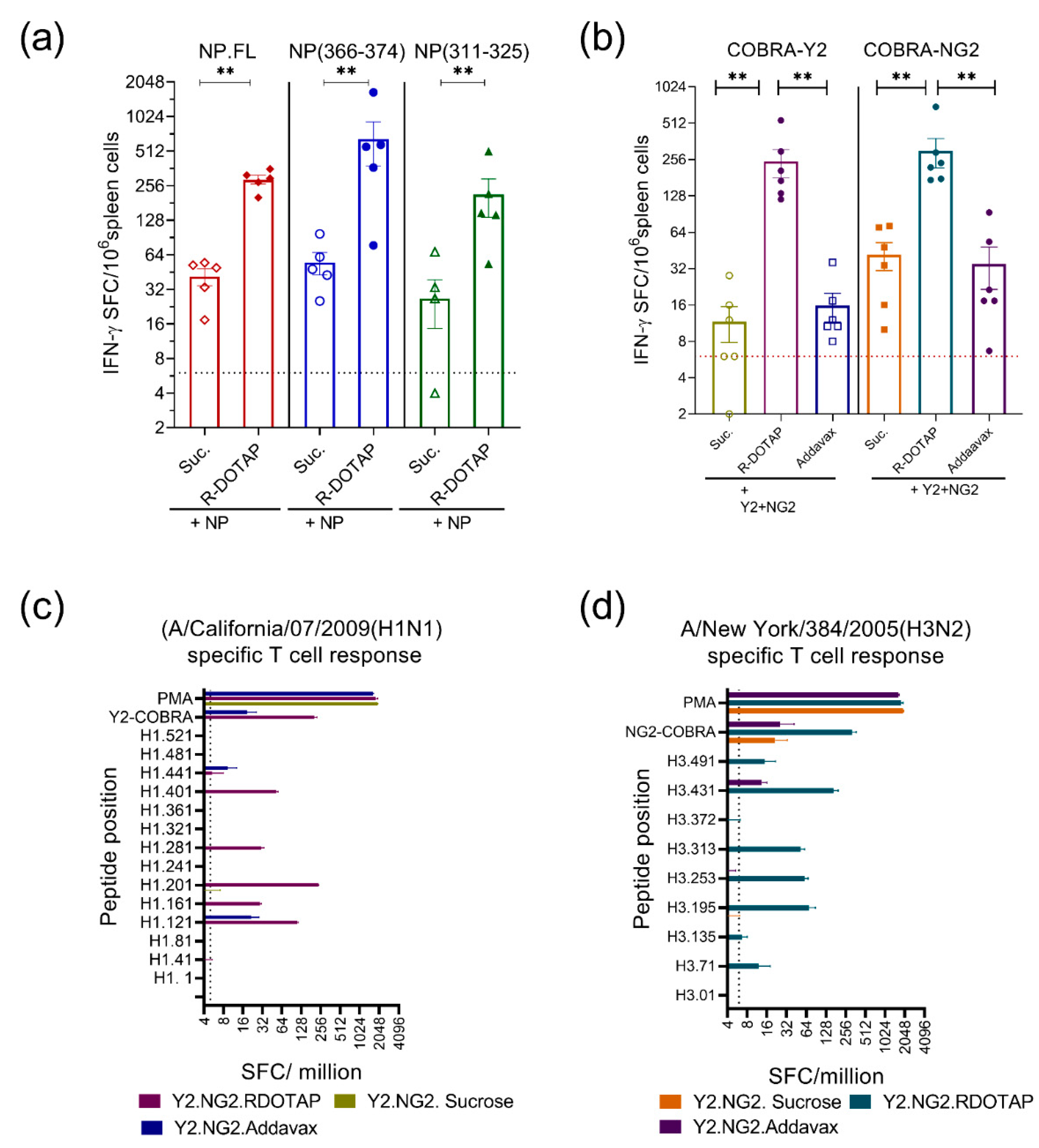
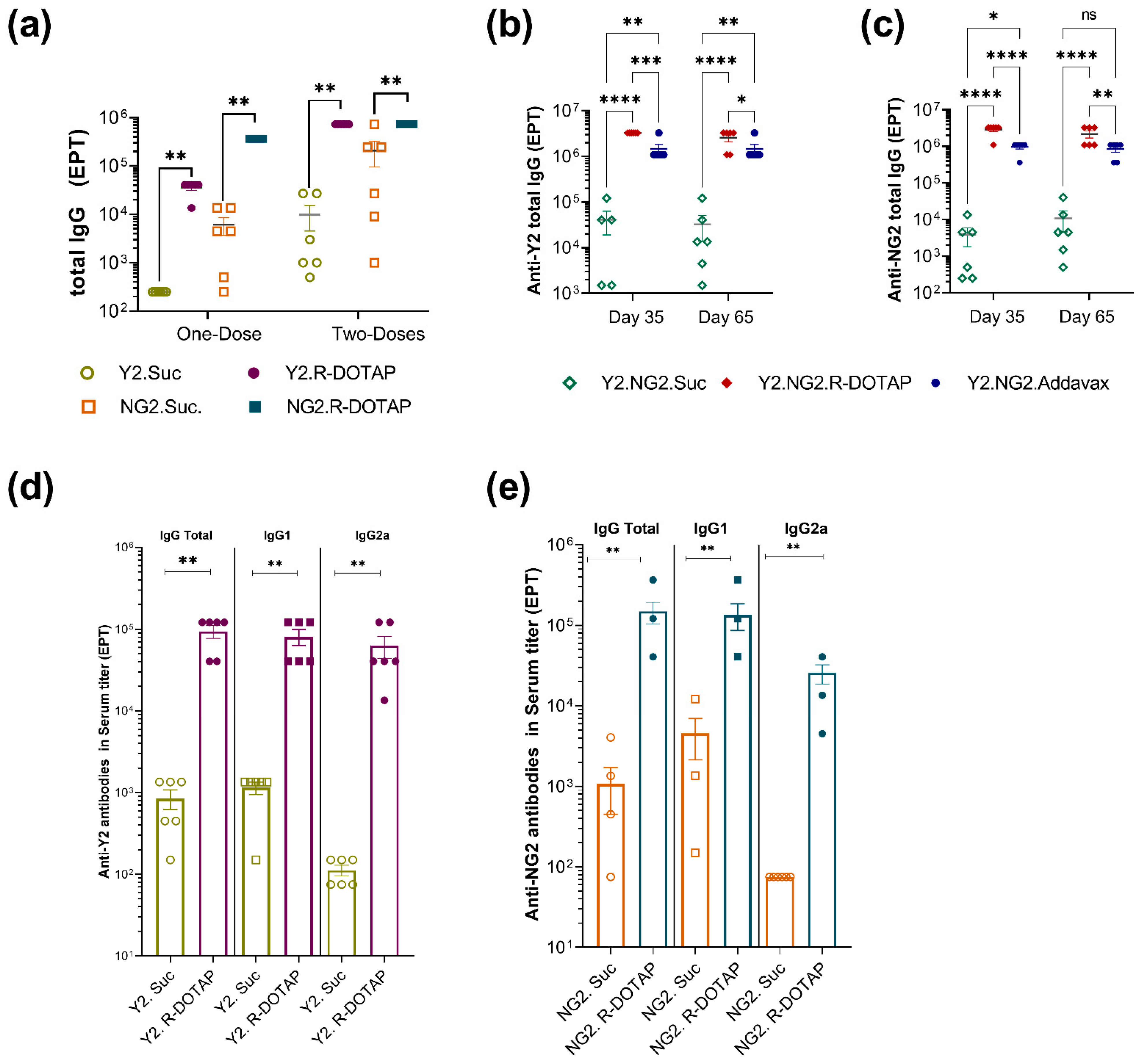
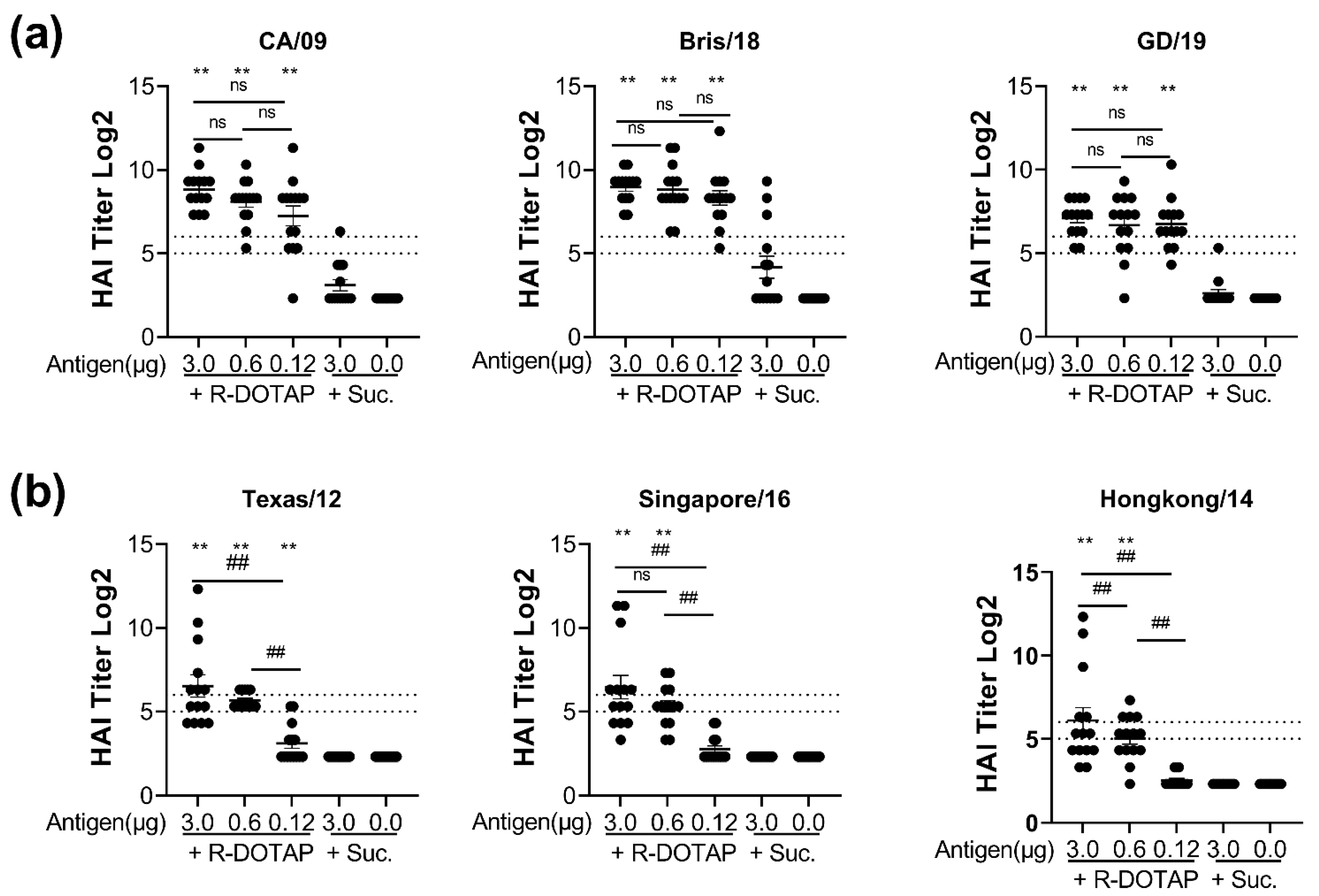
2.2. Immunogenicity of Recombinant Influenza Protein Formulated with R-DOTAP Nanoparticles
3. Discussion
4. Materials and Methods
4.1. Animals and Viruses
4.2. Reagents and Antibodies
4.3. Preparation of R-DOTAP Nanoparticles and Vaccine Formulations
4.4. Physical Characterization of Vaccine Formulation
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Enzyme-Linked Immunospot Assay (ELISpot)
4.7. Intracellular Cytokine and Cell Surface Staining
4.8. Mouse Vaccination
4.9. Hemagglutination Inhibition Assay
4.10. Mouse Challenge Experiments
4.11. Equipment, Software and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Tom, J.K.; Albin, T.J.; Manna, S.; Moser, B.A.; Steinhardt, R.C.; Esser-Kahn, A.P. Applications of Immunomodulatory Immune Synergies to Adjuvant Discovery and Vaccine Development. Trends Biotechnol. 2019, 37, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Harandi, A.M. Systems analysis of human vaccine adjuvants. Semin. Immunol. 2018, 39, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Woodruff, M.C.; Grigoryan, L.; Maier, B.; Lee, S.H.; Mandal, P.; Cortese, M.; Natrajan, M.S.; Ravindran, R.; Ma, H.; et al. Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody responses, through a RIPK3-dependent pathway. Elife 2020, 9, e52687. [Google Scholar] [CrossRef]
- Knudsen, N.P.; Olsen, A.; Buonsanti, C.; Follmann, F.; Zhang, Y.; Coler, R.N.; Fox, C.B.; Meinke, A.; D’Oro, U.; Casini, D.; et al. Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens. Sci. Rep. 2016, 6, 19570. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Cox, R.J. Correlates of protection to influenza virus, where do we go from here? Hum. Vaccin Immunother. 2013, 9, 405–408. [Google Scholar] [CrossRef]
- Jansen, J.M.; Gerlach, T.; Elbahesh, H.; Rimmelzwaan, G.F.; Saletti, G. Influenza virus-specific CD4+ and CD8+ T cell-mediated immunity induced by infection and vaccination. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2019, 119, 44–52. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Koutsakos, M.; Illing, P.T.; Nguyen, T.H.O.; Mifsud, N.A.; Crawford, J.C.; Rizzetto, S.; Eltahla, A.A.; Clemens, E.B.; Sant, S.; Chua, B.Y.; et al. Human CD8(+) T cell cross-reactivity across influenza A, B and C viruses. Nat. Immunol. 2019, 20, 613–625. [Google Scholar] [CrossRef]
- Clemens, E.B.; van de Sandt, C.; Wong, S.S.; Wakim, L.M.; Valkenburg, S.A. Harnessing the Power of T Cells: The Promising Hope for a Universal Influenza Vaccine. Vaccines 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Caplen, N.J. Nucleic acid transfer using cationic lipids. Methods Mol. Biol. 2000, 133, 1–19. [Google Scholar] [PubMed]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Stewart, D.J.; Lee, J.J.; Ji, L.; Ramesh, R.; Jayachandran, G.; Nunez, M.I.; Wistuba, I.I.; Erasmus, J.J.; Hicks, M.E.; et al. Phase I clinical trial of systemically administered TUSC2(FUS1)-nanoparticles mediating functional gene transfer in humans. PLoS ONE 2012, 7, e34833. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release Off. J. Control. Release Soc. 2021, 333, 511–520. [Google Scholar] [CrossRef]
- Barnier-Quer, C.; Elsharkawy, A.; Romeijn, S.; Kros, A.; Jiskoot, W. Adjuvant effect of cationic liposomes for subunit influenza vaccine: Influence of antigen loading method, cholesterol and immune modulators. Pharmaceutics 2013, 5, 392–410. [Google Scholar] [CrossRef]
- Vasievich, E.A.; Chen, W.; Huang, L. Enantiospecific adjuvant activity of cationic lipid DOTAP in cancer vaccine. Cancer Immunol. Immunother. 2011, 60, 629–638. [Google Scholar] [CrossRef]
- Christensen, D.; Korsholm, K.S.; Andersen, P.; Agger, E.M. Cationic liposomes as vaccine adjuvants. Expert Rev. Vaccines 2011, 10, 513–521. [Google Scholar] [CrossRef]
- Chen, W.; Yan, W.; Huang, L. A simple but effective cancer vaccine consisting of an antigen and a cationic lipid. Cancer Immunol. Immunother. 2008, 57, 517–530. [Google Scholar] [CrossRef]
- Walker, C.; Selby, M.; Erickson, A.; Cataldo, D.; Valensi, J.P.; Van Nest, G.V. Cationic lipids direct a viral glycoprotein into the class I major histocompatibility complex antigen-presentation pathway. Proc. Natl. Acad. Sci. USA 1992, 89, 7915–7918. [Google Scholar] [CrossRef]
- Henriksen-Lacey, M.; Christensen, D.; Bramwell, V.W.; Lindenstrom, T.; Agger, E.M.; Andersen, P.; Perrie, Y. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J. Control. Release Off. J. Control. Release Soc. 2010, 145, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.; Korsholm, K.S.; Rosenkrands, I.; Lindenstrom, T.; Andersen, P.; Agger, E.M. Cationic liposomes as vaccine adjuvants. Expert Rev. Vaccines 2007, 6, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Zaks, K.; Jordan, M.; Guth, A.; Sellins, K.; Kedl, R.; Izzo, A.; Bosio, C.; Dow, S. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J. Immunol. 2006, 176, 7335–7345. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Z.; Zuhorn, I.S.; Hoekstra, D. How cationic lipids transfer nucleic acids into cells and across cellular membranes: Recent advances. J. Control. Release Off. J. Control. Release Soc. 2013, 166, 46–56. [Google Scholar] [CrossRef]
- Rejman, J.; Conese, M.; Hoekstra, D. Gene transfer by means of lipo- and polyplexes: Role of clathrin and caveolae-mediated endocytosis. J. Liposome Res. 2006, 16, 237–247. [Google Scholar] [CrossRef]
- Rejman, J.; Bragonzi, A.; Conese, M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol. Ther. 2005, 12, 468–474. [Google Scholar] [CrossRef]
- Zabner, J.; Fasbender, A.J.; Moninger, T.; Poellinger, K.A.; Welsh, M.J. Cellular and molecular barriers to gene transfer by a cationic lipid. J. Biol. Chem. 1995, 270, 18997–19007. [Google Scholar] [CrossRef]
- Sun, W.; McCroskery, S.; Liu, W.C.; Leist, S.R.; Liu, Y.; Albrecht, R.A.; Slamanig, S.; Oliva, J.; Amanat, F.; Schafer, A.; et al. A Newcastle Disease Virus (NDV) Expressing a Membrane-Anchored Spike as a Cost-Effective Inactivated SARS-CoV-2 Vaccine. Vaccines 2020, 8, 771. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Mathiazzi, B.I.; Perez-Betancourt, Y. Cationic Nanostructures as Adjuvants for Vaccines. Methods Mol. Biol. 2022, 2412, 233–245. [Google Scholar]
- Lonez, C.; Bessodes, M.; Scherman, D.; Vandenbranden, M.; Escriou, V.; Ruysschaert, J.M. Cationic lipid nanocarriers activate Toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomedicine 2014, 10, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Lonez, C.; Vandenbranden, M.; Ruysschaert, J.M. Cationic liposomal lipids: From gene carriers to cell signaling. Prog. Lipid Res. 2008, 47, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Gandhapudi, S.K.; Ward, M.; Bush, J.P.C.; Bedu-Addo, F.; Conn, G.; Woodward, J.G. Antigen Priming with Enantiospecific Cationic Lipid Nanoparticles Induces Potent Antitumor CTL Responses through Novel Induction of a Type I IFN Response. J. Immunol. 2019, 202, 3524–3536. [Google Scholar] [CrossRef]
- Vasievich, E.A.; Ramishetti, S.; Zhang, Y.; Huang, L. Trp2 peptide vaccine adjuvanted with (R)-DOTAP inhibits tumor growth in an advanced melanoma model. Mol. Pharm. 2012, 9, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.V.; Gandhapudi, S.K.; Sundarapandiyan, K.; Bedu-Addo, F.K.; Conn, G.; Woodward, J.G. R-DOTAP (Versamune): A novel enantiospecific cationic lipid nanoparticle that induces CD4 and CD8 cellular immune responses to whole protein and tumor-specific peptide antigens. J. Clin. Oncol. 2020, 38, e15211. [Google Scholar] [CrossRef]
- Allen, J.D.; Ross, T.M. Bivalent H1 and H3 COBRA Recombinant Hemagglutinin Vaccines Elicit Seroprotective Antibodies against H1N1 and H3N2 Influenza Viruses from 2009 to 2019. J. Virol. 2022, 96, e0165221. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Zhuang, Z.; Lai, X.; Sun, J.; Chen, Z.; Zhang, Z.; Dai, J.; Liu, D.; Li, Y.; Li, F.; Wang, Y.; et al. Mapping and role of T cell response in SARS-CoV-2-infected mice. J. Exp. Med. 2021, 218, e20202187. [Google Scholar] [CrossRef]
- Carmen, J.M.; Shrivastava, S.; Lu, Z.; Anderson, A.; Morrison, E.B.; Sankhala, R.S.; Chen, W.H.; Chang, W.C.; Bolton, J.S.; Matyas, G.R.; et al. SARS-CoV-2 ferritin nanoparticle vaccine induces robust innate immune activity driving polyfunctional spike-specific T cell responses. NPJ Vaccines 2021, 6, 151. [Google Scholar] [CrossRef]
- La Gruta, N.L.; Kedzierska, K.; Pang, K.; Webby, R.; Davenport, M.; Chen, W.; Turner, S.J.; Doherty, P.C. A virus-specific CD8+ T cell immunodominance hierarchy determined by antigen dose and precursor frequencies. Proc. Natl. Acad. Sci. USA 2006, 103, 994–999. [Google Scholar] [CrossRef]
- Hornick, E.E.; Zacharias, Z.R.; Legge, K.L. Kinetics and Phenotype of the CD4 T Cell Response to Influenza Virus Infections. Front. Immunol. 2019, 10, 2351. [Google Scholar] [CrossRef]
- Allen, J.D.; Ross, T.M. Next generation methodology for updating HA vaccines against emerging human seasonal influenza A(H3N2) viruses. Sci. Rep. 2021, 11, 4554. [Google Scholar] [CrossRef]
- Huber, V.C.; McKeon, R.M.; Brackin, M.N.; Miller, L.A.; Keating, R.; Brown, S.A.; Makarova, N.; Perez, D.R.; Macdonald, G.H.; McCullers, J.A. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin. Vaccine Immunol. CVI 2006, 13, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Visciano, M.L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Effects of adjuvants on IgG subclasses elicited by virus-like particles. J. Transl. Med. 2012, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Darrah, P.A.; Patel, D.T.; De Luca, P.M.; Lindsay, R.W.; Davey, D.F.; Flynn, B.J.; Hoff, S.T.; Andersen, P.; Reed, S.G.; Morris, S.L.; et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007, 13, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Vangasseri, D.P.; Cui, Z.; Chen, W.; Hokey, D.A.; Falo, L.D., Jr.; Huang, L. Immunostimulation of dendritic cells by cationic liposomes. Mol. Membr. Biol. 2006, 23, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, I.; Garg, R.; van Drunen Littel-van den Hurk, S. Selection of adjuvants for vaccines targeting specific pathogens. Expert Rev. Vaccines 2019, 18, 505–521. [Google Scholar] [CrossRef]
- Beijnen, E.M.S.; van Haren, S.D. Vaccine-Induced CD8(+) T Cell Responses in Children: A Review of Age-Specific Molecular Determinants Contributing to Antigen Cross-Presentation. Front. Immunol. 2020, 11, 607977. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Feser, J.; Naficy, A.; Bernstein, D.I.; Guptill, J.; Walter, E.B.; Berlanda-Scorza, F.; Stadlbauer, D.; Wilson, P.C.; Aydillo, T.; et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 2021, 27, 106–114. [Google Scholar] [CrossRef]
- World Health Organization. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza; World Health Organization press: Geneva, Switzerland, 2011; pp. 59–62. ISBN 978-92-4154809-0. [Google Scholar]
- European Medicine Agency (EMA) Guideline on Influenza Vaccines. Non-Clinical and Clinical Module. EMA/CHMP/VWP/457259/2014. 2016. Available online: https://www.ema.europa.eu/en/influenza-vaccines-non-clinical-clinical-module-scientific-guideline (accessed on 10 January 2023).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhapudi, S.K.; Shi, H.; Ward, M.R.; Bush, J.P.; Avdiushko, M.; Sundarapandiyan, K.; Wood, L.V.; Dorrani, M.; Fatima, A.; Dervan, J.; et al. Recombinant Protein Vaccines Formulated with Enantio-Specific Cationic Lipid R-DOTAP Induce Protective Cellular and Antibody-Mediated Immune Responses in Mice. Viruses 2023, 15, 432. https://doi.org/10.3390/v15020432
Gandhapudi SK, Shi H, Ward MR, Bush JP, Avdiushko M, Sundarapandiyan K, Wood LV, Dorrani M, Fatima A, Dervan J, et al. Recombinant Protein Vaccines Formulated with Enantio-Specific Cationic Lipid R-DOTAP Induce Protective Cellular and Antibody-Mediated Immune Responses in Mice. Viruses. 2023; 15(2):432. https://doi.org/10.3390/v15020432
Chicago/Turabian StyleGandhapudi, Siva K., Hua Shi, Martin R. Ward, John Peyton Bush, Margarita Avdiushko, Karuna Sundarapandiyan, Lauren V. Wood, Mania Dorrani, Afsheen Fatima, Joe Dervan, and et al. 2023. "Recombinant Protein Vaccines Formulated with Enantio-Specific Cationic Lipid R-DOTAP Induce Protective Cellular and Antibody-Mediated Immune Responses in Mice" Viruses 15, no. 2: 432. https://doi.org/10.3390/v15020432
APA StyleGandhapudi, S. K., Shi, H., Ward, M. R., Bush, J. P., Avdiushko, M., Sundarapandiyan, K., Wood, L. V., Dorrani, M., Fatima, A., Dervan, J., Bedu-Addo, F., Conn, G., Ross, T. M., & Woodward, J. G. (2023). Recombinant Protein Vaccines Formulated with Enantio-Specific Cationic Lipid R-DOTAP Induce Protective Cellular and Antibody-Mediated Immune Responses in Mice. Viruses, 15(2), 432. https://doi.org/10.3390/v15020432





