Abstract
The Asian lineage of Zika virus (ZIKV), a mosquito-borne pathogen originally from Africa, caused an epidemic into Brazil in 2015 and subsequently spread throughout the Americas. Local transmission in the U.S. is a public health concern, especially for Florida where the mosquito vectors Aedes aegypti and Ae. albopictus are widespread, abundant, and there is a high potential for virus introduction due to imported cases. Here we evaluate relative susceptibility to infection and transmission of Zika virus among geographic populations of Ae. aegypti and Ae. albopictus in Florida. Both species have been implicated as ZIKV vectors elsewhere, but both virus and vector genotype are known to influence transmission capacities and, hence, the risk of outbreaks. We test the hypothesis that Ae. aegypti and Ae. albopictus show geographic differences in midgut and salivary gland barriers that limit ZIKV transmission, using local populations of the two vector species recently colonized from three regions of Florida to compare their susceptibility to ZIKV infection, disseminated infection, and transmission potential. Susceptibility to infection was higher in Ae. aegypti (range 76–92%) than Ae. albopictus (range 47–54%). Aedes aegypti exhibited 33–44% higher susceptibility to infection than Ae. albopictus, with Ae. aegypti from Okeechobee, FL having 17% higher susceptibility to infection than Ae. aegypti from Miami, FL. Similarly, disseminated infection was higher in Ae. aegypti (range 87–89%) than Ae. albopictus (range 31–39%), although did not vary by region. Enhanced infection and disseminated infection in Ae. aegypti were associated with higher viral loads in mosquito samples than in Ae. albopictus. Transmission rates did not vary by species or region (range 26–47%). The results support the hypothesis that Ae. aegypti, but not Ae. albopictus, exhibited regional differences in midgut infection barriers. Our observation of higher vector competence for Ae. aegypti than Ae. albopictus, together with this species greater propensity to feed on humans, lends support to the notion that Ae. aegypti is regarded as the primary vector for ZIKV and public health concern in continental U.S.
1. Introduction
Native to Africa, the first signs of ZIKV emergence, in terms of increased human cases, began in Yap Island, Micronesia in 2007, followed by outbreaks in French Polynesia in 2013 [1] and an epidemic in northeastern Brazil associated with many humans infected, ranging from 440,000–1.3 million cases in 2015 [2]. During this time, ZIKV became a pandemic throughout the Americas, raising the concern about Zika transmission in the continental U.S., especially Gulf Coast states such as Florida [3].
A major epidemic of ZIKV in Florida would have terrible consequences for public health because of potential birth defects in babies and other neurological complications, such as Guillain-Barré syndrome. During 2015–2018, there were 1455 imported cases and 302 locally acquired Zika fever cases in Florida. Although travel associated Zika fever cases in Florida are sporadically identified, local transmission of ZIKV by mosquitoes has not been detected in recent years. Additionally, Florida is at a relatively higher risk than other states for local transmission of ZIKV because the putative vectors Ae. aegypti and Ae. albopictus [4,5] are abundant throughout much of the year. Most local transmission of ZIKV in the U.S. has occurred in the territories of Puerto Rico (91% of total), followed by American Samoa and the U.S. Virgin Islands [6]. Travel and commerce between U.S. territories where there is local transmission of ZIKV, and Florida is common and increases risks for imported Zika cases and a large-scale epidemic in the future. Furthermore, most cases of Zika fever are asymptomatic (80%) [6], which may further facilitate ZIKV transmission because the daily routine of the infected individuals will not be altered by illness. This study will provide essential and currently unknown information on the risk of ZIKV emergence in Florida and facilitate the development of a risk prediction map of Florida.
Zika virus (Family Flaviviridae, genus Flavivirus) is native to Africa and consists of three genetically distinct strains (lineages), one from Asia and two from Africa [7]. In December 2015 the first autochthonous transmission of ZIKV in South America was documented in northeastern Brazil. Phylogenetic analysis indicated that the ZIKV belonged to the Asian clade [8], which subsequently spread throughout the Americas [9]. Human infection with ZIKV causes high fever, rash, joint pain, conjunctivitis, headaches, and muscle aches, but complications may include serious life-threatening disease (Guillain-Barré syndrome and birth defects) [10,11]. Laboratory studies have shown that Ae. aegypti and Ae. albopictus are competent vectors of Zika virus [12,13,14,15,16,17]. In addition, Ae. aegypti has been found to be naturally infected with ZIKV in Malaysia, Senegal, Ivory Coast, and Brazil [5,18,19,20,21] and Ae. albopictus in Gabon [4], which implicates these species as probable ZIKV vectors to humans in the Americas.
A few studies, mostly from Africa, have demonstrated distinct differences in vector competence of Ae. aegypti and Ae. albopictus depending on ZIKV strain and geographic origin of the mosquitoes [22,23,24]. To date, only a few studies have assessed ZIKV infection and transmission in Ae. aegypti and Ae. albopictus from the Americas, including Florida [13,17]. An assessment of vector competence was made for Ae. aegypti from Brazil, French Guiana, Guadeloupe, Martinique, and Florida (Orlando) and for Ae. albopictus from Brazil and Florida (Vero Beach) at 28 °C. This study showed higher infection of Ae. aegypti (70–80%) than Ae. albopictus (20–85%) and variations in infection between the geographic populations were observed for Ae. albopictus, but not Ae. aegypti. Disseminated infection rates were much lower and varied by geographic population (Ae. aegypti 10–50%, Ae. albopictus 10–15%). Similarly, transmission rates were much lower (Brazilian Ae. aegypti 10%, Vero Beach Ae. albopictus 3%) than infection rates but were only tested for a subset of the geographic populations. Transmission rates for Ae. aegypti from Florida were not tested in this study [13]. While this study used F1-F2 generation Aedes from American populations, the Florida-derived Ae. aegypti and Ae. albopictus had been maintained as laboratory colonies for several generations (F7–10) and may not be representative of field populations. Vazeille et al. [25] showed that dengue-2 infection rates of Ae. albopictus were up to 4-fold lower in recently collected versus laboratory strains with more generations in the lab, suggesting that laboratory colonization altered susceptibility to infection. Laboratory colonization refers to the processes (acclimation and evolution) that likely occur during establishment of a caged population of mosquitoes for multiple generations. These former studies suggest huge variation in transmission potential among Aedes vector populations for ZIKV which is consistent with observations for transmission potential among vector populations for chikungunya virus [26]. Taken together, these observations suggest that midgut escape and salivary gland transmission barriers are the primary determinants of variation in vector competence among these Aedes vectors.
Few studies have evaluated vector competence of local populations of mosquitoes in Florida for ZIKV. Although Ae. aegypti has been implicated as the probable primary vector of ZIKV in the Americas [27], there are some circumstances in which Ae. albopictus may be the primary vector [4]. A study using Ae. aegypti and Ae. albopictus from Florida (F1-F2) and Ae. aegypti from the Dominican Republic identified differences in disseminated infection and transmission rates for two emergent lineages of chikungunya virus (CHIKV) belonging to the Asian and Indian Ocean lineages [28]. This study showed clear evidence of geographic differences in vector competence for Ae. albopictus between North, East, and West Florida (disseminated infection of Asian strain of CHIKV, range of 63–97%; transmission potential of Indian Ocean strain of CHIKV, range 12–71%) and for Ae. aegypti between South, East, West Florida and the Dominican Republic (disseminated infection of Indian Ocean strain of CHIKV, range of 64–91%; transmission potential of the Asian strain of CHIKV, range 18–63%). These results showed huge variation between the vector competence of these species depending on virus genotype as well as region-specific variation in infection and transmission across Florida. These results provide evidence that there may be variation in midgut and salivary gland barriers in Ae. aegypti and Ae. albopictus across Florida. Using freshly collected local populations from Florida, we test the hypothesis that Ae. aegypti and Ae. albopictus show regional differences in midgut and salivary gland barriers that limit ZIKV transmission [13].
2. Materials and Methods
2.1. Mosquitoes
Aedes aegypti and Ae. albopictus were collected as larvae from cemeteries, tire shops or domiciliary settings across Florida where these species are present alone (allopatric) or coexist (sympatric) [29] (Figure 1, Table 1). We deliberately chose collection sites based on areas with past outbreaks of arboviruses (dengue) transmitted by these vector species as well as high risk areas for importation of ZIKV. Collection sites for Ae. aegypti included Miami-Dade (Miami) and Okeechobee (Okeechobee) Counties. Collection sites for Ae. albopictus included Duval (Jacksonville), and Okeechobee (Okeechobee) Counties. At the time of this study, Ae. aegypti and Ae. albopictus coexist in Okeechobee (sympatric) and exist alone (allopatric) in Miami (Ae. aegypti) and Duval (Ae. albopictus). We have identified variation in vector competence of these species across Florida for emergent strains of CHIKV, which may also exist for ZIKV [28,30].
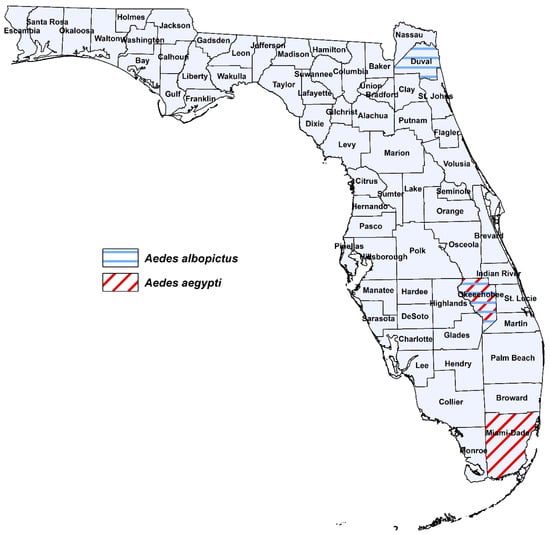
Figure 1.
Geographic origin for Florida populations of Ae. aegypti and Ae. albopictus used in this study.

Table 1.
Geographic origin for Florida populations of Ae. aegypti (F4) and Ae. albopictus (F4) used in this experiment.
Field-collected larvae were reared to adulthood on a diet of liver powder and lactalbumin at 28 °C. Pupae were transferred to vials with a cotton seal and upon eclosion identified to species. Female and male adults were provided with 10% sucrose solution, allowed to mate, and females ingested blood through membranes on commercially purchased bovine blood once per week in order to propagate eggs. The fourth-generation progeny of field-collected Ae. aegypti and Ae. albopictus were used for the ZIKV infection studies in the biosafety level-3 virology facility and Arthropod Containment level-3 at the Florida Medical Entomology Laboratory, in Vero Beach, FL.
2.2. Virus Isolates and Propagation
A low passage strain of ZIKV from Puerto Rico (Asian lineage, GenBank: KU501215.1, strain PRVABC59) was used to infect the mosquitoes. An isolate of ZIKV was kindly provided to us by Centers for Disease Control and Prevention. The virus isolate was obtained in December 2015 from human serum. We deliberately chose this genotype of ZIKV because it is responsible for outbreaks in the Americas (starting in December 2015), and it is a risk for importation to Florida. Zika virus was propagated by inoculating monolayers of African green monkey (Vero) cells with 500 μL of diluted ZIKV from stock at a multiplicity of infection of 0.01 (number of viruses to cells). Viral inoculum was allowed to incubate for 1 h at 37 °C and 5% carbon dioxide atmosphere after which 25 mL media (Medium 199 supplemented with 10% fetal bovine serum, 0.2% penicillin/streptomycin, and 0.2% of the antifungal, Mycostatin) was added to each flask (T-175 cm2) with cells and incubated for an additional six days, after which ZIKV was combined with bovine blood and used in oral infection studies with mosquitoes.
2.3. Per Os Challenge of Mosquitoes
All oral challenge experiments used the same Zika virus genotype and 8- to 9-day old adult females were allowed to feed on ZIKV infected defibrinated bovine blood (Hemostat Laboratories, Dixon, CA, USA) mixed with adenosine triphosphate (ATP) at 0.005 M added as a phagostimulant to the infected blood meal. A Hemotek membrane feeding system (Discovery Workshop, Lancashire, UK) was used to allow mosquitoes to imbibe ZIKV infected blood [31]. We used a dose of ZIKV which is within the range of viremia levels experienced by infected humans (7log10 PFU/mL) [32,33]. Aliquots of blood were stored at −80 °C for later determination of virus titer in the oral challenge experiments. Zika virus was freshly propagated in monolayers of Vero cells in T-175 cm2 flasks for preparing the infected blood to feed to the mosquitoes (previous section). After the feeding trials, fully engorged females were held in cylindrical cages (10 cm height × 10 cm top diameter × 7 cm bottom diameter) along with an oviposition substrate and maintained under a 14 h: 10 h light: dark photo regime and 28 °C for 14 days. Adults were provided with 10% sucrose solution. The temperature chosen is representative of the average daily temperature observed in central Florida during late summer/early fall when arbovirus transmission is expected to occur [34].
To assess transmission potential and incidence of transmission, expectorated saliva was collected from mosquitoes. For this procedure, mosquitoes were individually transferred to 37-mL plastic tubes (h by d: 8 by 3 cm) 14 days post infection (dpi). Each tube held one mosquito and was fitted with a removable screen lid. No sucrose was provided to the mosquitoes for one day before the transmission trial. Each tube containing a mosquito was presented with a honey-soaked filter paper card fastened to the inside of the lid. The honey was dyed with blue food coloring which provided a visual marker. Mosquitoes were sorted based on the presence or absence of the blue marker in their crop, indicating that a mosquito fed on the honey and expectorated saliva during feeding. This system has been successfully used to measure transmission potential of ZIKV and CHIKV for Ae. aegypti and Ae. albopictus mosquitoes [17,28] as well as a field surveillance system to detect arboviruses that exploits the fact that female mosquitoes expectorate virus in their saliva during feeding on sugar sources [35]. Here we use this methodology as a proxy for potential to transmit ZIKV. Mosquitoes were examined using a flashlight for blue coloring in their crop after 24 h of the transmission assay. Mosquitoes and filter paper cards were collected 15 dpi and frozen at −80 °C for later analysis of expectorated saliva and virus using quantitative (q) RT-PCR methods [17]. Mosquitoes that did not feed on the blue honey were not tested for ZIKV transmission potential. Additionally, mosquitoes were individually dissected to separate the legs, and the bodies and legs were tested separately to determine the incidences of susceptibility to infection and disseminated infection through the presence of ZIKV RNA by quantitative RT-PCR used the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) and primers and probes specific to the Asian lineage of ZIKV. Mosquito samples were homogenized in centrifuge tubes with 1 mL of media and two steel BBs at 26 HZ for 3 min using a TissueLyser II (Qiagen, Germantown, MD, USA). Viral RNA was extracted using the QIAamp® Viral RNA Mini Kit from 140 µL samples (Qiagen, Germantown, MD, USA). The Superscript III One-Step qRT-PCR with Platinum® Taq Kit by Invitrogen (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s protocol, was used to prepare RNA-extracted samples for quantitative real-time polymerase chain reaction (qRT-PCR) methods. Primers were designed to target the NS5 gene with the following sequences: forward primer, 5′-CTTCTTATCCACAGCCGTCTC-3′; reverse primer, 5′-CCAGGCTTCAACTCGTTAT-3′; and probe 5′-/56-FAM/AGAAGGAGACGAGATGCGGTACAGG/3BHQ_1/-3′ (Integrated DNA Technologies, Coralville, IA, USA). The program for qRT-PCR was as follows: 30 min at 50 °C, 2.0 min at 94 °C, 12 s at 94 °C, 1 min at 58 °C, and lastly repeated for 39 cycles. The cutoff for positive ZIKV samples was set at a Cq detection of 35 PCR cycles. Viral titer in mosquito samples were determined using a standard curve method that compares cDNA synthesis to a range of ZIKV serial dilutions in parallel with plaque assays of the same dilution of the virus, expressed as plaque forming unit equivalents (PFUE)/mL [36]. Testing for the presence of ZIKV RNA in bodies, legs, and saliva of mosquitoes enabled us to identify barriers to transmission (e.g., midgut infection and escape barriers, salivary gland barriers).
2.4. Statistical Analyses
Separate analyses were performed to test for treatment effects on susceptibility to infection (body samples), disseminated infection (leg samples), and transmission potential (saliva expectorates). Logistic regression analysis tested for species by region differences in infection parameters of mosquito samples (PROC LOGISTIC, version 9.4, SAS Institute Inc., Cary, NC, USA) based on the number of mosquitoes categorized for the presence or absence of ZIKV RNA. Viral loads in bodies, legs, and saliva were assessed for individual mosquitoes and analyzed by ANOVA (PROC GLM, version 9.4, SAS Institute Inc., Cary, NC, USA). Significant effects were followed by Tukey-Kramer multiple comparisons among treatment least-squares means for pairwise comparisons. Viral load in saliva expectorates is a proxy for amount of virus inoculated in a host during biting.
3. Results
3.1. Susceptibility to Infection
Oral challenge with Zika virus infected blood led to the establishment of infection in both Ae. aegypti and Ae. albopictus. Bodies were tested for the presence of ZIKV infection to determine the permissibility of infection (1120 mosquitoes: 559 Ae. aegypti, 561 Ae. albopictus) (Figure 2). Logistic regression analysis showed a significant effect indicating differences among the mosquito species and geographic populations (χ2 = 129.05, df = 3, p < 0.0001). Populations of Ae. aegypti (range, 75–90%) exhibited significantly higher rates of susceptibility to infection than populations of Ae. albopictus (range, 48–54%) (Figure 2, Table 2). Individuals of Ae. aegypti from Okeechobee were significantly more susceptible to ZIKV infection that Ae. aegypti from Miami (Figure 2, Table 2). The Jacksonville and Okeechobee populations of Ae. albopictus had similar infection rates to each other (range, 48–52%).
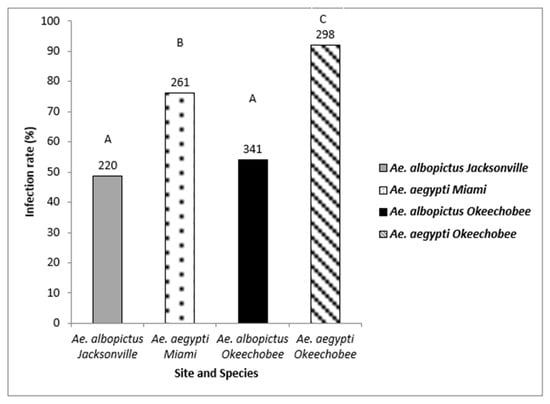
Figure 2.
Zika virus infection (bodies) in Ae. aegypti and Ae. albopictus mosquitoes from different geographic populations in Florida were examined to determine susceptibility to Zika virus infection. The number of individual mosquitoes tested are shown above the bars. Comparisons of infection rates among treatment groups sharing the same letter are not significantly different from one another.

Table 2.
Logistic regression of mosquito species and geographic site on susceptibility to infection (body), disseminated infection (legs), and transmission potential (saliva). The results show the means (probability scale), standard errors, and 95% confidence intervals (lower and upper means).
3.2. Disseminated Infection
The legs of mosquitoes whose bodies were determined to be ZIKV positive were tested for disseminated infection (744 mosquitoes: 455 Ae. aegypti, 289 Ae. albopictus) to gauge differences in midgut escape barriers among treatment groups (Figure 3). Logistic regression analysis showed a significant effect indicating differences in disseminated infection among mosquito species and geographic populations (χ2 = 185.81, df = 3, p < 0.0001). Populations of Ae. aegypti (range, 88–90%) exhibited significantly higher rates of disseminated infection than populations of Ae. albopictus (range, 32–38%) (Figure 3, Table 2). Although the species exhibited distinct patterns of disseminated infection from each other, the populations within each species responded similarly. (Figure 3, Table 2).
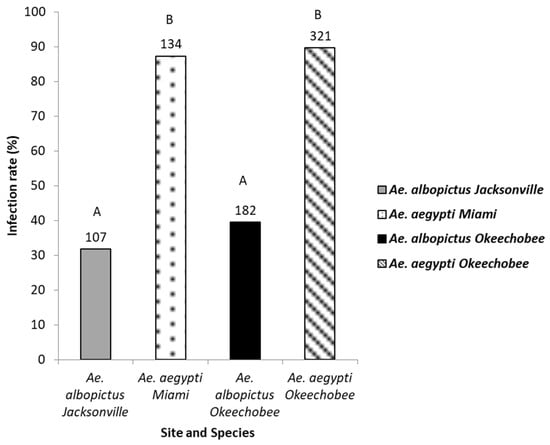
Figure 3.
The legs of the Zika virus positive bodies of mosquitoes were tested for the presence of Zika virus. Leg infection in Ae. aegypti and Ae. albopictus mosquitoes from different geographic populations in Florida were examined to determine disseminated infection. The number of individual mosquitoes tested are shown above the bars. Comparisons of disseminated infection rates among treatment groups sharing the same letter are not significantly different from one another.
3.3. Transmission Potential
Detection of Zika virus in the saliva of Ae. aegypti and Ae. albopictus reinforced the belief that both these invasive mosquitoes are capable of transmitting Zika virus to humans. The saliva of mosquitoes that tested positive for disseminated infection and contained blue in the crop were tested for the presence of ZIKV (128 mosquitoes: 66 Ae. aegypti, 62 Ae. albopictus) (Figure 4). Logistic regression analysis showed no significant differences in saliva infection in Ae. aegypti (range, 0–48%) and Ae. albopictus (range, 28–29%) from the geographic populations (χ2 = 4.46, df = 3, p = 0.21, Table 2).
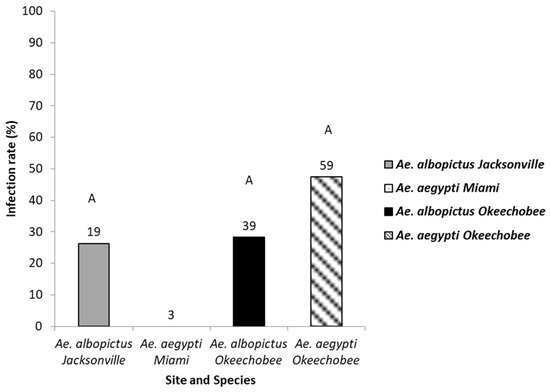
Figure 4.
The saliva of mosquitoes with disseminated infection were tested as a proxy for transmission. Saliva infection in Ae. aegypti and Ae. albopictus mosquitoes from different geographic populations in Florida were examined to determine transmission potential. The number of individual mosquitoes tested are shown above the bars. Comparisons of saliva infection rates among treatment groups sharing the same letter are not significantly different from one another.
3.4. Zika Virus Titer in Mosquito Samples
ANOVA showed significant differences in body viral titer between treatment groups for mosquitoes exhibiting disseminated infection (F3,543 = 5.69, p > 0.0008). Overall, body titers were higher among Ae. aegypti than Ae. albopictus females. Specifically, body titer was significantly higher in mosquitoes of Ae. aegypti originating from Miami than individuals of Ae. albopictus originating from Okeechobee (Figure 5). Similarly, titer was significantly higher in individuals of Ae. aegypti originating from Okeechobee than individuals of Ae. albopictus from Okeechobee (Figure 5). All remaining pairwise comparisons were not significantly different from each other.
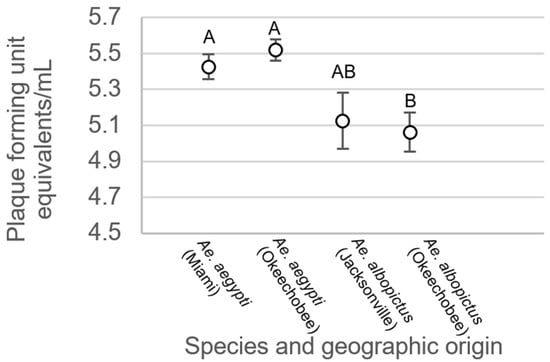
Figure 5.
Zika virus titer in the bodies of Ae. aegypti and Ae. albopictus mosquitoes with disseminated infection from different geographic populations in Florida. Comparisons of viral titers among treatments sharing the same letter are not significantly different from one another.
For mosquitoes with non-disseminated infections, there were no significant differences between treatment groups (F3,214 = 0.34, p = 0.7997, Figure 6). Analysis of variance on viral titer in mosquito legs showed significant treatment differences (F3,508 = 19.48, p < 0.0001, Figure 7). Leg titer was significantly higher in individuals of Ae. aegypti from Okeechobee than titers of Ae. aegypti from Miami and Ae. albopictus from Okeechobee (Figure 7). No other significant differences were observed. Analysis of variance on saliva titer showed no significant differences between treatment groups (F2,38 = 1.38, p = 0.2629, Figure 8). However, too few samples were available to test for individuals of Ae. aegypti from Miami, which was left out of the analysis.
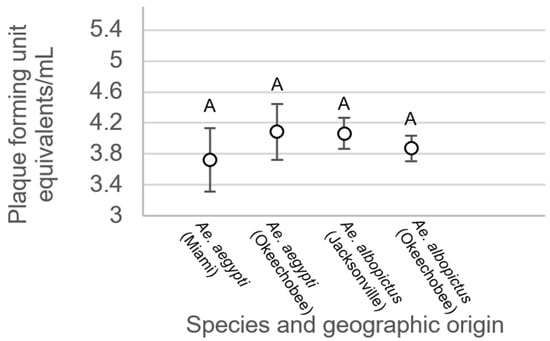
Figure 6.
Zika virus titer in the bodies of Ae. aegypti and Ae. albopictus mosquitoes with non-disseminated infection from different geographic populations in Florida. Comparisons of viral titers among treatments sharing the same letter are not significantly different from one another.
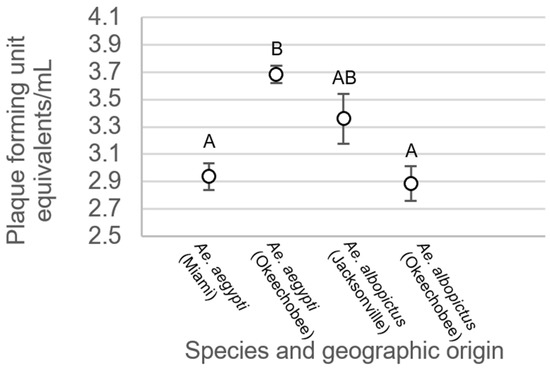
Figure 7.
Zika virus titer in legs of Ae. aegypti and Ae. albopictus mosquitoes from different geographic populations in Florida. Comparisons of viral titers among treatments sharing the same letter are not significantly different from one another.
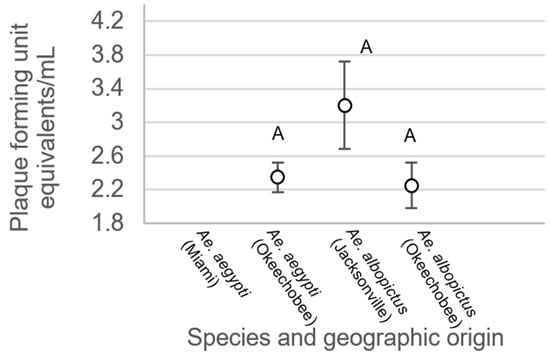
Figure 8.
Zika virus titer in saliva of Ae. aegypti and Ae. albopictus mosquitoes from different geographic populations in Florida. Comparisons of viral titers among treatments sharing the same letter are not significantly different from one another.
4. Discussion
The introduction of Zika virus from travel related cases continues to be reported in the U.S. Local transmission in the U.S. is a major public health risk, especially for Florida where mosquito vectors are abundant and there is a high potential for virus re-introduction. This study provides information on emergence potential of ZIKV in Florida and can help improve risk prediction for ZIKV in Florida by characterizing transmission efficiency of local populations of Ae. aegypti and Ae. albopictus. All geographic populations tested were susceptible to ZIKV and displayed disseminated infection, demonstrating the virus can pass through the midgut barrier(s) and progress to an advanced state of infection. We found limited support for our hypothesis of geographic differences in midgut and salivary gland barriers that limits Zika virus transmission. Specifically, support for the hypothesis was only observed for measurements of susceptibility to Zika virus infection in Ae. aegypti, and it did not change infectivity.
The observations from our study showed the Okeechobee population of Ae. aegypti as being highly susceptible to ZIKV infection. However, the same individuals from the Okeechobee population did not have enhanced disseminated infection and transmission rates compared to the other Ae. aegypti population, suggesting only a small enhancement of Zika transmission risk. However, the Okeechobee population of Ae. aegypti had similar or higher viral titers of legs and bodies of mosquitoes with disseminated infections than other groups of mosquitoes, perhaps attributable to enhanced viral replication. The current study did not assess the extrinsic incubation period of ZIKV, differences in behaviors (human biting rate), or other life history traits (adult survivorship) between geographic populations which limits our interpretation of anticipated changes to measures of risk of transmission (e.g., vectorial capacity), given that the observed difference being solely for susceptibility to ZIKV infection between two geographic populations of Ae. aegypti. However, we have identified variation in components of vector competence on a smaller spatial scale than previously observed for Zika [28]. However, we did not find any geographic population associated with higher risk for ZIKV transmission based on salivary infection. Lack of differences in saliva infection may be attributable to the low sample size and heterogeneity in the dataset, or that little variation exists for this phenotypic trait among these Florida mosquito populations. These observations suggest that midgut escape barriers may be playing an important role in the variation among these Aedes vectors. The relative higher vector competence for ZIKV by Ae. aegypti, and associated infectivity, can contribute to the likelihood of the two species transmitting ZIKV to humans in nature.
Chouin-Carneiro et al. [13] found variation in susceptibility to ZIKV infection and disseminated infection in Ae. aegypti and Ae. albopictus from the continental United States and Brazil. Chouin-Carneiro et al. [13] observed higher infection and disseminated infection rates in Ae. aegypti from Brazil compared to Ae. albopictus from the United States. However, transmission potential between Ae. aegypti and Ae. albopictus were similar. These results are consistent with our findings, in that Ae. aegypti was more susceptible to ZIKV infection and disseminated infection compared to Ae. albopictus. The observed higher leg and body titers in Ae. aegypti with disseminated infections is in accordance with observations of higher infection and dissemination rates than Ae. albopictus, an indicator of higher permissibility of the former mosquito species for ZIKV. In our study, we provide evidence of variation in midgut and salivary gland barriers in Ae. aegypti and Ae. albopictus across Florida.
Higher susceptibility to infection and disseminated infection of Zika virus exhibited in Florida populations of Ae. aegypti, together with a preference for human blood [37] and gonotrophic discordance behavior, raises Ae. aegypti as the primary vector of Zika virus in Florida, and elsewhere. We can infer from our observations that the higher rates of disseminated infection among Florida Ae. aegypti at 14-dpi than Ae. albopictus suggests a shorter extrinsic incubation period (EIP) for Ae. aegypti, further enhancing risk of Zika transmission. A similar study comparing vector competence measures among Italian populations of Ae. albopictus and a long-established colony of Ae. aegypti (collected in Reynosa, Mexico, in 1998) and the Asian genotype of Zika virus (similar titers used) observed an EIP50 of 7- and 11-days for Ae. aegypti and Ae. albopictus, respectively [14]. In contrast, the estimated EIP values were longer (16–17 days) for Ae. albopictus than Ae. aegypti among populations from Florida [38]. A comparison of Australian populations of mosquitoes and a Brazilian ZIKV strain, laboratory colonies of Ae. aegypti (Poza Rica, Mexico) and Ae. albopictus (Florida) were allowed to ingest ZIKV and held under multiple constant temperatures ranging from 25–35 °C [39]. Vector competence estimates assumed a unimodal pattern with peaks from 28–32 °C, and higher transmission rates occurred on 10-dpi for Ae. aegypti and 14-dpi for Ae. albopictus. Extrinsic incubation periods were consistently shorter for Ae. aegypti than Ae. albopictus over a range of temperatures [39]. Discrepancies in results may be attributable, in part, to mosquito and virus strains used and experimental procedures. Overall, our results are consistent with most other studies and extend these patterns of Zika infection to invasive mosquito vectors in Florida.
This project showed relative differences in susceptibility to ZIKV, disseminated infection and transmission rates of Ae. aegypti and Ae. albopictus vectors, and differences in these rates across Florida. Future studies on vectorial capacity parameters of Ae. aegypti and Ae. albopictus should be carried out to determine variation among geographic populations to assist with emergence and risk prediction of ZIKV in Florida.
Author Contributions
Conceptualization, R.A.Z. and B.W.A.; writing—original draft preparation, R.A.Z.; writing—review and editing, R.A.Z. and B.W.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Florida Department of Agriculture and Consumer Services, contract number 024378.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank Shawna Bellamy, Bradley Eastmond, Jebidiah Light, Sara Ortiz, and Keenan Wiggins for assistance with the experiments; Phil Lounibos, Sarah Murr, Tom Swan, and María Cristina Carrasquilla for collection and assistance in establishing the mosquitoes used in this study. Zika virus (strain PRVABC59) was kindly provided by the Centers of Disease Control. The University of Florida Graduate Student fellowship provided support for this research and the Florida Department of Agriculture Consumer Services grant 024378.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cao-Lormeau, V.M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.L.; Mallet, H.P.; Sall, A.A.; Musso, D. Zika virus, French Polynesia, South Pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika virus outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef]
- Bogoch, I.I.; Brady, O.J.; Kraemer, M.U.G.; German, M.; Creatore, M.I.; Kulkarni, M.A.; Brownstein, J.S.; Mekaru, S.R.; Hay, S.I.; Groot, E.; et al. Anticipating the international spread of Zika virus from Brazil. Lancet 2016, 387, 335–336. [Google Scholar] [CrossRef]
- Grard, G.; Caron, M.; Mombo, I.M.; Nkoghe, D.; Ondo, S.M.; Jiolee, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika virus in Gabon (Central Africa)-2007: A new threat from Aedes albopictus? PLoS Neg. Trop. Dis. 2014, 8, e2681. [Google Scholar] [CrossRef]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–415. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Zika-Affected Areas. Available online: www.cdc.gov/zika/geo/index.html (accessed on 3 February 2016).
- Faye, O.; Freire, C.C.; Iamarino, A.; Faye, O.; de Oliveira, J.V.; Diallo, M.; Zanotto, P.M. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl. Trop. Dis. 2014, 8, e2636. [Google Scholar] [CrossRef]
- Zanluca, C.; Melo, V.C.A.D.; Mosimann, A.L.P.; Santos, G.I.V.D.; Santos, C.N.D.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Lambert, A.J.; Holodniy, M.; Saavedra, S.; Signor, L. Phylogeny of Zika virus in Western Hemisphere, 2015. Emerg. Infect. Dis. 2016, 22, 933–935. [Google Scholar] [CrossRef]
- PAHO/WHO. Epidemiological Alert: Increase of Microcephaly in the Northeast of Brazil. 2015. Available online: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32285&lang=en (accessed on 30 December 2022).
- PAHO/WHO. Epidemiological Update: Neurological Syndrome, Congenital Anomalies and Zika Virus Infection. 2016. Available online: www.paho.org (accessed on 30 December 2022).
- Wong, P.S.J.; Li, M.Z.I.; Chong, C.S.; Ng, L.C.; Tan, C.H. Aedes (Stegomyia) albopictus (Skuse): A potential vector of Zika virus in Singapore. PLoS Negl. Trop. Dis. 2013, 7, e2348. [Google Scholar] [CrossRef]
- Chouin-Carneiro, T.; Vega-Rua, A.; Vazeille, M.; Yebakima, A.; Girod, R.; Goindin, D.; Dupont-Rouzeyrol, M.; Lourenço-De-Oliveira, R.; Failloux, A.B. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl. Trop. Dis. 2016, 10, e0004543. [Google Scholar] [CrossRef]
- DI Luca, M.; Severini, F.; Toma, L.; Boccolini, D.; Romi, R.; Remoli, M.E.; Sabbatucci, M.; Rizzo, C.; Venturi, G.; Rezza, G.; et al. Experimental studies of susceptibility of Italian Aedes albopictus to Zika virus. Eurosurveillance 2016, 21, 30223. [Google Scholar] [CrossRef]
- Ciota, A.T.; Bialosuknia, S.M.; Zink, S.D.; Brecher, M.; Ehrbar, D.J.; Morrissette, M.N.; Kramer, L.D. Effects of Zika virus strain and Aedes mosquito species on vector competence. Emerg. Infect. Dis. 2017, 23, 1110–1117. [Google Scholar] [CrossRef]
- Roundy, C.M.; Azar, S.R.; Rossi, S.L.; Huang, J.H.; Al, C.M.R.E.; Yun, R.; Fernandez-Salas, I.; Vitek, C.J.; Paploski, I.A.; Kitron, U.; et al. Variation in Aedes aegypti mosquito competence for Zika virus transmission. Emerg. Infect. Dis. 2017, 23, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Zimler, R.A.; Alto, B.W. Florida Aedes aegypti (Diptera: Culicidae) and Aedes albopictus vector competency for Zika virus. J. Med. Èntomol. 2019, 56, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Diallo, D.; Sall, A.A.; Diagne, C.T.; Faye, O.; Faye, O.; Ba, Y.; Hanley, K.A.; Buenemann, M.; Weaver, S.C.; Diallo, M. Zika virus emergence in mosquitoes in Southeastern Senegal, 2011. PLoS ONE 2014, 9, e109442. [Google Scholar] [CrossRef]
- Akoua-Koffi, C.; Diarrassouba, S.; Benie, V.B.; Ngbichi, J.M.; Bozoua, T.; Bosson, A.; Akran, V.; Carnevale, P.; Ehouman, A. Investigation surrounding a fatal case of yellow fever in Cote d’Ivoire in 1999. Bull. Soc. Pathol. Exot. 2001, 94, 227–230. [Google Scholar]
- Monlun, E.; Zeller, H.; Le Guenno, B.; Traoré-Lamizana, M.; Hervy, J.P.; Adam, F.; Ferrara, L.; Fontenille, D.; Sylla, R.; Mondo, M. Surveillance of the circulation of arbovirus of medical interest in the region of eastern Senegal. Bull. Soc. Pathol. Exot. Fil. 1993, 86, 21–28. [Google Scholar]
- Ayllón, T.; de Mendonça Campos, R.; Brasil, P.; Morone, F.C.; Câmara, D.C.P.; Meira, G.L.S.; Tannich, E.; Yamamota, K.A.; Carvolho, M.S.; Pedro, R.S.; et al. Early evidence for Zika virus circulation among Aedes aegypti mosquitoes, Rio de Janeiro, Brazil. Emerg. Infect. Dis. 2017, 23, 1411–1412. [Google Scholar] [CrossRef]
- Boorman, J.P.T.; Porterfield, J.S. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans. R. Soc. Trop. Med. Hyg. 1956, 50, 238–242. [Google Scholar] [CrossRef]
- Cornet, M.; Robin, Y.; Adam, C.; Valade, M.; Calvo, M.A. Transmission expérimentale comparée du virus amaril et du virus Zika chez Aedes aegypti. L Cah. ORSTOM Série Entomol. Médicale Et Parasitol. 1979, 17, 47–53. [Google Scholar]
- Diagne, C.T.; Diallo, D.; Faye, O.; Ba, Y.; Faye, O.; Gaye, A.; Dia, I.; Weaver, S.C.; Sall, A.A.; Diallo, M. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infect. Dis. 2015, 15, 492. [Google Scholar] [CrossRef] [PubMed]
- Vazeille, M.; Mousson, L.; Rosen, L.; Failloux, A.B. Low oral receptivity for dengue type 2 viruses of Aedes albopictus from southeast Asia compared with that of Aedes aegypti. Am. J. Trop. Med. Hyg. 2003, 68, 203–208. [Google Scholar] [CrossRef]
- Vega-Rúa, A.; Zouache, K.; Girod, R.; Failloux, A.B.; Lourenço-De-Oliveira, R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J. Virol. 2014, 88, 6294–6306. [Google Scholar] [CrossRef]
- Hennessey, M.; Fischer, M.; Staples, J.E. Zika virus spreads to new regions—Region of the Americas, May 2015–January 2016. Am. J. Transplant. 2016, 65, 55–58. [Google Scholar]
- Alto, B.W.; Wiggins, K.; Eastmond, B.; Velez, D.; Lounibos, L.P.; Lord, C.C. Transmission risk of two chikungunya lineages by invasive mosquito vectors from Florida and the Dominican Republic. PLoS Negl. Trop. Dis. 2017, 11, e0005724. [Google Scholar] [CrossRef]
- Murrell, E.G.; Damal, K.; Lounibos, L.P.; Juliano, S.A. Distributions of competing container mosquitoes depend on detritus types, nutrient ratios, and food availability. Ann. Èntomol. Soc. Am. 2011, 104, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Honório, N.A.; Wiggins, K.; Câmara, D.C.P.; Eastmond, B.; Alto, B.W. Chikungunya virus vector competency of Brazilian and Florida mosquito vectors. PLoS Negl. Trop. Dis. 2018, 12, e0006521. [Google Scholar] [CrossRef] [PubMed]
- Alto, B.W.; Lord, C.C. Transstadial effects of Bti on traits of Aedes aegypti and infection with Dengue virus. PLoS Negl. Trop. Dis. 2016, 10, e0004370. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Lanteri, M.C.; Kleinman, S.H.; Glynn, S.A.; Musso, D.; Hoots, W.K.; Custer, B.S.; Sabino, E.C.; Busch, M.P. Zika virus: A new threat to the safety of the blood supply with worldwide impact and implications. Transfusion 2016, 56, 1907–1914. [Google Scholar] [CrossRef]
- National Oceanic Administration Association (NOAA). Available online: http://cdo.ncdc.noaa.gov/ulcd/ULCD (accessed on 6 February 2020).
- Hall-Mendelin, S.; Ritchie, S.A.; Johansen, C.A.; Zborowski, P.; Cortis, G.; Dandridge, S.; Hall, R.A.; Hurk, A.F.V.D. Exploiting mosquito sugar feeding to detect mosquito-borne pathogens. Proc. Natl. Acad. Sci. USA 2010, 107, 11255–11259. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef]
- Stenn, T.; Peck, K.J.; Pereira, G.R.; Burkett-Cadena, N.D. Vertebrate hosts of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus (Diptera: Culicidae) as potential vectors of Zika virus in Florida. J. Med. Entomol. 2019, 56, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zimler, R.A.; Alto, B.W. The extrinsic incubation period of Zika virus in Florida mosquitoes Aedes aegypti and Ae. albopictus. Pathogens 2021, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Murrieta, R.A.; Garcia-Luna, S.M.; Murrieta, D.J.; Halladay, G.; Young, M.C.; Fauver, J.R.; Gendernalik, A.; Weger-Lucarelli, J.; Rückert, C.; Ebel, G.D. Impact of extrinsic incubation temperature on natural selection during Zika virus infection of Aedes aegypti and Aedes albopictus. PLoS Pathog. 2021, 17, e1009433. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).