High Concordance between D:A:Dr and the Framingham Risk Score in Brazilians Living with HIV
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Risk Stratification
2.3. Exclusion Criteria
2.4. Statistical Analysis
2.5. Agreement between the CVR Stratification Instruments
2.6. Recommendation for the Use of Statins According to CPTG
3. Results
3.1. Patient Characteristics
3.2. Risk Stratification
3.3. Agreement between the CVR Stratification Instruments
3.4. Recommendation for the Use of Statins According to CPTG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sommer Bittencourt, M. Estimando o Risco Cardiovascular em Pacientes Infectados pelo HIV. Arq. Bras. Cardiol. 2019, 114, 76–77. [Google Scholar] [CrossRef]
- de Barros, S.G.; Vieira-da-Silva, L.M. A terapia antirretroviral combinada, a política de controle da Aids e as transformações do Espaço Aids no Brasil dos anos 1990. Saúde Debate 2017, 41 (Suppl. 3), 114–128. [Google Scholar] [CrossRef]
- Boccara, F.; Cohen, A. HIV and Heart Disease: What Cardiologists Should Know. Rev. Española Cardiol. 2016, 69, 1126–1130. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Waters, D.D. Time to Recognize HIV Infection as a Major Cardiovascular Risk Factor. Circulation 2018, 138, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Todowede, O.O.; Sartorius, B.; Magula, N.; Schutte, A.E. Association of predicted 10 years cardiovascular mortality risk with duration of HIV infection and antiretroviral therapy among HIV-infected individuals in Durban, South Africa. Diabetol. Metab. Syndr. 2019, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Neto, M.G.; Zwirtes, R.; Brites, C. A literature review on cardiovascular risk in human immunodeficiency virus-infected patients: Implications for clinical management. Braz. J. Infect. Dis. 2013, 17, 691–700. [Google Scholar]
- Vendruscolo, O.; Majdalani, M.; Mota, R.; Luz, E.; Brites, C. Frequency of adverse events associated to antiretroviral drugs in patients starting therapy in Salvador, Brazil. Braz. J. Infect. Dis. 2015, 19, 108–109. [Google Scholar] [CrossRef]
- Krikke, M.; Hoogeveen, R.; Hoepelman, A.; Visseren, F.; Arends, J.E. Cardiovascular risk prediction in HIV-infected patients: Comparing the Framingham, atherosclerotic cardiovascular disease risk score (ASCVD), Systematic Coronary Risk Evaluation for the Netherlands (SCORE-NL) and Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) risk prediction models. HIV Med. 2016, 17, 289–297. [Google Scholar]
- Lotufo, P.A. O escore de risco de Framingham para doenças cardiovasculares. Rev. Med. 2008, 87, 232. [Google Scholar] [CrossRef]
- De Filippis, A.P.; Young, R.; Carrubba, C.J.; McEvoy, J.W.; Budoff, M.J.; Blumenthal, R.S.; Kronmal, R.A.; McClelland, R.L.; Nasir, K.; Blaha, M.J. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann. Intern. Med. 2015, 162, 266–275. [Google Scholar] [CrossRef]
- Silva, A.G.; Paulo, R.V.; Silva-Vergara, M.L. Subclinical carotid atherosclerosis and reduced dad score for cardiovascular risk stratification in HIV-positive patients. Arq. Bras. Cardiol. 2020, 114, 68–75. [Google Scholar] [PubMed]
- Friis-Møller, N.; Ryom, L.; Smith, C.; Weber, R.; Reiss, P.; Dabis, F.; de Wit, S.; Monforte, A.D.; Kirk, O.; Fontas, E.; et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur. J. Prev. Cardiol. 2016, 23, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Nery, M.W.; Martelli, C.M.T.; Silveira, E.A.; de Sousa, C.A.; de Oliveira Falco, M.; de Cássia Oliveira de Castro, A.; Esper, J.T.; Silva e Souza, L.C.; Turchi, M.D. Cardiovascular risk assessment: A comparison of the Framingham, PROCAM, and DAD equations in HIV-infected persons. Sci. World J. 2013, 2013, 969281. [Google Scholar] [CrossRef] [PubMed]
- Achhra, A.C.; Lyass, A.; Borowsky, L.; Bogorodskaya, M.; Plutzky, J.; Massaro, J.M.; D’Agostino Sr, R.B.; Triant, V.A. Assessing Cardiovascular Risk in People Living with HIV: Current Tools and Limitations. Curr. HIV/AIDS Rep. 2021, 18, 271–279. [Google Scholar] [CrossRef] [PubMed]
- BRASIL. Protocolo Clínico e Diretrizes Terapêuticas para Manejo da Infecção pelo HIV em Adultos; Ministério da Saúde: Brasília, Brazil, 2018; 412 p, ISBN 978-85-334-2640-5. [Google Scholar]
- Friis-Møller, N.; Weber, R.; Reiss, P.; Thiébaut, R.; Kirk, O.; D’Arminio Monforte, A.; Pradier, C.; Morfeldt, L.; Mateu, S.; Law, M.; et al. Cardiovascular disease risk factors in HIV patients—Association with antiretroviral therapy. Results from the DAD study. Aids 2003, 17, 1179–1193. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. Available online: http://www.jstor.org/stable/2529310 (accessed on 23 August 2021). [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Lancet 1977, 38, 97–107. [Google Scholar] [CrossRef]
- da Silva Pinto Neto, L.F.; Dias, F.R.; Bressan, F.F.; Santos, C.R.O. Comparison of the ACC/AHA and Framingham algorithms to assess cardiovascular risk in HIV-infected patients. Braz. J. Infect. Dis. 2017, 21, 577–580. [Google Scholar] [CrossRef]
- Kelesidis, T.; Currier, J.S. Dyslipidemia and cardiovascular risk in human immunodeficiency virus infection. Endocrinol. Metab. Clin. North Am. 2014, 43, 665–684. [Google Scholar] [CrossRef]
- Bailin, S.S.; Gabriel, C.L.; Wanjalla, C.N.; Koethe, J.R. Obesity and Weight Gain in Persons with HIV. Curr HIV/AIDS Rep. 2020, 17, 138–150. [Google Scholar] [CrossRef]
- Hemkens, L.G.; Bucher, H.C. HIV infection and cardiovascular disease. Eur. Heart J. 2014, 35, 1373–1381. [Google Scholar] [CrossRef]
- Wu, P.Y.; Chen, M.Y.; Sheng, W.H.; Hsieh, S.M.; Chuang, Y.C.; Cheng, A.; Pan, S.-C.; Wu, U.-I.; Chang, H.-Y.; Luo, Y.-Z.; et al. Estimated risk of cardiovascular disease among the HIV-positive patients aged 40 years or older in Taiwan. J. Microbiol. Immunol. Infect. 2019, 52, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Sánchez, M.; Perelló, R.; Pérez, I.; Mateo, M.G.; Junyent, M.; Laguno, M.; Martínez-Rebollar, M.; Sánchez, M.; Mallolas, J.; Gatell, J.M.; et al. Differences between HIV-infected and uninfected adults in the contributions of smoking, diabetes and hypertension to acute coronary syndrome: Two parallel case-control studies. HIV Med. 2013, 14, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.; Sabin, C.A.; Alagaratnam, J.; Bagkeris, E.; Post, F.A.; Boffito, M.; Anderson, J.; Vera, J.; Williams, I.; Johnson, M.; et al. Level of agreement between frequently used cardiovascular risk calculators in people living with HIV. HIV Med. 2019, 20, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Bazo-Alvarez, J.C.; Quispe, R.; Peralta, F.; Poterico, J.A.; Valle, G.A.; Burroughs, M.; Pillay, T.; Gilman, R.H.; Checkley, W.; Malaga, G.; et al. Agreement between cardiovascular disease risk scores in resource-limited settings: Evidence from 5 Peruvian sites. Crit. Pathw. Cardiol. 2015, 14, 74–80. [Google Scholar] [CrossRef]
- Edwards-Jackson, N.; Kerr, S.J.; Tieu, H.V.; Ananworanich, J.; Hammer, S.M.; Ruxrungtham, K.; Phanuphak, P.; Avihingsanon, A. HIV-NAT 006 Study Team. Cardiovascular risk assessment in persons with HIV infection in the developing world: Comparing three risk equations in a cohort of HIV-infected Thais. HIV Med. 2011, 12, 510–515. [Google Scholar] [CrossRef]
- Triant, V.A.; Perez, J.; Regan, S.; Massaro, J.M.; Meigs, J.B.; Grinspoon, S.K.; D’Agostino Sr, R.B. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation 2018, 137, 2203–2214. [Google Scholar] [CrossRef]
- Thompson-Paul, A.M.; Lichtenstein, K.A.; Armon, C.; Palella, F.J.; Skarbinski, J.; Chmiel, J.S.; Hart, R.; Wei, S.C.; Loustalot, F.; Brooks, J.T.; et al. Cardiovascular disease risk prediction in the HIV outpatient study. Clin. Infect. Dis. 2016, 63, 1508–1516. [Google Scholar] [CrossRef]
- Goff, D.C.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 2014, 129 (Suppl. 1), 49–73. [Google Scholar] [CrossRef]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Phan, B.A.P.; Weigel, B.; Ma, Y.; Scherzer, R.; Li, D.; Hur, S.; Kalapus, S.C.; Deeks, S.; Hsue, P. Utility of 2013 American College of Cardiology/American Heart Association Cholesterol Guidelines in HIV-Infected Adults with Carotid Atherosclerosis. Circ. Cardiovasc. Imaging 2017, 10, e005995. [Google Scholar] [CrossRef] [PubMed]

| FRS | ASCVD | D:A:Dr | |
|---|---|---|---|
| Cohort | Framingham Heart Study | New pooled cohort | D:A:D study |
| Predictors | Age, systolic blood pressure, use of antihypertensive medication, current smoking, DM, HDL cholesterol. | Age, sex, race (white, African-American, others), systolic and diastolic BP, total cholesterol, HDL, DM, smoking, and antihypertensive therapy. | Gender, age, smoking, DM (diagnosed or on antidiabetic treatment), family history of early CVD, systolic BP, total cholesterol, HDL, CD4+ count. |
| Age group | 30–75 | 40–74 | 18–75 |
| Cardiovascular outcomes | Coronary heart disease, cerebrovascular and peripheral arterial disease, and heart failure. | First occurrence of nonfatal myocardial infarction, death due to coronary artery disease, and stroke. | Myocardial infarction, stroke, invasive coronary artery procedure or death due to coronary heart disease. |
| n (%) | FRS | p * | ASCVD | p * | D:A:Dr | p * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low CVR | Moderate CVR | High CVR | Low CVR | Moderate CVR | High CVR | Low CVR | Moderate CVR | High CVR | |||||
| Sex (%) | 0.001 | <0.001 | 0.051 | ||||||||||
| Male | 156 (58.9) | 60 (46.9) | 55 (68.8) | 41 (71.9) | 79 (51.3) | 69 (75.0) | 8 (42.1) | 65 (51.2) | 56 (65.9) | 35 (66.0) | |||
| Female | 109 (41.1) | 68 (53.1) | 25 (31.2) | 16 (28.1) | 75 (48.7) | 23 (25.0) | 11 (57.9) | 62 (48.8) | 29 (34.1) | 18 (34.0) | |||
| Race (%) | 0.110 | 0.494 | 0.166 | ||||||||||
| White | 33 (12.5) | 12 (9.4) | 11 (13.8) | 10 (17.5) | 17 (11.0) | 13 (14.1) | 3 (15.8) | 12 (9.4) | 11 (12.9) | 10 (18.9) | |||
| Black | 110 (41.5) | 63 (49.2) | 30 (35.7) | 17 (29.8) | 71 (46.1) | 33 (35.9) | 6 (31.6) | 61 (48.0) | 33 (38.8) | 16 (30.2) | |||
| Miscegenated | 122 (46.0) | 53 (41.4) | 39 (48.8) | 30 (52.6) | 66 (42.9) | 46 (50.0) | 10 (52.6) | 54 (42.5) | 41 (48.2) | 27 (50.9) | |||
| Age † | 52 (47–58) | 48 (43.2–52.7) | 55 (50–60.7) | 60 (56–66.5) | <0.001 | 49 (44–53) | 58 (53.2–65) | 66 (58-69) | <0.001 | 47 (43–52) | 56 (50–60) | 64 (56.5–68) | <0.001 |
| BMI (%) | 0.581 | 0.272 | 0.907 | ||||||||||
| Overweight | 83 (31.3) | 38 (29.7) | 26 (32.5) | 19 (33.3) | 51 (33.1) | 23 (25.0) | 9 (47.4) | 40 (31.5) | 24 (28.2) | 19 (35.8) | |||
| Obesity | 50 (18.9) | 20 (15.6) | 17 (21.3) | 13 (22.8) | 26 (16.9) | 20 (21.7) | 4 (21.1) | 25 (19.7) | 16 (18.8) | 9 (17.0) | |||
| Changed AC (%) (n = 259) | 85 (32.8) | 37 (29.6) | 23 (29.9) | 25 (43.9) | 0.132 | 49 (32.5) | 25 (28.1) | 11 (57.9) | 0.042 | 39 (31.7) | 25 (29.8) | 21 (40.4) | 0.412 |
| Smoking (%) | <0.001 | 0.001 | <0.001 | ||||||||||
| Never | 172 (64.9) | 96 (55.8) | 51 (29.6) | 25 (14.5) | 116 (75.3) | 47 (51.0) | 9 (47.3) | 105 (82.6) | 46 (54.1) | 21 (39.6) | |||
| Past | 63 (23.8) | 19 (30.1) | 26 (41.2) | 18 (28.5) | 25 (16.2) | 31 (33.6) | 7 (36.8) | 17 (13.3) | 25 (29.4) | 21 (399.6) | |||
| Current | 30 (11.3) | 13 (43.3) | 3 (10) | 14 (46.7) | 13 (8.4) | 14 (15.2) | 3 (15.7) | 5 (3.9) | 14 (16.4) | 11 (20.7) | |||
| Alcohol abuse (%) | 68 (25.7) | 36 (52.9) | 19 (27.9) | 13 (19.1) | 0.669 | 39 (57.3) | 25 (36.7) | 4 (5.8) | 0.847 | 38 (55.8) | 19 (27.9) | 11 (16.1) | 0.307 |
| Sedentary life (%) | 139 (52.5) | 62 (44.6) | 43 (30.9) | 34 (24.4) | 0.356 | 84 (60.4) | 45 (32.3) | 10 (7.1) | 0.693 | 62 (44.6) | 47 (33.8) | 30 (21.5) | 0.518 |
| Previous comorbidities (%) | |||||||||||||
| AH | 90 (34.0) | 17 (18.8) | 35 (38.8) | 38 (42.2) | <0.001 | 23 (25.5) | 50 (55.5) | 17 (18.8) | <0.001 | 25 (27.7) | 36 (40.0) | 29 (32.2) | <0.001 |
| DM | 24 (9.1) | 1 (4.1) | 9 (37.5) | 14 (56.0) | <0.001 | 2 (8.3) | 12 (50.0) | 10 (41.6) | <0.001 | 2 (8.3) | 7 (29.1) | 15 (62.5) | <0.001 |
| CKD | 6 (2.3) | 2 (33.3) | 2 (33.3) | 2 (33.3) | 0.703 | 2 (33.3) | 3 (50.0) | 1 (16.6) | 0.400 | 1 (16.6) | 3 (50.0) | 2 (33.3) | 0.299 |
| Family history of early CAD (%) | 42 (15.8) | 22 (52.3) | 12 (28.5) | 8 (19.0) | 0.837 | 27 (64.2) | 13 (30.9) | 2 (4.7) | 0.627 | 15 (35.7) | 18 (42.8) | 9 (21.1) | 0.182 |
| Use of lipid-lowering drugs (%) | 33 (12.5) | 9 (27.2) | 17 (51.5) | 7 (21.2) | 0.010 | 14 (42.2) | 14 (42.2) | 5 (15.1) | 0.061 | 9 (27.2) | 15 (45.4) | 9 (27.2) | 0.040 |
| Changed lipid profile (%) | |||||||||||||
| CT > 190 mg/dL | 149 (56.2) | 64 (50.0) | 47 (58.8) | 38 (66.7) | 0.093 | 84 (54.5) | 49 (53.3) | 16 (84.2) | 0.038 | 60 (47.2) | 51 (60.0) | 38 (71.7) | 0.007 |
| HDL < 40 mg/dL | 84 (31.7) | 32 (25.0) | 27 (33.8) | 25 (43.9) | 0.035 | 43 (27.9) | 31 (33.7) | 10 (52.6) | 0.081 | 36 (28.3) | 28 (32.9) | 20 (37.7) | 0.447 |
| LDL > 130 mg/dL | 110 (41.5) | 48 (37.5) | 34 (42.5) | 28 (49.1) | 0.326 | 64 (41.6) | 35 (38.0) | 11 (57.9) | 0.279 | 45 (35.4) | 39 (45.9) | 26 (49.1) | 0.146 |
| TG > 150 mg/dL | 118 (44.5) | 41 (32.0) | 41 (51.2) | 36 (63.2) | <0.001 | 56 (36.4) | 47 (51.1) | 15 (78.9) | 0.001 | 45 (35.4) | 36 (42.4) | 37 (69.8) | <0.001 |
| Glucose ≥100 mg/dL (%) (n = 254) | 94 (37.0) | 34 (27.2) | 35 (46.1) | 25 (47.2) | 0.006 | 43 (28.9) | 40 (46.0) | 11 (61.1) | 0.003 | 35 (28.2) | 35 (42.7) | 24 (50.0) | 0.013 |
| Creatinine (mg/dL)† (n = 249) | 0,92 (0.8–1.1) | 0.9 (0.8–1.1) | 1 (0.8–1.1) | 1 (0.8–1.2) | 0.008 | 0.9 (0.8–1.1) | 1.0 (0.8–1.2) | 1.1 (0.8–1.2) | 0.006 | 1.0 (0.8–1.1) | 0.9 (0.8–1.1) | 1.0 (0.9–1.2) | 0.097 |
| Blood pressure | |||||||||||||
| SBP (mmHg) † | 130 (120–140) | 120 (110–130) | 130 (120–140) | 14 (130–160) | <0.001 | 120 (110–130) | 130 (120–140) | 160 (150–180) | <0.001 | 120 (110–130) | 130 (120–140) | 140 (129.5–160) | <0.001 |
| DBP (mmHg) † | 80 (70–81) | 80 (70–80) | 80 (70–90) | 80 (80–90) | <0.001 | 80 (70–90) | 80 (70–80) | 90 (80–100) | <0.001 | 80 (70–80) | 80 (70–90) | 80 (70–90) | <0.001 |
| MBP (mmHg) † | 93.3(86.7–103.3) | 90 (83.3–96.7) | 96,7 (90–106.7) | 103.3 (96.7–113.3) | <0.001 | 93.3 (83.3–96.7) | 96.7 (90–106.7) | 113.3 (103.3–126.7) | <0.001 | 93.3 (83.3–96.7) | 96.7 (90-103) | 103.3 (91.7–113.3) | <0.001 |
| Time since diagnosis † | 15.5 (7–22) | 11 (5–21) | 16.5 (11–22.7) | 18 (11–22) | 0.003 | 11.5 (6–21) | 18 (11–22) | 21 (13–27) | <0.001 | 12 (6–21) | 16 (9.5–22) | 19 (11–22) | 0.013 |
| Time of antiretroviral therapy † | 15 (7–21) | 10.5 (5–21) | 15 (10.2–21) | 17 (10–21.5) | 0.003 | 11 (5–21) | 17 (11–21) | 20 (11–24) | <0.001 | 11 (6–21) | 15 (9–21) | 18 (10–21) | 0.015 |
| Sexual orientation (%) | 0.543 | 0.345 | 0.852 | ||||||||||
| Heterosexual | 71 (26.8) | 29 (10.9) | 25 (9.4) | 17 (6.4) | 42 (15.8) | 26 (9.8) | 3 (1.1) | 35 (13.2) | 21 (7.9) | 15 (5.7) | |||
| MSM | 140 (52.8) | 73 (27.5) | 37 (14.0) | 30 (11.3) | 83 (30.9) | 44 (16.6) | 14 (5.3) | 65 (24.5) | 45 (17.0) | 30 (11.3) | |||
| Not defined | 54 (20.4) | 26 (9.8) | 18 (6.8) | 10 (3.8) | 30 (11.3) | 22 (8.3) | 2 (0.8) | 27 (10.2) | 19 (7.2) | 8 (3.0) | |||
| CD4+ (cells/mL) (%) | 0.490 | 0.329 | 0.929 | ||||||||||
| <200 | 12 (4.5) | 7 (5.4) | 2 (3) | 3 (4.1) | 7 (4.5) | 5 (5.4) | 0 (0.0) | 5 (3.9) | 4 (4.7) | 3 (5.6) | |||
| 200–499 | 73 (27.5) | 40 (31.2) | 21 (32.3) | 12 (16.6) | 44 (28.5) | 27 (29.3) | 2 (10.5) | 36 (28.3) | 21 (24.7) | 16 (30.1) | |||
| ≥500 | 180 (67.9) | 81 (63.2) | 42 (64.6) | 57 (79.1) | 103 (66.8) | 60 (65.2) | 17 (89.4) | 86 (67.7) | 60 (70.5) | 34 (64.1) | |||
| Detectable viral load | 22 (8.3) | 12 (54.5) | 9 (40.9) | 1 (4.5) | 0.089 | 15 (68.2) | 7 (31.8) | 0 (0.0) | 0.493 | 13 (59.1) | 7 (31.8) | 2 (9.1) | 0.413 |
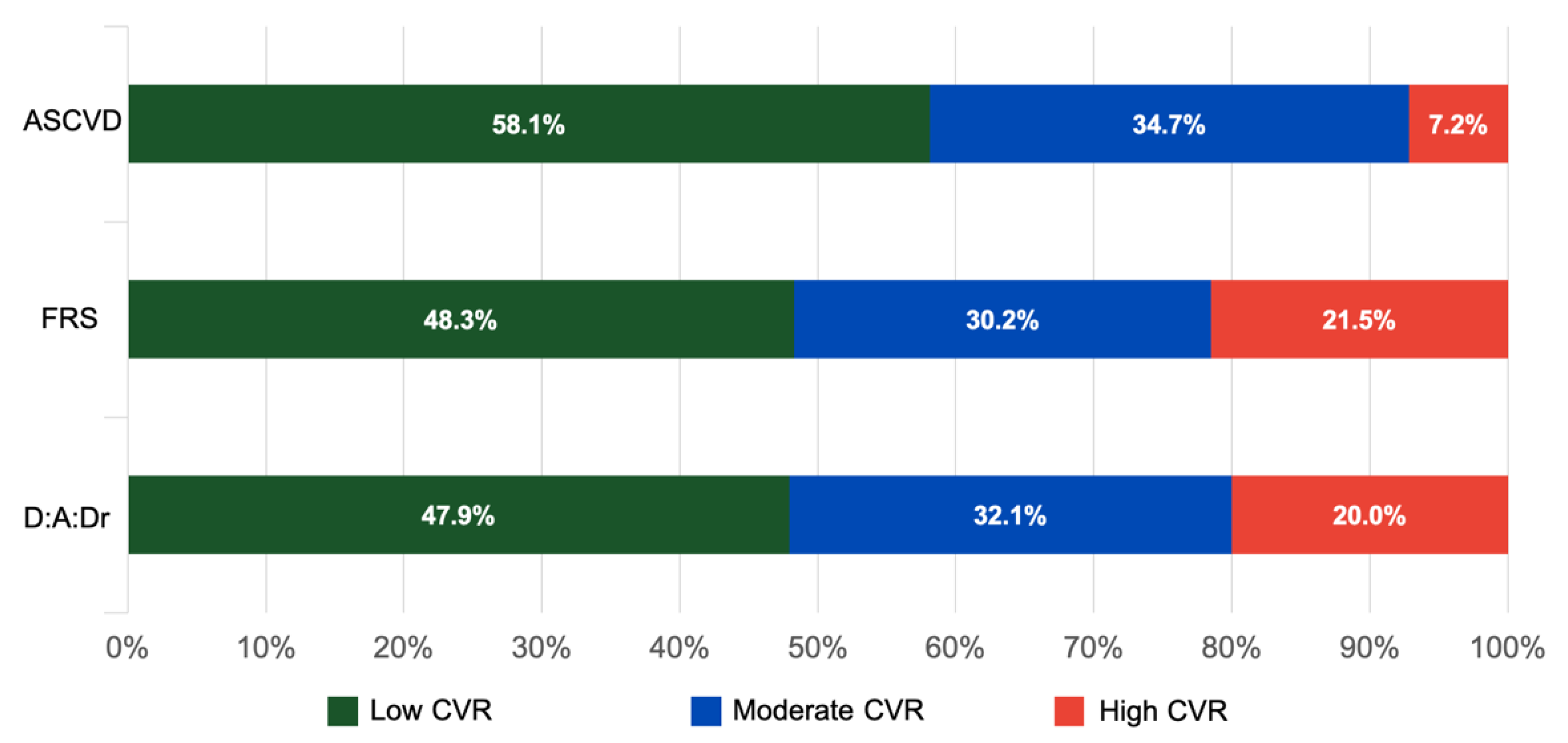
| Total | 265 | 128 (48.3) | 80 (30.2) | 57 (21.5) | 154 (58.1) | 92 (34.7) | 19 (7.2) | 127 (47.9) | 85 (32.1) | 53 (20.0) | |||
| FRS | ASCVD | ||||||
|---|---|---|---|---|---|---|---|
| Low CVR (%) | Moderate CVR (%) | High CVR (%) | Low CVR (%) | Moderate CVR (%) | High CVR (%) | ||
| D:A:Dr | Low CVR (%) | 110 (41.5) | 17 (6.4) | 0 (0.0) | 118 (44.5) | 9 (3.4) | 0 (0.0) |
| Moderate CVR (%) | 18 (6.8) | 54 (20.4) | 13 (4.9) | 35 (13.2) | 50 (18.9) | 0 (0.0) | |
| High CVR (%) | 0 (0.0) | 9 (3.4) | 44 (16.6) | 1 (0.4) | 33 (12.5) | 19 (7.2) | |
| ASCVD | Low CVR (%) | 123 (46.4) | 5 (1.9) | 0 (0.0) | - | - | - |
| Moderate CVR (%) | 31 (11.7) | 49 (18.5) | 0 (0.0) | - | - | - | |
| High CVR (%) | 0 (0.0) | 38 (14.3) | 19 (7.2) | - | - | - | |
| CVR Score | |||
|---|---|---|---|
| FRS | ASCVD | D:A:Dr | |
| Expected risk in 10 years (%) (median (IQR)) | 10.00 (5.60–18.40) | 6.20 (3.35–11.30) | 5.23 (2.85–8.74) |
| Agreement between scores | |||
| FRS | |||
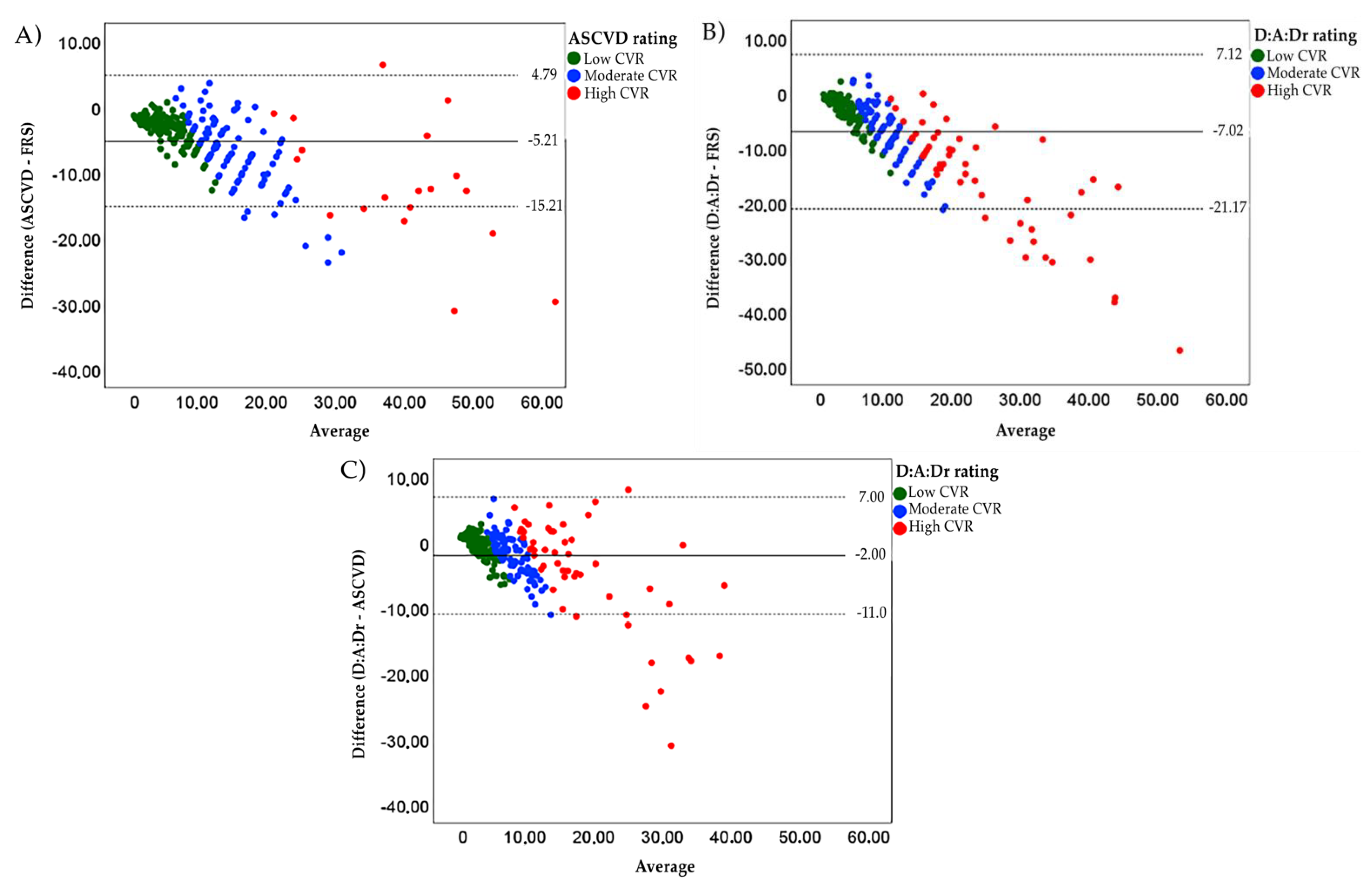
| Observed agreement (%) | - | 72.1 | 78.5 |
| Weighted kappa (CI 95%) | - | 0.74 (0.69–0.79) | 0.82 (0.77–0.87) |
| p value | - | p < 0.001 | p < 0.001 |
| ASCVD | |||
| Observed agreement (%) | - | - | 70.6 |
| Weighted kappa (CI 95%) | - | - | 0.70 (0.64–0.76) |
| p value | - | - | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, V.; Valadares, V.; Dias, T.; Brites, C. High Concordance between D:A:Dr and the Framingham Risk Score in Brazilians Living with HIV. Viruses 2023, 15, 348. https://doi.org/10.3390/v15020348
Souza V, Valadares V, Dias T, Brites C. High Concordance between D:A:Dr and the Framingham Risk Score in Brazilians Living with HIV. Viruses. 2023; 15(2):348. https://doi.org/10.3390/v15020348
Chicago/Turabian StyleSouza, Vitor, Victória Valadares, Thais Dias, and Carlos Brites. 2023. "High Concordance between D:A:Dr and the Framingham Risk Score in Brazilians Living with HIV" Viruses 15, no. 2: 348. https://doi.org/10.3390/v15020348
APA StyleSouza, V., Valadares, V., Dias, T., & Brites, C. (2023). High Concordance between D:A:Dr and the Framingham Risk Score in Brazilians Living with HIV. Viruses, 15(2), 348. https://doi.org/10.3390/v15020348







