Swine-to-Ferret Transmission of Antigenically Drifted Contemporary Swine H3N2 Influenza A Virus Is an Indicator of Zoonotic Risk to Humans
Abstract
1. Introduction
2. Material and Methods
2.1. Genetic Analysis and Strain Selection
2.2. Viruses and Ferret Antisera
2.3. Hemagglutination Inhibition
2.4. Swine-to-Ferret Transmission Study Design
2.5. Virus Replication and Shedding
2.6. Pathologic Examination
2.7. Microbiological Assays
2.8. Data Analysis
3. Results
3.1. Genetic Characterization of Dominant U.S. Swine H3N2 Strains
3.2. Loss in Cross-Reactivity between Dominant Swine H3N2 Strains and CVV or HuVac
3.3. Swine-to-Ferret Transmission of Antigenically Drifted Swine H3N2 Lineages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jester, B.J.; Uyeki, T.M.; Jernigan, D.B. Fifty Years of Influenza A(H3N2) Following the Pandemic of 1968. Am. J. Public Health 2020, 110, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Long, J.S.; Mistry, B.; Haslam, S.M.; Barclay, W.S. Publisher Correction: Host and viral determinants of influenza A virus species specificity. Nat. Rev. Microbiol. 2019, 17, 124, Correction in Nat. Rev. Microbiol. 2019, 17, 67–81. [Google Scholar] [CrossRef]
- Garten, R.J.; Davis, C.T.; Russell, C.A.; Shu, B.; Lindstrom, S.; Balish, A.; Sessions, W.M.; Xu, X.; Skepner, E.; Deyde, V.; et al. Antigenic and Genetic Characteristics of Swine-Origin 2009 A(H1N1) Influenza Viruses Circulating in Humans. Science 2009, 325, 197–201. [Google Scholar] [CrossRef]
- Olsen, C.W.; Karasin, A.I.; Carman, S.; Li, Y.; Bastien, N.; Ojkic, D.; Alves, D.; Charbonneau, G.; Henning, B.M.; Low, D.E.; et al. Triple Reassortant H3N2 Influenza A Viruses, Canada, 2005. Emerg. Infect. Dis. 2006, 12, 1132–1135. [Google Scholar] [CrossRef]
- Rajao, D.S.; Vincent, A.L.; Perez, D. Adaptation of Human Influenza Viruses to Swine. Front. Veter- Sci. 2018, 5, 347. [Google Scholar] [CrossRef]
- Zhou, N.N.; Senne, D.A.; Landgraf, J.S.; Swenson, S.L.; Erickson, G.; Rossow, K.; Liu, L.; Yoon, K.; Krauss, S.; Webster, R.G. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J. Virol. 1999, 73, 8851–8856. [Google Scholar] [CrossRef] [PubMed]
- Webby, R.J.; Swenson, S.L.; Krauss, S.L.; Gerrish, P.J.; Goyal, S.M.; Webster, R.G. Evolution of Swine H3N2 Influenza Viruses in the United States. J. Virol. 2000, 74, 8243–8251. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.I.; Vincent, A.L.; Kitikoon, P.; Holmes, E.C.; Gramer, M.R. Evolution of Novel Reassortant A/H3N2 Influenza Viruses in North American Swine and Humans, 2009–2011. J. Virol. 2012, 86, 8872–8878. [Google Scholar] [CrossRef]
- Vincent, A.L.; Ma, W.; Lager, K.M.; Janke, B.H.; Richt, J.A. Swine influenza viruses a North American perspective. Adv. Virus Res. 2008, 72, 127–154. [Google Scholar]
- Anderson, T.K.; Chang, J.; Arendsee, Z.W.; Venkatesh, D.; Souza, C.K.; Kimble, J.B.; Lewis, N.S.; Davis, C.T.; Vincent, A.L. Swine Influenza A Viruses and the Tangled Relationship with Humans. Cold Spring Harb. Perspect. Med. 2020, 11, a038737. [Google Scholar] [CrossRef] [PubMed]
- Bolton, M.J.; Abente, E.J.; Venkatesh, D.; Stratton, J.A.; Zeller, M.; Anderson, T.K.; Lewis, N.S.; Vincent, A.L. Antigenic evolution of H3N2 influenza A viruses in swine in the United States from 2012 to 2016. Influ. Other Respir. Viruses 2018, 13, 83–90. [Google Scholar] [CrossRef]
- Lewis, N.S.; Anderson, T.K.; Kitikoon, P.; Skepner, E.; Burke, D.F.; Vincent, A.L. Substitutions near the Hemagglutinin Receptor-Binding Site Determine the Antigenic Evolution of Influenza A H3N2 Viruses in U.S. Swine. J. Virol. 2014, 88, 4752–4763. [Google Scholar] [CrossRef]
- Rajão, D.S.; Gauger, P.C.; Anderson, T.K.; Lewis, N.S.; Abente, E.J.; Killian, M.L.; Perez, D.R.; Sutton, T.C.; Zhang, J.; Vincent, A.L. Novel Reassortant Human-Like H3N2 and H3N1 Influenza A Viruses Detected in Pigs Are Virulent and Antigenically Distinct from Swine Viruses Endemic to the United States. J. Virol. 2015, 89, 11213–11222. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zeller, M.A.; Souza, C.K.; Anderson, T.K.; Vincent, A.L.; Harmon, K.; Li, G.; Zhang, J.; Gauger, P.C. Characterization of a 2016–2017 Human Seasonal H3 Influenza A Virus Spillover Now Endemic to U.S. Swine. Msphere 2022, 7, e00809-21. [Google Scholar] [CrossRef] [PubMed]
- Zeller, M.; Li, G.; Harmon, K.M.; Zhang, J.; Vincent, A.L.; Anderson, T.K.; Gauger, P.C. Complete Genome Sequences of Two Novel Human-Like H3N2 Influenza A Viruses, A/swine/Oklahoma/65980/2017 (H3N2) and A/Swine/Oklahoma/65260/2017 (H3N2), Detected in Swine in the United States. Genome Announc. 2018, 7, e01203-18. [Google Scholar] [CrossRef]
- Robertson, J.S.; Nicolson, C.; Harvey, R.; Johnson, R.; Major, D.; Guilfoyle, K.; Roseby, S.; Newman, R.; Collin, R.; Wallis, C.; et al. The development of vaccine viruses against pandemic A(H1N1) influenza. Vaccine 2011, 29, 1836–1843. [Google Scholar] [CrossRef]
- Burke, S.A.; Trock, S.C. Use of Influenza Risk Assessment Tool for Prepandemic Preparedness. Emerg. Infect. Dis. 2018, 24, 471–477. [Google Scholar] [CrossRef]
- Souza, C.K.; Anderson, T.K.; Chang, J.; Venkatesh, D.; Lewis, N.S.; Pekosz, A.; Shaw-Saliba, K.; Rothman, R.E.; Chen, K.-F.; Vincent, A.L. Antigenic Distance between North American Swine and Human Seasonal H3N2 Influenza A Viruses as an Indication of Zoonotic Risk to Humans. J. Virol. 2022, 96, e01374-21. [Google Scholar] [CrossRef]
- Shu, Y.; McCauley, J. GISAID: Global initiative on sharing all influenza data—From vision to reality. Euro Surveill 2017, 22, 30494. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Arendsee, Z.W.; Baker, A.L.V.; Anderson, T.K. smot: A python package and CLI tool for contextual phylogenetic subsampling. J. Open Source Softw. 2022, 7, 4193. [Google Scholar] [CrossRef]
- Chang, J.; Anderson, T.K.; Zeller, M.; Gauger, P.C.; Vincent, A.L. octoFLU: Automated Classification for the Evolutionary Origin of Influenza A Virus Gene Sequences Detected in U.S. Swine. Microbiol. Resour. Announc. 2019, 8, e00673-19. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.S.; Kimble, J.B.; Chang, J.; Anderson, T.K.; Gauger, P.C.; Janas-Martindale, A.; Killian, M.L.; Bowman, A.S.; Vincent, A.L. Aerosol Transmission from Infected Swine to Ferrets of an H3N2 Virus Collected from an Agricultural Fair and Associated with Human Variant Infections. J. Virol. 2020, 94, e01009-20. [Google Scholar] [CrossRef] [PubMed]
- Kitikoon, P.; Gauger, P.C.; Vincent, A.L. Hemagglutinin Inhibition Assay with Swine Sera. Methods Mol. Biol. 2014, 1161, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Gauger, P.C.; Vincent, A.L.; Loving, C.L.; Henningson, J.N.; Lager, K.M.; Janke, B.H.; Kehrli, M.E., Jr.; Roth, J.A. Kinetics of Lung Lesion Development and Pro-Inflammatory Cytokine Response in Pigs with Vaccine-Associated Enhanced Respiratory Disease Induced by Challenge with Pandemic (2009) A/H1N1 Influenza Virus. Vet. Pathol. 2012, 49, 900–912. [Google Scholar] [CrossRef]
- Kitikoon, P.; Nilubol, D.; Erickson, B.J.; Janke, B.H.; Hoover, T.C.; Sornsen, S.A.; Thacker, E.L. The immune response and maternal antibody interference to a heterologous H1N1 swine influenza virus infection following vaccination. Vet. Immunol. Immunopathol. 2006, 112, 117–128. [Google Scholar] [CrossRef]
- Vincent, L.L.; Janke, B.H.; Paul, P.S.; Halbur, P.G. A Monoclonal-Antibody-Based Immunohistochemical Method for the Detection of Swine Influenza Virus in Formalin-Fixed, Paraffin-Embedded Tissues. J. Vet. Diagn. Investig. 1997, 9, 191–195. [Google Scholar] [CrossRef]
- Opriessnig, T.; Yu, S.; Gallup, J.M.; Evans, R.B.; Fenaux, M.; Pallares, F.; Thacker, E.L.; Brockus, C.W.; Ackermann, M.R.; Thomas, P.; et al. Effect of Vaccination with Selective Bacterins on Conventional Pigs Infected with Type 2 Porcine Circovirus. Vet. Pathol. 2003, 40, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC) Reported Infections with Variant Influenza Viruses in the United States. Available online: https://www.cdc.gov/flu/swineflu/variant-cases-us.htm (accessed on 3 January 2020).
- Henritzi, D.; Petric, P.P.; Lewis, N.S.; Graaf, A.; Pessia, A.; Starick, E.; Breithaupt, A.; Strebelow, G.; Luttermann, C.; Parker, L.M.K.; et al. Surveillance of European Domestic Pig Populations Identifies an Emerging Reservoir of Potentially Zoonotic Swine Influenza A Viruses. Cell Host Microbe 2020, 28, 614–627.e6. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.; Harder, T.C.; SchwemmLe, M.; Ciminski, K. Influenza A Viruses and Zoonotic Events-Are We Creating Our Own Reservoirs? Viruses 2021, 13, 2250. [Google Scholar] [PubMed]
- Nelson, M.I.; Worobey, M. Origins of the 1918 Pandemic: Revisiting the Swine “Mixing Vessel” Hypothesis. Am. J. Epidemiol. 2018, 187, 2498–2502. [Google Scholar] [CrossRef]
- Short, K.R.; Richard, M.; Verhagen, J.H.; van Riel, D.; Schrauwen, E.J.; Brand, J.M.V.D.; Mänz, B.; Bodewes, R.; Herfst, S. One health, multiple challenges: The inter-species transmission of influenza A virus. One Health 2015, 1, 1–13. [Google Scholar] [CrossRef]
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans. N. Engl. J. Med. 2009, 360, 2605–2615. [Google Scholar] [CrossRef]
- Neveau, M.N.; Zeller, M.A.; Kaplan, B.S.; Souza, C.K.; Gauger, P.C.; Vincent, A.L.; Anderson, T.K. Genetic and Antigenic Characterization of an Expanding H3 Influenza A Virus Clade in U.S. Swine Visualized by Nextstrain. Msphere 2022, 7, e00994-21. [Google Scholar] [CrossRef]
- Zeller, M.; Anderson, T.K.; Walia, R.W.; Vincent, A.L.; Gauger, P.C. ISU FLUture: A veterinary diagnostic laboratory web-based platform to monitor the temporal genetic patterns of Influenza A virus in swine. BMC Bioinform. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Kimble, J.B.; Souza, C.K.; Anderson, T.K.; Arendsee, Z.W.; Hufnagel, D.E.; Young, K.M.; Lewis, N.S.; Davis, C.T.; Thor, S.; Baker, A.L.V. Interspecies Transmission from Pigs to Ferrets of Antigenically Distinct Swine H1 Influenza A Viruses with Reduced Reactivity to Candidate Vaccine Virus Antisera as Measures of Relative Zoonotic Risk. Viruses 2022, 14, 2398. [Google Scholar] [CrossRef]
- DiPiazza, A.T.; Richards, K.A.; Liu, W.-C.; Albrecht, R.A.; Sant, A.J. Analyses of Cellular Immune Responses in Ferrets following Influenza Virus Infection. Methods Mol. Biol. 2018, 1836, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.B.; Jayaraman, A.; Pappas, C.; Belser, J.A.; Zeng, H.; Gustin, K.M.; Maines, T.R.; Sun, X.; Raman, R.; Cox, N.J.; et al. Pathogenesis and transmission of swine origin A(H3N2)v influenza viruses in ferrets. Proc. Natl. Acad. Sci. USA 2012, 109, 3944–3949. [Google Scholar] [CrossRef]
- Neumann, G.; Kawaoka, Y. Host range restriction and pathogenicity in the context of influenza pandemic. Emerg. Infect. Dis. 2006, 12, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Koel, B.F.; Burke, D.F.; Bestebroer, T.M.; van der Vliet, S.; Zondag, G.C.M.; Vervaet, G.; Skepner, E.; Lewis, N.S.; Spronken, M.I.J.; Russell, C.A.; et al. Substitutions Near the Receptor Binding Site Determine Major Antigenic Change during Influenza Virus Evolution. Science 2013, 342, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.F.; Smith, D.J. A Recommended Numbering Scheme for Influenza A HA Subtypes. PLoS ONE 2014, 9, e112302. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shen, H.; Liu, Q.; Bawa, B.; Qi, W.; Duff, M.; Lang, Y.; Lee, J.; Yu, H.; Bai, J.; et al. Pathogenicity and Transmissibility of Novel Reassortant H3N2 Influenza Viruses with 2009 Pandemic H1N1 Genes in Pigs. J. Virol. 2015, 89, 2831–2841. [Google Scholar] [CrossRef]
- Rajão, D.S.; Walia, R.R.; Campbell, B.; Gauger, P.C.; Janas-Martindale, A.; Killian, M.L.; Vincent, A.L. Reassortment between Swine H3N2 and 2009 Pandemic H1N1 in the United States Resulted in Influenza A Viruses with Diverse Genetic Constellations with Variable Virulence in Pigs. J. Virol. 2017, 91, e01763-16. [Google Scholar] [CrossRef]
- Genzow, M.; Goodell, C.; Kaiser, T.J.; Johnson, W.; Eichmeyer, M. Live attenuated influenza virus vaccine reduces virus shedding of newborn piglets in the presence of maternal antibody. Influ. Other Respir. Viruses 2017, 12, 353–359. [Google Scholar] [CrossRef]
- Kaiser, T.J.; Smiley, R.A.; Fergen, B.; Eichmeyer, M.; Genzow, M. Influenza A virus shedding reduction observed at 12 weeks post-vaccination when newborn pigs are administered live-attenuated influenza virus vaccine. Influ. Other Respir. Viruses 2019, 13, 274–278. [Google Scholar] [CrossRef]
- Sharma, A.; Zeller, M.; Li, G.; Harmon, K.M.; Zhang, J.; Hoang, H.; Anderson, T.K.; Vincent, A.L.; Gauger, P.C. Detection of live attenuated influenza vaccine virus and evidence of reassortment in the U.S. swine population. J. Vet. Diagn. Investig. 2020, 32, 301–311. [Google Scholar] [CrossRef]
- Chou, Y.-Y.; Albrecht, R.A.; Pica, N.; Lowen, A.C.; Richt, J.A.; García-Sastre, A.; Palese, P.; Hai, R. The M Segment of the 2009 New Pandemic H1N1 Influenza Virus Is Critical for Its High Transmission Efficiency in the Guinea Pig Model. J. Virol. 2011, 85, 11235–11241. [Google Scholar] [CrossRef]
- Epperson, S.; Jhung, M.; Richards, S.; Quinlisk, P.; Ball, L.; Moll, M.; Boulton, R.; Haddy, L.; Biggerstaff, M.; Brammer, L.; et al. Human Infections with Influenza A(H3N2) Variant Virus in the United States, 2011–2012. Clin. Infect. Dis. 2013, 57, S4–S11. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Kawashita, N.; Daidoji, T.; Ibrahim, M.S.; Elgendy, E.; Takagi, T.; Takahashi, K.; Suzuki, Y.; Ikuta, K.; Nakaya, T.; et al. Novel Polymerase Gene Mutations for Human Adaptation in Clinical Isolates of Avian H5N1 Influenza Viruses. PLoS Pathog. 2016, 12, e1005583. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Wills, S.; Bussey, K.A.; Takimoto, T. Identification of Influenza A Virus PB2 Residues Involved in Enhanced Polymerase Activity and Virus Growth in Mammalian Cells at Low Temperatures. J. Virol. 2015, 89, 8042–8049. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qiao, C.; Marjuki, H.; Bawa, B.; Ma, J.; Guillossou, S.; Webby, R.J.; Richt, J.A.; Ma, W. Combination of PB2 271A and SR polymorphism at positions 590/591 is critical for viral replication and virulence of swine influenza virus in cultured cells and in vivo. J. Virol. 2012, 86, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Pulit-Penaloza, J.A.; Belser, J.A.; Pappas, C.; Pearce, M.B.; Brock, N.; Zeng, H.; Creager, H.M.; Zanders, N.; Jang, Y.; et al. Pathogenesis and Transmission of Genetically Diverse Swine-Origin H3N2 Variant Influenza A Viruses from Multiple Lineages Isolated in the United States, 2011–2016. J. Virol. 2018, 92, e00665-18. [Google Scholar] [CrossRef]
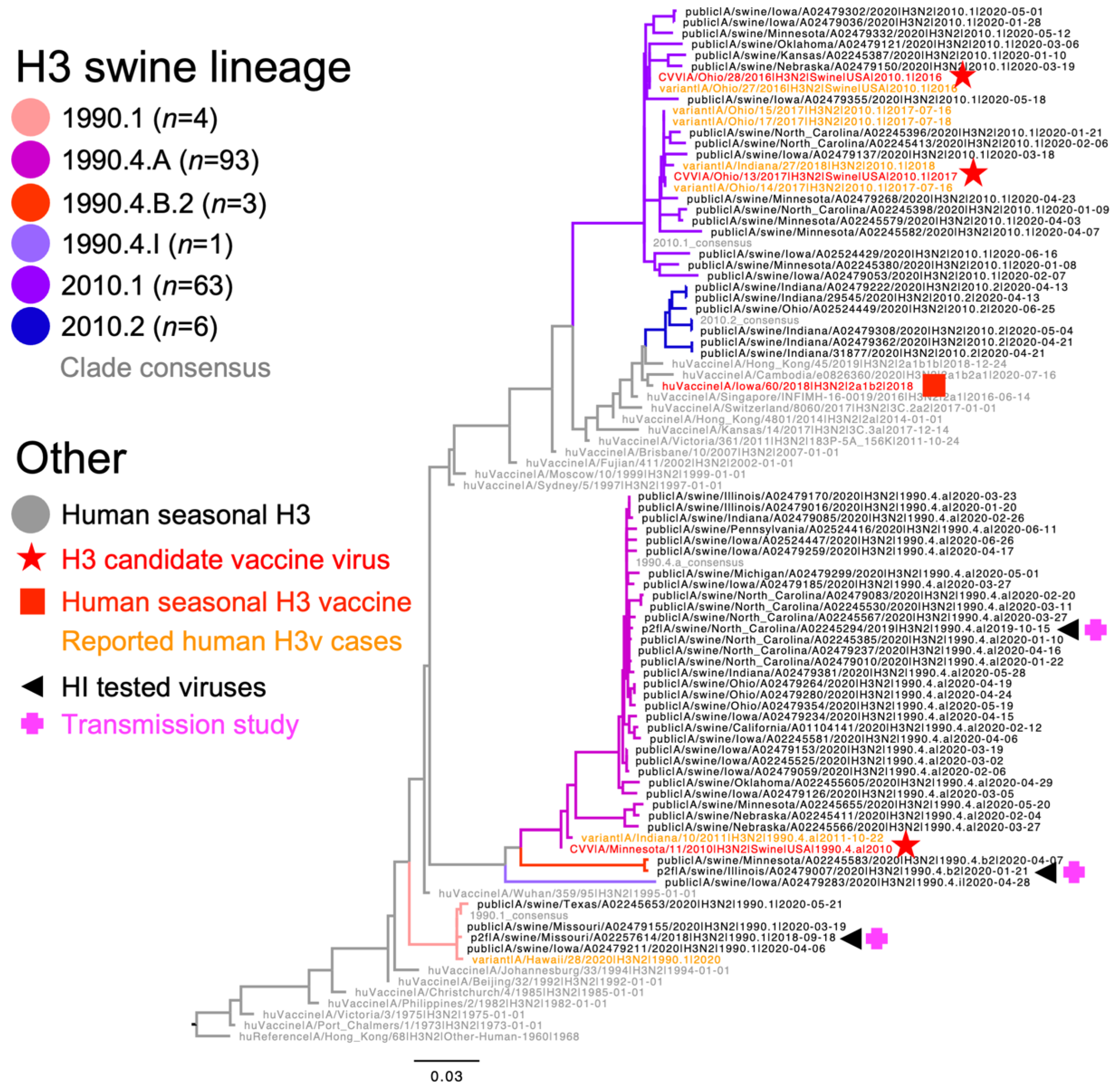

| 1990.1 Consensus | A/swine/Missouri/A02257614/2018 | 1990.4.A Consensus | A/Minnesota/11/2010 CVV | A/swine/North Carolina/A02245294/2019 | 1990.4.B.2. Consensus | A/swine/Illinois/A02479007/2020 | 2010.1 Consensus | A/Ohio/28/2016 | A/Indiana/27/2018 * | A/Iowa/60/2018 HuVac | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1990.1 consensus | 99.70 | 85.71 | 87.23 | 85.71 | 87.23 | 86.02 | 84.19 | 84.19 | 83.89 | 83.89 | |
| A/swine/Missouri/A02257614/2018 | 99.70 | 86.02 | 87.54 | 86.02 | 87.54 | 86.32 | 84.19 | 84.19 | 83.89 | 84.19 | |
| 1990.4.A consensus | 85.71 | 86.02 | 97.26 | 100 | 90.88 | 89.67 | 83.59 | 82.98 | 82.37 | 81.46 | |
| A/Minnesota/11/2010 CVV | 87.23 | 87.54 | 97.26 | 97.26 | 93.01 | 91.79 | 83.89 | 83.89 | 82.98 | 83.28 | |
| A/swine/North Carolina/A02245294/2019 | 85.71 | 86.02 | 100 | 97.26 | 90.88 | 89.67 | 83.59 | 82.98 | 82.37 | 81.46 | |
| 1990.4.B.2 consensus | 87.23 | 87.54 | 90.88 | 93.01 | 90.88 | 95.14 | 84.5 | 85.11 | 82.98 | 83.89 | |
| A/swine/Illinois/A02479007/2020 | 86.02 | 86.32 | 89.67 | 91.79 | 89.67 | 95.14 | 82.98 | 83.28 | 82.37 | 82.67 | |
| 2010.1 consensus | 84.19 | 84.19 | 83.59 | 83.89 | 83.59 | 84.5 | 82.98 | 99.39 | 98.18 | 89.36 | |
| A/Ohio/28/2016 | 84.19 | 84.19 | 82.98 | 83.89 | 82.98 | 85.11 | 83.28 | 99.39 | 97.57 | 89.67 | |
| A/Indiana/27/2018 * | 83.89 | 83.89 | 82.37 | 82.98 | 82.37 | 82.98 | 82.37 | 98.18 | 97.57 | 88.15 | |
| A/Iowa/60/2018 HuVac | 83.89 | 84.19 | 81.46 | 83.28 | 81.46 | 83.89 | 82.67 | 89.36 | 89.67 | 88.15 |
| Strain | Lineage | Antigenic Motif | A/Minnesota/11/2010 × 203 | IDCDC-RG55C A/Ohio/28/2016-like | A/Indiana/27/2018 * | A/Iowa/60/ 2018 |
|---|---|---|---|---|---|---|
| A/swine/Missouri/A02257614/2018 | 1990.1 | KHKEYS | 40 | <10 | 20 | <10 |
| A/Minnesota/11/2010 × 203 | 1990.4.A | NYNNYK | 1280 | <10 | 10 | <10 |
| A/swine/North Carolina/A02245294/2019 | 1990.4.A | NYHNYK | 80 | <10 | 20 | <10 |
| A/swine/Illinois/A02479007/2020 | 1990.4.B.2 | SYHNYK | 40 | <10 | 10 | 20 |
| IDCDC-RG55C A/Ohio/28/2016-like | 2010.1 | KTHNFK | <10 | 1280 | 80 | 20 |
| A/Indiana/27/2018 * | 2010.1 | NTRDFT | <10 | 10 | 640 | 10 |
| A/Iowa/60/2018 | HuVac | STHNYK | <10 | <10 | 10 | 320 |
| Viral Clade | Ferret # | Change in Bodyweight (%) from 0–12 dpc | dpc with Nasal Detection | Peak Titer log10 TCID50/mL | 12 dpc HI Titer | NP-ELISA S/N |
|---|---|---|---|---|---|---|
| 1990.1 | 53 | 7.9 | 3,5,7 | 3.5 | 320 | 0.171 |
| 55 | 12.1 | 3 | 0.5 | 10 | 1.003 | |
| 56 | 9.4 | 1 | 0.5 | 20 | 1.116 | |
| 64 | 3.5 | 1,3,5,7 | 4.4 | 640 | 0.188 | |
| 1990.4.A | 57 | 4.9 | 3,5,7 | 5.5 | 160 | 0.335 |
| 58 | 5.5 | 3,5,7,9 | 5.8 | 160 | 0.314 | |
| 59 | 10.5 | 3,5,7,9,11 | 5.2 | 160 | 0.410 | |
| 60 | 1.5 | 3,5,7 | 4.8 | 160 | 0.272 | |
| 1990.4.B.2 | 61 | 10.3 | 3,5,7,9 | 4.8 | 640 | 0.213 |
| 62 | 2.0 | 3,5,7 | 6.5 | 640 | 0.265 | |
| 63 | 3.9 | 3,5,7,9 | 5.5 | 1280 | 0.511 | |
| 54 | 11.8 | 1,3,5,7,9 | 6.5 | 320 | 0.555 | |
| No virus | 13 | 5.2 | none | 0 | <10 | 1.082 |
| 14 | 3.5 | none | 0 | <10 | 0.829 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, C.K.; Kimble, J.B.; Anderson, T.K.; Arendsee, Z.W.; Hufnagel, D.E.; Young, K.M.; Gauger, P.C.; Lewis, N.S.; Davis, C.T.; Thor, S.; et al. Swine-to-Ferret Transmission of Antigenically Drifted Contemporary Swine H3N2 Influenza A Virus Is an Indicator of Zoonotic Risk to Humans. Viruses 2023, 15, 331. https://doi.org/10.3390/v15020331
Souza CK, Kimble JB, Anderson TK, Arendsee ZW, Hufnagel DE, Young KM, Gauger PC, Lewis NS, Davis CT, Thor S, et al. Swine-to-Ferret Transmission of Antigenically Drifted Contemporary Swine H3N2 Influenza A Virus Is an Indicator of Zoonotic Risk to Humans. Viruses. 2023; 15(2):331. https://doi.org/10.3390/v15020331
Chicago/Turabian StyleSouza, Carine K., J. Brian Kimble, Tavis K. Anderson, Zebulun W. Arendsee, David E. Hufnagel, Katharine M. Young, Phillip C. Gauger, Nicola S. Lewis, C. Todd Davis, Sharmi Thor, and et al. 2023. "Swine-to-Ferret Transmission of Antigenically Drifted Contemporary Swine H3N2 Influenza A Virus Is an Indicator of Zoonotic Risk to Humans" Viruses 15, no. 2: 331. https://doi.org/10.3390/v15020331
APA StyleSouza, C. K., Kimble, J. B., Anderson, T. K., Arendsee, Z. W., Hufnagel, D. E., Young, K. M., Gauger, P. C., Lewis, N. S., Davis, C. T., Thor, S., & Vincent Baker, A. L. (2023). Swine-to-Ferret Transmission of Antigenically Drifted Contemporary Swine H3N2 Influenza A Virus Is an Indicator of Zoonotic Risk to Humans. Viruses, 15(2), 331. https://doi.org/10.3390/v15020331







