Elevated Plasma Protein Carbonyl Concentration Is Associated with More Abnormal White Matter in People with HIV
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R., Jr.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Lu, Q.; Farrell, M.; Lappin, J.M.; Shi, J.; Lu, L.; Bao, Y. Global prevalence and burden of HIV-associated neurocognitive disorder: A meta-analysis. Neurology 2020, 95, e2610–e2621. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.E.; Sullivan, E.V.; Chang, L.; Hammoud, D.A.; Wilson, T.W.; Ragin, A.B.; Meade, C.S.; Coughlin, J.; Ances, B.M. Imaging of Brain Structural and Functional Effects in People with Human Immunodeficiency Virus. J. Infect. Dis. 2023, 227, S16–S29. [Google Scholar] [CrossRef] [PubMed]
- Mina, Y.; Wu, T.; Hsieh, H.C.; Hammoud, D.A.; Shah, S.; Lau, C.Y.; Ham, L.; Snow, J.; Horne, E.; Ganesan, A.; et al. Association of White Matter Hyperintensities with HIV Status and Vascular Risk Factors. Neurology 2021, 96, e1823–e1834. [Google Scholar] [CrossRef] [PubMed]
- Seider, T.R.; Gongvatana, A.; Woods, A.J.; Chen, H.; Porges, E.C.; Cummings, T.; Correia, S.; Tashima, K.; Cohen, R.A. Age exacerbates HIV-associated white matter abnormalities. J. Neurovirol. 2016, 22, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H.; Caan, M.W.A.; Underwood, J.; De Francesco, D.; van Zoest, R.A.; Wit, F.; Mutsaerts, H.; Leech, R.; Geurtsen, G.J.; Portegies, P.; et al. No Evidence for Accelerated Aging-Related Brain Pathology in Treated Human Immunodeficiency Virus: Longitudinal Neuroimaging Results From the Comorbidity in Relation to AIDS (COBRA) Project. Clin. Infect. Dis. 2018, 66, 1899–1909. [Google Scholar] [CrossRef]
- Cardenas, V.; Meyerhoff, D.; Studholme, C.; Kornak, J.; Rothlind, J.; Lampiris, H.; Neuhaus, J.; Grant, R.; Chao, L.; Truran, D. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J. Neurovirol. 2009, 15, 324–333. [Google Scholar] [CrossRef]
- Jokinen, H.; Koikkalainen, J.; Laakso, H.M.; Melkas, S.; Nieminen, T.; Brander, A.; Korvenoja, A.; Rueckert, D.; Barkhof, F.; Scheltens, P.; et al. Global Burden of Small Vessel Disease-Related Brain Changes on MRI Predicts Cognitive and Functional Decline. Stroke 2020, 51, 170–178. [Google Scholar] [CrossRef]
- West, N.A.; Windham, B.G.; Knopman, D.S.; Shibata, D.K.; Coker, L.H.; Mosley, T.H., Jr. Neuroimaging findings in midlife and risk of late-life dementia over 20 years of follow-up. Neurology 2019, 92, e917–e923. [Google Scholar] [CrossRef]
- Herrmann, L.L.; Le Masurier, M.; Ebmeier, K.P. White matter hyperintensities in late life depression: A systematic review. J. Neurol. Neurosurg. Psychiatry 2008, 79, 619–624. [Google Scholar] [CrossRef]
- Wang, L.; Leonards, C.O.; Sterzer, P.; Ebinger, M. White matter lesions and depression: A systematic review and meta-analysis. J. Psychiatr. Res. 2014, 56, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Fennema-Notestine, C.; McEvoy, L.K.; Notestine, R.; Panizzon, M.S.; Yau, W.W.; Franz, C.E.; Lyons, M.J.; Eyler, L.T.; Neale, M.C.; Xian, H.; et al. White matter disease in midlife is heritable, related to hypertension, and shares some genetic influence with systolic blood pressure. Neuroimage Clin. 2016, 12, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Sanderson-Cimino, M.; Panizzon, M.S.; Elman, J.A.; Tu, X.; Gustavson, D.E.; Puckett, O.; Cross, K.; Notestine, R.; Hatton, S.N.; Eyler, L.T.; et al. Periventricular and deep abnormal white matter differ in associations with cognitive performance at midlife. Neuropsychology 2021, 35, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Busovaca, E.; Foley, J.M.; Allen, I.E.; Schwarz, C.G.; Jahanshad, N.; Nir, T.M.; Esmaeili-Firidouni, P.; Milanini, B.; Rosen, H.; et al. White matter hyperintensities correlate to cognition and fiber tract integrity in older adults with HIV. J. Neurovirol. 2017, 23, 422–429. [Google Scholar] [CrossRef]
- Hoare, J.; Fouche, J.P.; Spottiswoode, B.; Joska, J.A.; Schoeman, R.; Stein, D.J.; Carey, P.D. White matter correlates of apathy in HIV-positive subjects: A diffusion tensor imaging study. J. Neuropsychiatry Clin. Neurosci. 2010, 22, 313–320. [Google Scholar] [CrossRef]
- Kamat, R.; Brown, G.G.; Bolden, K.; Fennema-Notestein, C.; Archibald, S.; Marcotte, T.D.; Letendre, S.L.; Ellis, R.J.; Woods, S.P.; Grant, I.; et al. Apathy is associated with white matter abnormalities in anterior, medial brain regions in persons with HIV infection. J. Clin. Exp. Neuropsychol. 2014, 36, 854–866. [Google Scholar] [CrossRef][Green Version]
- Underwood, J.; Cole, J.H.; Caan, M.; De Francesco, D.; Leech, R.; van Zoest, R.A.; Su, T.; Geurtsen, G.J.; Schmand, B.A.; Portegies, P.; et al. Gray and White Matter Abnormalities in Treated Human Immunodeficiency Virus Disease and Their Relationship to Cognitive Function. Clin. Infect. Dis. 2017, 65, 422–432. [Google Scholar] [CrossRef]
- Saloner, R.; Heaton, R.K.; Campbell, L.M.; Chen, A.; Franklin, D., Jr.; Ellis, R.J.; Collier, A.C.; Marra, C.; Clifford, D.B.; Gelman, B.; et al. Effects of comorbidity burden and age on brain integrity in HIV. AIDS 2019, 33, 1175–1185. [Google Scholar] [CrossRef]
- Alakkas, A.; Ellis, R.J.; Watson, C.W.; Umlauf, A.; Heaton, R.K.; Letendre, S.; Collier, A.; Marra, C.; Clifford, D.B.; Gelman, B.; et al. White matter damage, neuroinflammation, and neuronal integrity in HAND. J. Neurovirol. 2019, 25, 32–41. [Google Scholar] [CrossRef]
- Moulignier, A.; Savatovsky, J.; Assoumou, L.; Lescure, F.X.; Lamirel, C.; Godin, O.; Valin, N.; Tubiana, R.; Canestri, A.; Roux, P.; et al. Silent Cerebral Small-Vessel Disease Is Twice as Prevalent in Middle-Aged Individuals with Well-Controlled, Combination Antiretroviral Therapy-Treated Human Immunodeficiency Virus (HIV) than in HIV-Uninfected Individuals. Clin. Infect. Dis. 2018, 66, 1762–1769. [Google Scholar] [CrossRef]
- van Zoest, R.A.; Underwood, J.; De Francesco, D.; Sabin, C.A.; Cole, J.H.; Wit, F.W.; Caan, M.W.A.; Kootstra, N.A.; Fuchs, D.; Zetterberg, H.; et al. Structural Brain Abnormalities in Successfully Treated HIV Infection: Associations with Disease and Cerebrospinal Fluid Biomarkers. J. Infect. Dis. 2017, 217, 69–81. [Google Scholar] [CrossRef] [PubMed]
- McMurtray, A.; Nakamoto, B.; Shikuma, C.; Valcour, V. Small-vessel vascular disease in human immunodeficiency virus infection: The Hawaii aging with HIV cohort study. Cerebrovasc. Dis. 2007, 24, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Sanford, R.; Strain, J.; Dadar, M.; Maranzano, J.; Bonnet, A.; Mayo, N.E.; Scott, S.C.; Fellows, L.K.; Ances, B.M.; Collins, D.L. HIV infection and cerebral small vessel disease are independently associated with brain atrophy and cognitive impairment. AIDS 2019, 33, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Caan, M.W.; Wit, F.W.; Schouten, J.; Geurtsen, G.J.; Cole, J.H.; Sharp, D.J.; Vos, F.M.; Prins, M.; Portegies, P.; et al. White matter structure alterations in HIV-1-infected men with sustained suppression of viraemia on treatment. AIDS 2016, 30, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Fatukasi, O.; Yang, S.; Alger, J.; Barker, P.B.; Hetherington, H.; Kim, T.; Levine, A.; Martin, E.; Munro, C.A.; et al. HIV disease and diabetes interact to affect brain white matter hyperintensities and cognition. AIDS 2018, 32, 1803–1810. [Google Scholar] [CrossRef]
- Jernigan, T.L.; Archibald, S.L.; Fennema-Notestine, C.; Taylor, M.J.; Theilmann, R.J.; Julaton, M.D.; Notestine, R.J.; Wolfson, T.; Letendre, S.L.; Ellis, R.J.; et al. Clinical factors related to brain structure in HIV: The CHARTER study. J. Neurovirol. 2011, 17, 248–257. [Google Scholar] [CrossRef]
- Fennema-Notestine, C.; Ellis, R.J.; Archibald, S.L.; Jernigan, T.L.; Letendre, S.L.; Notestine, R.J.; Taylor, M.J.; Theilmann, R.J.; Julaton, M.D.; Croteau, D.J.; et al. Increases in brain white matter abnormalities and subcortical gray matter are linked to CD4 recovery in HIV infection. J. Neurovirol. 2013, 19, 393–401. [Google Scholar] [CrossRef][Green Version]
- Kolgiri, V.; Patil, V.W. Protein carbonyl content: A novel biomarker for aging in HIV/AIDS patients. Braz. J. Infect. Dis. 2017, 21, 35–41. [Google Scholar] [CrossRef]
- Bandaru, V.V.; McArthur, J.C.; Sacktor, N.; Cutler, R.G.; Knapp, E.L.; Mattson, M.P.; Haughey, N.J. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology 2007, 68, 1481–1487. [Google Scholar] [CrossRef]
- Buckley, S.; Byrnes, S.; Cochrane, C.; Roche, M.; Estes, J.D.; Selemidis, S.; Angelovich, T.A.; Churchill, M.J. The role of oxidative stress in HIV-associated neurocognitive disorders. Brain Behav. Immun. Health 2021, 13, 100235. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef]
- Vassimon, H.S.; Deminice, R.; Machado, A.A.; Monteiro, J.P.; Jordao, A.A. The association of lipodystrophy and oxidative stress biomarkers in HIV-infected men. Curr. HIV Res. 2010, 8, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Uzasci, L.; Nath, A.; Cotter, R. Oxidative stress and the HIV-infected brain proteome. J. Neuroimmune Pharmacol. 2013, 8, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Schank, M.; Zhao, J.; Wang, L.; Nguyen, L.N.T.; Zhang, Y.; Wu, X.Y.; Zhang, J.; Jiang, Y.; Ning, S.; El Gazzar, M.; et al. ROS-Induced Mitochondrial Dysfunction in CD4 T Cells from ART-Controlled People Living with HIV. Viruses 2023, 15, 1061. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.K.; Roth, L.M.; Grinspan, J.B.; Jordan-Sciutto, K.L. White matter loss and oligodendrocyte dysfunction in HIV: A consequence of the infection, the antiretroviral therapy or both? Brain Res. 2019, 1724, 146397. [Google Scholar] [CrossRef] [PubMed]
- Aukrust, P.; Svardal, A.M.; Muller, F.; Lunden, B.; Nordoy, I.; Froland, S.S. Markedly disturbed glutathione redox status in CD45RA+CD4+ lymphocytes in human immunodeficiency virus type 1 infection is associated with selective depletion of this lymphocyte subset. Blood 1996, 88, 2626–2633. [Google Scholar] [CrossRef]
- Guerville, F.; Vialemaringe, M.; Cognet, C.; Duffau, P.; Lazaro, E.; Cazanave, C.; Bonnet, F.; Leleux, O.; Rossignol, R.; Pinson, B.; et al. Mechanisms of systemic low-grade inflammation in HIV patients on long-term suppressive antiretroviral therapy: The inflammasome hypothesis. AIDS 2023, 37, 1035–1046. [Google Scholar] [CrossRef]
- Ellis, R.J.; Moore, D.J.; Sundermann, E.E.; Heaton, R.K.; Mehta, S.; Hulgan, T.; Samuels, D.; Fields, J.A.; Letendre, S.L. Nucleic acid oxidation is associated with biomarkers of neurodegeneration in CSF in people with HIV. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e902. [Google Scholar] [CrossRef]
- Guo, L.; Xing, Y.; Pan, R.; Jiang, M.; Gong, Z.; Lin, L.; Wang, J.; Xiong, G.; Dong, J. Curcumin protects microglia and primary rat cortical neurons against HIV-1 gp120-mediated inflammation and apoptosis. PLoS ONE 2013, 8, e70565. [Google Scholar] [CrossRef]
- Shah, A.; Kumar, S.; Simon, S.D.; Singh, D.P.; Kumar, A. HIV gp120- and methamphetamine-mediated oxidative stress induces astrocyte apoptosis via cytochrome P450 2E1. Cell Death Dis. 2013, 4, e850. [Google Scholar] [CrossRef]
- Haughey, N.J.; Cutler, R.G.; Tamara, A.; McArthur, J.C.; Vargas, D.L.; Pardo, C.A.; Turchan, J.; Nath, A.; Mattson, M.P. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann. Neurol. 2004, 55, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Jordan-Sciutto, K.L. Effects of Antiretroviral Therapy in the Central Nervous System: Beyond Viral Suppression. J. Neuroimmune Pharmacol. 2021, 16, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Cabiscol, E.; Tamarit, J.; Ros, J. Protein carbonylation: Proteomics, specificity and relevance to aging. Mass. Spectrom. Rev. 2014, 33, 21–48. [Google Scholar] [CrossRef] [PubMed]
- Kehm, R.; Baldensperger, T.; Raupbach, J.; Hohn, A. Protein oxidation—Formation mechanisms, detection and relevance as biomarkers in human diseases. Redox Biol. 2021, 42, 101901. [Google Scholar] [CrossRef]
- Dasgupta, A.; Zheng, J.; Bizzozero, O.A. Protein carbonylation and aggregation precede neuronal apoptosis induced by partial glutathione depletion. ASN Neuro. 2012, 4, AN20110064. [Google Scholar] [CrossRef]
- Turchan, J.; Pocernich, C.B.; Gairola, C.; Chauhan, A.; Schifitto, G.; Butterfield, D.A.; Buch, S.; Narayan, O.; Sinai, A.; Geiger, J.; et al. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology 2003, 60, 307–314. [Google Scholar] [CrossRef]
- Perrotte, M.; Le Page, A.; Fournet, M.; Le Sayec, M.; Rassart, E.; Fulop, T.; Ramassamy, C. Blood-based redox-signature and their association to the cognitive scores in MCI and Alzheimer’s disease patients. Free Radic. Biol. Med. 2019, 130, 499–511. [Google Scholar] [CrossRef]
- Sharma, A.; Weber, D.; Raupbach, J.; Dakal, T.C.; Fliessbach, K.; Ramirez, A.; Grune, T.; Wullner, U. Advanced glycation end products and protein carbonyl levels in plasma reveal sex-specific differences in Parkinson’s and Alzheimer’s disease. Redox Biol. 2020, 34, 101546. [Google Scholar] [CrossRef]
- Son, S.; Arai, M.; Miyata, J.; Toriumi, K.; Mizuta, H.; Hayashi, T.; Aso, T.; Itokawa, M.; Murai, T. Enhanced carbonyl stress and disrupted white matter integrity in schizophrenia. Schizophr. Res. 2020, 223, 242–248. [Google Scholar] [CrossRef]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxid. Med. Cell Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef]
- Morris, G.; Walder, K.R.; Berk, M.; Marx, W.; Walker, A.J.; Maes, M.; Puri, B.K. The interplay between oxidative stress and bioenergetic failure in neuropsychiatric illnesses: Can we explain it and can we treat it? Mol. Biol. Rep. 2020, 47, 5587–5620. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.A.; Castro-Faria-Neto, H.C. Systemic Response to Infection Induces Long-Term Cognitive Decline: Neuroinflammation and Oxidative Stress as Therapeutical Targets. Front. Neurosci. 2021, 15, 742158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bailey, J.T.; Xu, E.; Singh, K.; Lavaert, M.; Link, V.M.; D’Souza, S.; Hafiz, A.; Cao, J.; Cao, G.; et al. Mucosal-associated invariant T cells restrict reactive oxidative damage and preserve meningeal barrier integrity and cognitive function. Nat. Immunol. 2022, 23, 1714–1725. [Google Scholar] [CrossRef]
- Mullen, L.; Mengozzi, M.; Hanschmann, E.M.; Alberts, B.; Ghezzi, P. How the redox state regulates immunity. Free Radic. Biol. Med. 2020, 157, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Ellis, R.J.; Tang, B.; Marra, C.M.; Rubin, L.H.; Clifford, D.B.; McCutchan, J.A.; Gelman, B.B.; Morgello, S.; Franklin, D.R.; et al. Twelve-year neurocognitive decline in HIV is associated with comorbidities, not age: A CHARTER study. Brain 2023, 146, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available online: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv (accessed on 13 July 2023).
- Torres, R.A.; Lewis, W. Aging and HIV/AIDS: Pathogenetic role of therapeutic side effects. Lab. Investig. 2014, 94, 120–128. [Google Scholar] [CrossRef]
- Sattler, F.R.; He, J.; Letendre, S.; Wilson, C.; Sanders, C.; Heaton, R.; Ellis, R.; Franklin, D.; Aldrovandi, G.; Marra, C.M.; et al. Abdominal obesity contributes to neurocognitive impairment in HIV-infected patients with increased inflammation and immune activation. J. Acquir. Immune Defic. Syndr. 2015, 68, 281–288. [Google Scholar] [CrossRef]
- Inami, N.; Nomura, S.; Inami, O.; Kimura, Y.; Urase, F.; Maeda, Y.; Iwasaka, T. Significance of soluble CD40 ligand, adiponectin and reactive oxygen metabolites in aging. Arch. Gerontol. Geriatr. 2009, 49, 13–16. [Google Scholar] [CrossRef]
- Balducci, C.; Forloni, G. Novel targets in Alzheimer’s disease: A special focus on microglia. Pharmacol. Res. 2018, 130, 402–413. [Google Scholar] [CrossRef]
- Kallianpur, A.R.; Levine, A.J. Host Genetic Factors Predisposing to HIV-Associated Neurocognitive Disorder. Curr. HIV/AIDS Rep. 2014, 11, 336–352. [Google Scholar] [CrossRef]
- Armah, K.A.; McGinnis, K.; Baker, J.; Gibert, C.; Butt, A.A.; Bryant, K.J.; Goetz, M.; Tracy, R.; Oursler, K.K.; Rimland, D.; et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin. Infect. Dis. 2012, 55, 126–136. [Google Scholar] [CrossRef]
- Peterson, J.; Gisslen, M.; Zetterberg, H.; Fuchs, D.; Shacklett, B.L.; Hagberg, L.; Yiannoutsos, C.T.; Spudich, S.S.; Price, R.W. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: Hierarchy of injury and detection. PLoS ONE 2014, 9, e116081. [Google Scholar] [CrossRef]
- Anderson, A.M.; Jang, J.H.; Easley, K.A.; Fuchs, D.; Gisslen, M.; Zetterberg, H.; Blennow, K.; Ellis, R.J.; Franklin, D.; Heaton, R.K.; et al. Cognitive and Neuronal Link with Inflammation: A Longitudinal Study in People with and without HIV Infection. J. Acquir. Immune Defic. Syndr. 2020, 85, 617–625. [Google Scholar] [CrossRef]
- Chang, K.; Premeaux, T.A.; Cobigo, Y.; Milanini, B.; Hellmuth, J.; Rubin, L.H.; Javandel, S.; Allen, I.; Ndhlovu, L.C.; Paul, R.; et al. Plasma inflammatory biomarkers link to diffusion tensor imaging metrics in virally suppressed HIV-infected individuals. AIDS 2020, 34, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Gisslen, M.; Krut, J.; Andreasson, U.; Blennow, K.; Cinque, P.; Brew, B.J.; Spudich, S.; Hagberg, L.; Rosengren, L.; Price, R.W.; et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Fennema-Notestine, C.; Gamst, A.C.; Quinn, B.T.; Pacheco, J.; Jernigan, T.L.; Thal, L.; Buckner, R.; Killiany, R.; Blacker, D.; Dale, A.M.; et al. Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. Neuroinformatics 2007, 5, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Maes, F.; Collignon, A.; Vandermeulen, D.; Marchal, G.; Suetens, P. Multimodality image registration by maximization of mutual information. IEEE Trans. Med. Imaging 1997, 16, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Sled, J.G.; Zijdenbos, A.P.; Evans, A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging 1998, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.W. Parametric Statistical Modeling by Minimum Integrated Square Error. Technometrics 2001, 43, 274–285. [Google Scholar] [CrossRef]
- Yoo, T.S.; Ackerman, M.J.; Lorensen, W.E.; Schroeder, W.; Chalana, V.; Aylward, S.; Metaxas, D.; Whitaker, R. Engineering and algorithm design for an image processing Api: A technical report on ITK—the Insight Toolkit. Stud. Health Technol. Inform. 2002, 85, 586–592. [Google Scholar]
- Frohnert, B.I.; Sinaiko, A.R.; Serrot, F.J.; Foncea, R.E.; Moran, A.; Ikramuddin, S.; Choudry, U.; Bernlohr, D.A. Increased adipose protein carbonylation in human obesity. Obesity (Silver Spring) 2011, 19, 1735–1741. [Google Scholar] [CrossRef]
- Archibald, S.L.; McCutchan, J.A.; Sanders, C.; Wolfson, T.; Jernigan, T.L.; Ellis, R.J.; Ances, B.M.; Collier, A.C.; McArthur, J.C.; Morgello, S.; et al. Brain morphometric correlates of metabolic variables in HIV: The CHARTER study. J. Neurovirol. 2014, 20, 603–611. [Google Scholar] [CrossRef][Green Version]
- Iadecola, C.; Parikh, N.S. Framingham General Cardiovascular Risk Score and Cognitive Impairment: The Power of Foresight. J. Am. Coll. Cardiol. 2020, 75, 2535–2537. [Google Scholar] [CrossRef]
- Colombo, G.; Garavaglia, M.L.; Astori, E.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Protein carbonylation in human bronchial epithelial cells exposed to cigarette smoke extract. Cell Biol. Toxicol. 2019, 35, 345–360. [Google Scholar] [CrossRef]
- Nishimoto-Kusunose, S.; Sawa, M.; Inaba, Y.; Ushiyama, A.; Ishii, K.; Hattori, K.; Ogasawara, Y. Exposure to aerosol extract from heated tobacco products causes a drastic decrease of glutathione and protein carbonylation in human lung epithelial cells. Biochem. Biophys. Res. Commun. 2022, 589, 92–99. [Google Scholar] [CrossRef]
- Gorisse, L.; Pietrement, C.; Vuiblet, V.; Schmelzer, C.E.; Kohler, M.; Duca, L.; Debelle, L.; Fornes, P.; Jaisson, S.; Gillery, P. Protein carbamylation is a hallmark of aging. Proc. Natl. Acad. Sci. USA 2016, 113, 1191–1196. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Tang, W.H.; Hazen, S.L. Protein carbamylation and cardiovascular disease. Kidney Int. 2015, 88, 474–478. [Google Scholar] [CrossRef]
- Bizzozero, O.A. Protein Carbonylation in Neurodegenerative and Demyelinating CNS Diseases. In Handbook of Neurochemistry and Molecular Neurobiology: Brain and Spinal Cord Trauma; Lajtha, A., Banik, N., Ray, S.K., Eds.; Springer: Boston, MA, USA, 2009; pp. 543–562. [Google Scholar]
- Bizzozero, O.A.; DeJesus, G.; Callahan, K.; Pastuszyn, A. Elevated protein carbonylation in the brain white matter and gray matter of patients with multiple sclerosis. J. Neurosci. Res. 2005, 81, 687–695. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative Stress, Amyloid-beta Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimers Dis. 2018, 62, 1345–1367. [Google Scholar] [CrossRef]
- Franchina, D.G.; Dostert, C.; Brenner, D. Reactive Oxygen Species: Involvement in T Cell Signaling and Metabolism. Trends Immunol. 2018, 39, 489–502. [Google Scholar] [CrossRef]
- Eden, A.; Marcotte, T.D.; Heaton, R.K.; Nilsson, S.; Zetterberg, H.; Fuchs, D.; Franklin, D.; Price, R.W.; Grant, I.; Letendre, S.L.; et al. Increased Intrathecal Immune Activation in Virally Suppressed HIV-1 Infected Patients with Neurocognitive Impairment. PLoS ONE 2016, 11, e0157160. [Google Scholar] [CrossRef]
- Hagberg, L.; Eden, A.; Zetterberg, H.; Price, R.W.; Gisslen, M. Blood biomarkers for HIV infection with focus on neurologic complications-A review. Acta Neurol. Scand. 2022, 146, 56–60. [Google Scholar] [CrossRef]
- Hagberg, L.; Cinque, P.; Gisslen, M.; Brew, B.J.; Spudich, S.; Bestetti, A.; Price, R.W.; Fuchs, D. Cerebrospinal fluid neopterin: An informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res. Ther. 2010, 7, 15. [Google Scholar] [CrossRef]
- Fields, J.A.; Spencer, B.; Swinton, M.; Qvale, E.M.; Marquine, M.J.; Alexeeva, A.; Gough, S.; Soontornniyomkij, B.; Valera, E.; Masliah, E.; et al. Alterations in brain TREM2 and Amyloid-beta levels are associated with neurocognitive impairment in HIV-infected persons on antiretroviral therapy. J. Neurochem. 2018, 147, 784–802. [Google Scholar] [CrossRef]
- Giudici, K.V.; de Souto Barreto, P.; Guyonnet, S.; Li, Y.; Bateman, R.J.; Vellas, B.; Group, M.D. Assessment of Plasma Amyloid-beta42/40 and Cognitive Decline among Community-Dwelling Older Adults. JAMA Netw. Open 2020, 3, e2028634. [Google Scholar] [CrossRef]
- Jazvinscak Jembrek, M.; Hof, P.R.; Simic, G. Ceramides in Alzheimer’s Disease: Key Mediators of Neuronal Apoptosis Induced by Oxidative Stress and Abeta Accumulation. Oxid. Med. Cell Longev. 2015, 2015, 346783. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.C.; Zheng, H.; Huang, T.Y. Amyloid, tau, pathogen infection and antimicrobial protection in Alzheimer’s disease -conformist, nonconformist, and realistic prospects for AD pathogenesis. Transl. Neurodegener. 2018, 7, 34. [Google Scholar] [CrossRef]
- Mackiewicz, M.M.; Overk, C.; Achim, C.L.; Masliah, E. Pathogenesis of age-related HIV neurodegeneration. J. Neurovirol. 2019, 25, 622–633. [Google Scholar] [CrossRef]
- Solanky, D.; Fields, J.A.; Iudicello, J.E.; Ellis, R.J.; Franklin, D.; Clifford, D.B.; Gelman, B.B.; Marra, C.M.; Morgello, S.; Rubin, L.H.; et al. Higher buccal mitochondrial DNA and mitochondrial common deletion number are associated with markers of neurodegeneration and inflammation in cerebrospinal fluid. J. Neurovirol. 2022, 28, 281–290. [Google Scholar] [CrossRef]
- Sanchez Macarro, M.; Avila-Gandia, V.; Perez-Pinero, S.; Canovas, F.; Garcia-Munoz, A.M.; Abellan-Ruiz, M.S.; Victoria-Montesinos, D.; Luque-Rubia, A.J.; Climent, E.; Genoves, S.; et al. Antioxidant Effect of a Probiotic Product on a Model of Oxidative Stress Induced by High-Intensity and Duration Physical Exercise. Antioxidants 2021, 10, 323. [Google Scholar] [CrossRef]
- Prabhulkar, S.; Li, C.Z. Assessment of oxidative DNA damage and repair at single cellular level via real-time monitoring of 8-OHdG biomarker. Biosens. Bioelectron. 2010, 26, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, M. Protein carbonylation: Molecular mechanisms, biological implications, and analytical approaches. Free Radic. Res. 2021, 55, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Eden, A.; Rydberg, F.; Yilmaz, A.; Hagberg, L.; Gostner, J.; Nilsson, S.; Fuchs, D.; Gisslen, M. Residual Central Nervous System Immune Activation Is Not Prevented by Antiretroviral Therapy Initiated During Early Chronic HIV Infection. Open Forum Infect. Dis. 2023, 10, ofad064. [Google Scholar] [CrossRef] [PubMed]
- Blackstone, K.; Moore, D.J.; Franklin, D.R.; Clifford, D.B.; Collier, A.C.; Marra, C.M.; Gelman, B.B.; McArthur, J.C.; Morgello, S.; Simpson, D.M.; et al. Defining neurocognitive impairment in HIV: Deficit scores versus clinical ratings. Clin. Neuropsychol. 2012, 26, 894–908. [Google Scholar] [CrossRef]
- Carey, C.L.; Woods, S.P.; Gonzalez, R.; Conover, E.; Marcotte, T.D.; Grant, I.; Heaton, R.K.; Group, H. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J. Clin. Exp. Neuropsychol. 2004, 26, 307–319. [Google Scholar] [CrossRef]
- Casaletto, K.B.; Nichols, E.; Aslanyan, V.; Simone, S.M.; Rabin, J.S.; La Joie, R.; Brickman, A.M.; Dams-O’Connor, K.; Palta, P.; Kumar, R.G.; et al. Sex-specific effects of microglial activation on Alzheimer’s disease proteinopathy in older adults. Brain 2022, 145, 3536–3545. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Saredy, J.; Yuan, Z.; Yang, X.; Wang, H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020, 37, 101759. [Google Scholar] [CrossRef]
| Demographic Characteristics | |
|---|---|
| Age (years) | 55 [52, 63] |
| Sex (male) | 40 (88.9%) |
| Race | |
| White | 29 (64.4%) |
| Black | 16 (35.6%) |
| Ethnicity (Hispanic) | 6 (13.3%) |
| Education (years) | 13 [12, 15.5] |
| HIV Disease Characteristics | |
| CD4+ T Cell Count (cells/μL) | 527 [387, 810] |
| CD4+ T Cell Nadir (cells/μL) | 104 [19.5, 190] |
| AIDS Diagnosis | 40 (88.9%) |
| Estimated Duration of HIV (years) | 24.2 [17.0, 29.5] |
| Duration of Current Regimen (months) | 25.3 [8.2, 72.0] |
| Duration of All ART (months) | 188 [150, 239] |
| ART Regimen Class | |
| Integrase Inhibitor Use | 27 (60.0%) |
| Protease Inhibitor Use | 13 (28.9%) |
| Non-nucleoside Reverse Transcriptase Inhibitor Use | 11 (24.4%) |
| Comorbid Conditions | |
| Body Mass Index (kg/m2) | 25.7 [23.3, 29.8] |
| HCV Seropositive | 24 (53.3%) |
| Diabetes Mellitus | 8 (17.8%) |
| Hypertension | 25 (55.6%) |
| Hyperlipidemia | 19 (42.2%) |
| Framingham 10-year Risk Score | 15.7% [8.9, 24.0] |
| Chronic Pulmonary Disease | 8 (17.8%) |
| Current Substance Use Disorder | 2 (4.6%) |
| Lifetime Substance Use Disorder | 37 (82.2%) |
| Current Tobacco Use | 7 (15.6%) |
| Lifetime Tobacco Use | 35 (77.8%) |
| Current Major Depression | 2 (4.6%) |
| Lifetime Major Depression | 31 (68.9%) |
| Neurocognitive Impairment (global deficit score ≥ 0.5) | 17 (37.8%) |
| Scanner Only | Multivariable | |||||
|---|---|---|---|---|---|---|
| Std β | p Value | Std β | p Value | FDR p Value | Risk Direction | |
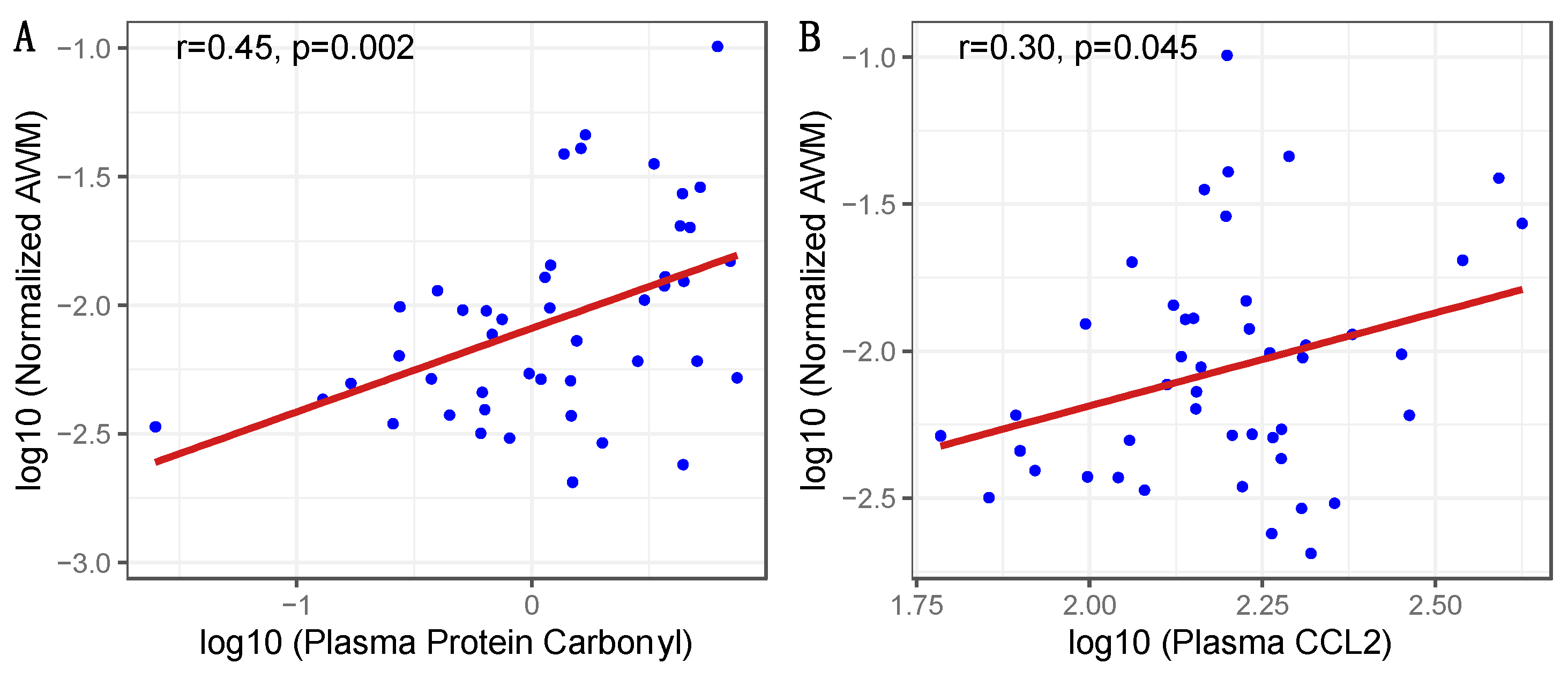
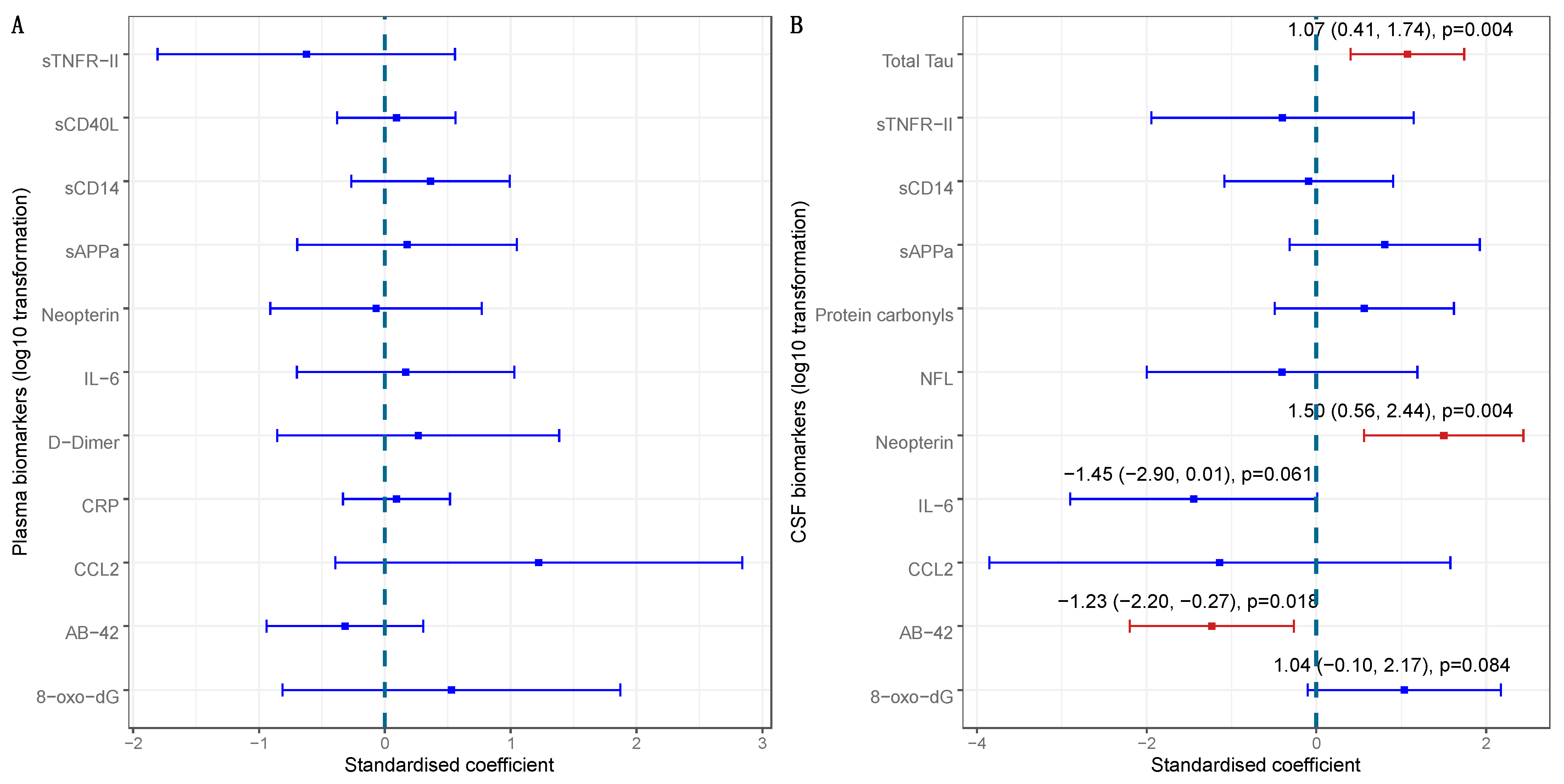
| Plasma Protein Carbonyls a | 0.423 | 0.002 | 0.340 | 0.006 | 0.018 | Higher |
| Lifetime Tobacco Use | 0.378 | 0.016 | 0.276 | 0.028 | 0.042 | Present |
| Race | 0.404 | 0.035 | Black | |||
| HCV Serostatus | 0.286 | 0.074 | Positive | |||
| Plasma D-Dimer a | 0.252 | 0.097 | Higher | |||
| Current Tobacco Smoking | 0.226 | 0.116 | Present | |||
| Plasma C-Reactive Protein a | 0.225 | 0.123 | 0.239 | 0.049 | 0.059 | Higher |
| Age | 0.213 | 0.176 | 0.312 | 0.023 | 0.042 | Older |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riggs, P.K.; Anderson, A.M.; Tang, B.; Rubin, L.H.; Morgello, S.; Marra, C.M.; Gelman, B.B.; Clifford, D.B.; Franklin, D.; Heaton, R.K.; et al. Elevated Plasma Protein Carbonyl Concentration Is Associated with More Abnormal White Matter in People with HIV. Viruses 2023, 15, 2410. https://doi.org/10.3390/v15122410
Riggs PK, Anderson AM, Tang B, Rubin LH, Morgello S, Marra CM, Gelman BB, Clifford DB, Franklin D, Heaton RK, et al. Elevated Plasma Protein Carbonyl Concentration Is Associated with More Abnormal White Matter in People with HIV. Viruses. 2023; 15(12):2410. https://doi.org/10.3390/v15122410
Chicago/Turabian StyleRiggs, Patricia K., Albert M. Anderson, Bin Tang, Leah H. Rubin, Susan Morgello, Christina M. Marra, Benjamin B. Gelman, David B. Clifford, Donald Franklin, Robert K. Heaton, and et al. 2023. "Elevated Plasma Protein Carbonyl Concentration Is Associated with More Abnormal White Matter in People with HIV" Viruses 15, no. 12: 2410. https://doi.org/10.3390/v15122410
APA StyleRiggs, P. K., Anderson, A. M., Tang, B., Rubin, L. H., Morgello, S., Marra, C. M., Gelman, B. B., Clifford, D. B., Franklin, D., Heaton, R. K., Ellis, R. J., Fennema-Notestine, C., & Letendre, S. L. (2023). Elevated Plasma Protein Carbonyl Concentration Is Associated with More Abnormal White Matter in People with HIV. Viruses, 15(12), 2410. https://doi.org/10.3390/v15122410




