Neutralization Determinants on Poxviruses
Abstract
1. Introduction
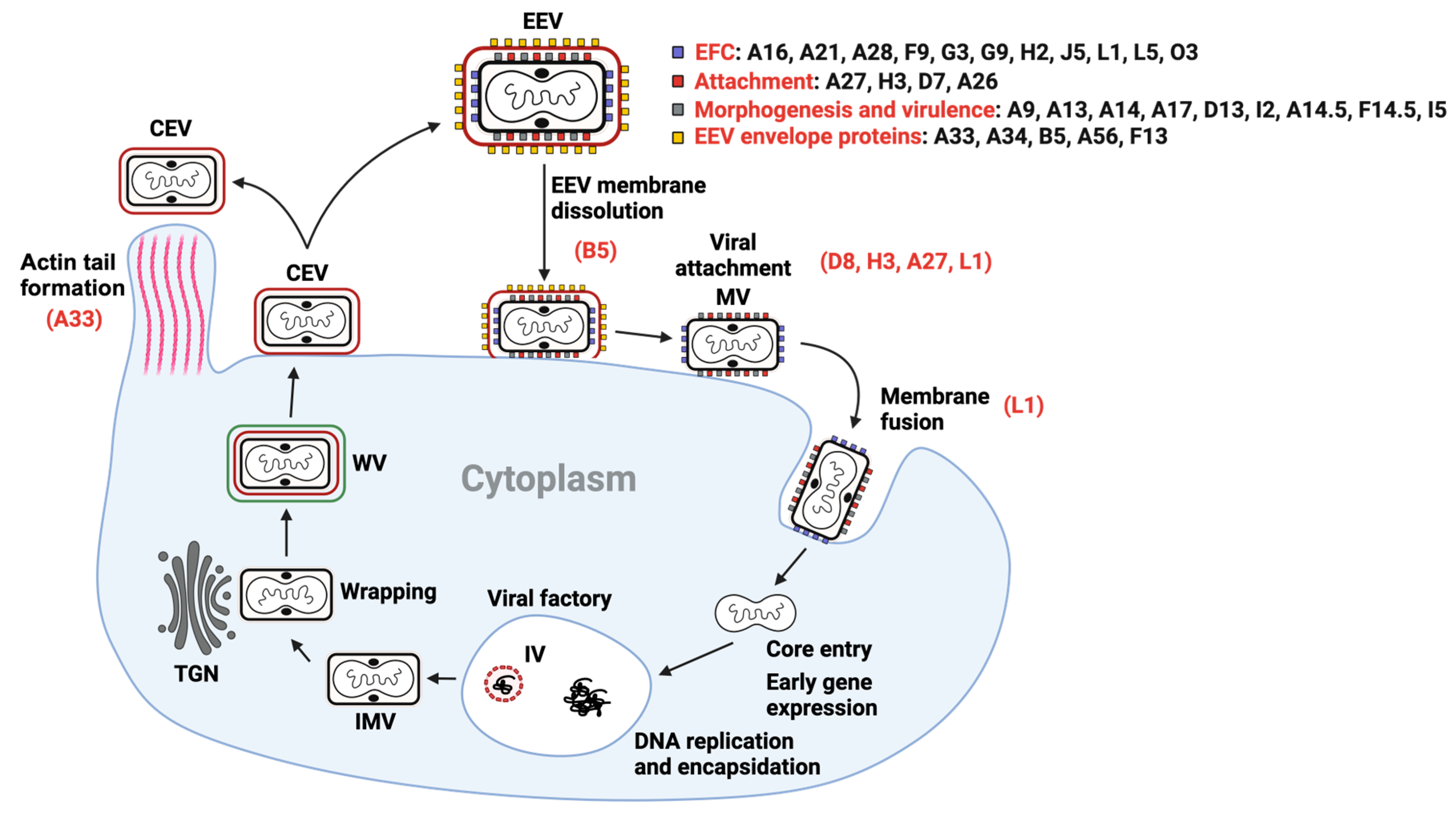
2. The Replication Cycle of OPXVs
3. Correlates of Protection
4. Immunodominant Antigens
5. Neutralization Determinants
6. Neutralization Determinants on MVs

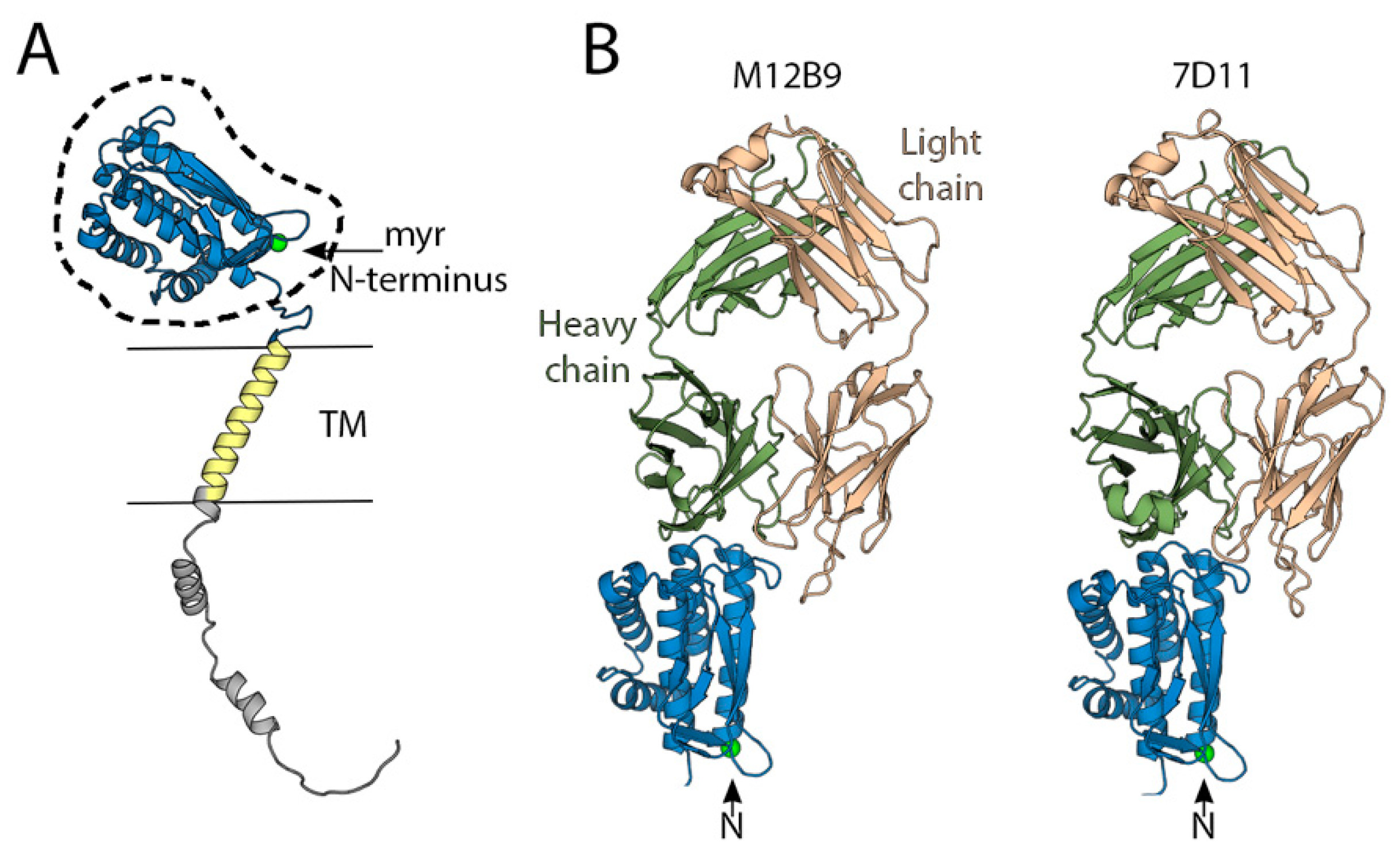
7. Neutralization Determinants on EVs
8. Vaccines and Perspectives
- (1)
- The neutralization determinants and their biogenesis. To date, six well-characterized neutralization determinants have been identified: four on the membrane of MV (D8, A27, L1, and H3) and two on the EV membrane (B5 and A33). mRNA and DNA vaccines require antigens to either be secreted or presented on the plasma membrane. However, MV surface antigens do not naturally transit the secretory pathway and, thus, require some protein engineering to be used as immunogens in these platforms. Key modifications include adding a signal peptide to direct the protein to the secretory pathway—for instance, the signal peptide from influenza hemagglutinin was used to produce secreted M1 and A29 of MPOX [55]; the insertion, removal, or replacement of the transmembrane region; eliminating free cysteine residues, such as those found in A27 (Figure 4); and removing any N-glycosylation motifs. This last modification is particularly crucial in L1 (M1 in MPOX), as the epitope recognized by potent neutralizing antibodies contains a N-glycosylation motif. Additionally, surface antigens on EVs, which naturally transit to the plasma membrane, can also be modified to enhance their expression on the cell surface, for example by removing cytoplasmic regions or modifying their transmembrane domains [14];
- (2)
- The number of antigens to be used. Immunizing mice with individual OPXV proteins may provide some protection against death, but in all cases, signs of disease are observed [14,36]. This is attributed to the complex nature of poxvirus infection, which results in the generation of two antigenically distinct viral particles: mature (MV) and enveloped (EV) virions, each playing distinct roles during infection. To achieve full protection, it is essential to neutralize both viral particles, which can only be accomplished by including MV and EV antigens. Following these guidelines, several subunit vaccines including between two and five antigens have demonstrated efficacy in protecting mice and nonhuman primates from lethal OPXV challenges (Table 2). The most recent mRNA vaccines, developed in response to the MPOX epidemics, use four antigens (the MPOX orthologs of A33, L1, B5, and A27), and not only confer complete survival to mice infected with VACV but also elicit higher neutralization titers than MVA and provide nearly total protection against disease [14,55].
Author Contributions
Funding
Conflicts of Interest
References
- Bray, M.; Buller, M. Looking back at smallpox. Clin. Infect. Dis. 2004, 38, 882–889. [Google Scholar] [CrossRef]
- Thurston, L.; Williams, G. An examination of John Fewster’s role in the discovery of smallpox vaccination. J. R. Coll. Physicians Edinb. 2015, 45, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Moss, B. Smallpox vaccines: Targets of protective immunity. Immunol. Rev. 2011, 239, 8–26. [Google Scholar] [CrossRef]
- Yang, Z.; Gray, M.; Winter, L. Why do poxviruses still matter? Cell Biosci. 2021, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Naleway, A.L. Smallpox vaccine: The good, the bad, and the ugly. Clin. Med. Res. 2003, 1, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Weltzin, R.; Liu, J.; Pugachev, K.V.; Myers, G.A.; Coughlin, B.; Blum, P.S.; Nichols, R.; Johnson, C.; Cruz, J.; Kennedy, J.S.; et al. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat. Med. 2003, 9, 1125–1130. [Google Scholar] [CrossRef]
- Monath, T.P.; Caldwell, J.R.; Mundt, W.; Fusco, J.; Johnson, C.S.; Buller, M.; Liu, J.; Gardner, B.; Downing, G.; Blum, P.S.; et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—A second-generation smallpox vaccine for biological defense. Int. J. Infect. Dis. 2004, 8 (Suppl. 2), S31–S44. [Google Scholar] [CrossRef]
- Earl, P.L.; Americo, J.L.; Wyatt, L.S.; Eller, L.A.; Whitbeck, J.C.; Cohen, G.H.; Eisenberg, R.J.; Hartmann, C.J.; Jackson, D.L.; Kulesh, D.A.; et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 2004, 428, 182–185. [Google Scholar] [CrossRef]
- Zaeck, L.M.; Lamers, M.M.; Verstrepen, B.E.; Bestebroer, T.M.; van Royen, M.E.; Gotz, H.; Shamier, M.C.; van Leeuwen, L.P.M.; Schmitz, K.S.; Alblas, K.; et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2023, 29, 270–278. [Google Scholar] [CrossRef]
- Hazra, A.; Zucker, J.; Bell, E.; Flores, J.; Gordon, L.; Mitja, O.; Suner, C.; Lemaignen, A.; Jamard, S.; Nozza, S.; et al. Mpox in people with past infection or a complete vaccination course: A global case series. Lancet Infect. Dis. 2023. [Google Scholar] [CrossRef]
- Xiao, Y.; Zeng, Y.; Schante, C.; Joshi, S.B.; Buchman, G.W.; Volkin, D.B.; Middaugh, C.R.; Isaacs, S.N. Short-term and longer-term protective immune responses generated by subunit vaccination with smallpox A33, B5, L1 or A27 proteins adjuvanted with aluminum hydroxide and CpG in mice challenged with vaccinia virus. Vaccine 2020, 38, 6007–6018. [Google Scholar] [CrossRef] [PubMed]
- Golden, J.W.; Josleyn, M.; Mucker, E.M.; Hung, C.F.; Loudon, P.T.; Wu, T.C.; Hooper, J.W. Side-by-side comparison of gene-based smallpox vaccine with MVA in nonhuman primates. PLoS ONE 2012, 7, e42353. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Zhang, Y.; Liu, X.; Murad, Y.M.; Xu, J.; Yu, Z.; Hua, X.; Song, Y.; Ding, J.; Huang, H.; et al. mRNA vaccines encoding fusion proteins of monkeypox virus antigens protect mice from vaccinia virus challenge. Nat. Commun. 2023, 14, 5925. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Zhang, Z.; Liu, F.; Lu, H.; Yu, C.; Sun, H.; Long, J.; Cao, Y.; Mai, J.; Miao, Y.; et al. Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus. Signal Transduct. Target. Ther. 2023, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Wang, Z.J.; Zhu, Y.L.; Tang, W.; Zhou, C.; Zhao, S.Q.; Wu, M.; Ming, T.; Deng, Y.Q.; Chen, Q.; et al. Rational development of multicomponent mRNA vaccine candidates against mpox. Emerg. Microbes Infect. 2023, 12, 2192815. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sampedro, L.; Perdiguero, B.; Mejias-Perez, E.; Garcia-Arriaza, J.; Di Pilato, M.; Esteban, M. The evolution of poxvirus vaccines. Viruses 2015, 7, 1726–1803. [Google Scholar] [CrossRef] [PubMed]
- Cyrklaff, M.; Risco, C.; Fernandez, J.J.; Jimenez, M.V.; Esteban, M.; Baumeister, W.; Carrascosa, J.L. Cryo-electron tomography of vaccinia virus. Proc. Natl. Acad. Sci. USA 2005, 102, 2772–2777. [Google Scholar] [CrossRef]
- Jordan, R.; Leeds, J.M.; Tyavanagimatt, S.; Hruby, D.E. Development of ST-246(R) for Treatment of Poxvirus Infections. Viruses 2010, 2, 2409–2435. [Google Scholar] [CrossRef]
- Law, M.; Carter, G.C.; Roberts, K.L.; Hollinshead, M.; Smith, G.L. Ligand-induced and nonfusogenic dissolution of a viral membrane. Proc. Natl. Acad. Sci. USA 2006, 103, 5989–5994. [Google Scholar] [CrossRef]
- Slifka, M.K. Immunological memory to viral infection. Curr. Opin. Immunol. 2004, 16, 443–450. [Google Scholar] [CrossRef]
- Davies, D.H.; McCausland, M.M.; Valdez, C.; Huynh, D.; Hernandez, J.E.; Mu, Y.; Hirst, S.; Villarreal, L.; Felgner, P.L.; Crotty, S. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 2005, 79, 11724–11733. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Putz, M.M.; Smith, G.L. An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J. Gen. Virol. 2005, 86 Pt 4, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Lustig, S.; Fogg, C.; Whitbeck, J.C.; Eisenberg, R.J.; Cohen, G.H.; Moss, B. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 2005, 79, 13454–13462. [Google Scholar] [CrossRef]
- Edghill-Smith, Y.; Golding, H.; Manischewitz, J.; King, L.R.; Scott, D.; Bray, M.; Nalca, A.; Hooper, J.W.; Whitehouse, C.A.; Schmitz, J.E.; et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005, 11, 740–747. [Google Scholar] [CrossRef]
- Chaudhri, G.; Panchanathan, V.; Bluethmann, H.; Karupiah, G. Obligatory requirement for antibody in recovery from a primary poxvirus infection. J. Virol. 2006, 80, 6339–6344. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Johnson, A.J.; Liggitt, D.; Bevan, M.J. Cellular and humoral immunity against vaccinia virus infection of mice. J. Immunol. 2004, 172, 6265–6271. [Google Scholar] [CrossRef] [PubMed]
- Orr, N.; Forman, M.; Marcus, H.; Lustig, S.; Paran, N.; Grotto, I.; Klement, E.; Yehezkelli, Y.; Robin, G.; Reuveny, S.; et al. Clinical and immune responses after revaccination of israeli adults with the Lister strain of vaccinia virus. J. Infect. Dis. 2004, 190, 1295–1302. [Google Scholar] [CrossRef]
- Kempe, C.H. Studies smallpox and complications of smallpox vaccination. Pediatrics 1960, 26, 176–189. [Google Scholar] [CrossRef]
- Davies, D.H.; Wyatt, L.S.; Newman, F.K.; Earl, P.L.; Chun, S.; Hernandez, J.E.; Molina, D.M.; Hirst, S.; Moss, B.; Frey, S.E.; et al. Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus ankara is comparable to that of Dryvax. J. Virol. 2008, 82, 652–663. [Google Scholar] [CrossRef]
- Davies, D.H.; Liang, X.; Hernandez, J.E.; Randall, A.; Hirst, S.; Mu, Y.; Romero, K.M.; Nguyen, T.T.; Kalantari-Dehaghi, M.; Crotty, S.; et al. Profiling the humoral immune response to infection by using proteome microarrays: High-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. USA 2005, 102, 547–552. [Google Scholar] [CrossRef]
- Noy-Porat, T.; Tamir, H.; Alcalay, R.; Rosenfeld, R.; Epstein, E.; Cherry, L.; Achdout, H.; Erez, N.; Politi, B.; Yahalom-Ronen, Y.; et al. Generation of recombinant mAbs to vaccinia virus displaying high affinity and potent neutralization. Microbiol. Spectr. 2023, 11, e0159823. [Google Scholar] [CrossRef]
- Manischewitz, J.; King, L.R.; Bleckwenn, N.A.; Shiloach, J.; Taffs, R.; Merchlinsky, M.; Eller, N.; Mikolajczyk, M.G.; Clanton, D.J.; Monath, T.; et al. Development of a novel vaccinia-neutralization assay based on reporter-gene expression. J. Infect. Dis. 2003, 188, 440–448. [Google Scholar] [CrossRef]
- Cosma, A.; Buhler, S.; Nagaraj, R.; Staib, C.; Hammarin, A.L.; Wahren, B.; Goebel, F.D.; Erfle, V.; Sutter, G. Neutralization assay using a modified vaccinia virus Ankara vector expressing the green fluorescent protein is a high-throughput method to monitor the humoral immune response against vaccinia virus. Clin. Diagn. Lab. Immunol. 2004, 11, 406–410. [Google Scholar] [CrossRef]
- Earl, P.L.; Americo, J.L.; Moss, B. Development and use of a vaccinia virus neutralization assay based on flow cytometric detection of green fluorescent protein. J. Virol. 2003, 77, 10684–10688. [Google Scholar] [CrossRef] [PubMed]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend, M.B.; et al. Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell 2016, 167, 684–694.e9. [Google Scholar] [CrossRef] [PubMed]
- Fogg, C.; Lustig, S.; Whitbeck, J.C.; Eisenberg, R.J.; Cohen, G.H.; Moss, B. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 2004, 78, 10230–10237. [Google Scholar] [CrossRef] [PubMed]
- Aldaz-Carroll, L.; Whitbeck, J.C.; Ponce de Leon, M.; Lou, H.; Hirao, L.; Isaacs, S.N.; Moss, B.; Eisenberg, R.J.; Cohen, G.H. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J. Virol. 2005, 79, 6260–6271. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Hollinshead, R.; Smith, G.L. Antibody-sensitive and antibody-resistant cell-to-cell spread by vaccinia virus: Role of the A33R protein in antibody-resistant spread. J. Gen. Virol. 2002, 83 Pt 1, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Putz, M.M.; Midgley, C.M.; Law, M.; Smith, G.L. Quantification of antibody responses against multiple antigens of the two infectious forms of Vaccinia virus provides a benchmark for smallpox vaccination. Nat. Med. 2006, 12, 1310–1315. [Google Scholar] [CrossRef]
- Lin, C.L.; Chung, C.S.; Heine, H.G.; Chang, W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 2000, 74, 3353–3365. [Google Scholar] [CrossRef]
- Berhanu, A.; Wilson, R.L.; Kirkwood-Watts, D.L.; King, D.S.; Warren, T.K.; Lund, S.A.; Brown, L.L.; Krupkin, A.K.; Vandermay, E.; Weimers, W.; et al. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J. Virol. 2008, 82, 3517–3529. [Google Scholar] [CrossRef]
- Niles, E.G.; Seto, J. Vaccinia virus gene D8 encodes a virion transmembrane protein. J. Virol. 1988, 62, 3772–3778. [Google Scholar] [CrossRef]
- Rodriguez, J.F.; Janeczko, R.; Esteban, M. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J. Virol. 1985, 56, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Wolffe, E.J.; Vijaya, S.; Moss, B. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology 1995, 211, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, K.; Wyatt, L.S.; Moss, B. The neutralizing antibody response to the vaccinia virus A28 protein is specifically enhanced by its association with the H2 protein. Virology 2010, 405, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wallengren, K.; Risco, C.; Krijnse-Locker, J.; Esteban, M.; Rodriguez, D. The A17L gene product of vaccinia virus is exposed on the surface of IMV. Virology 2001, 290, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Engelstad, M.; Howard, S.T.; Smith, G.L. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology 1992, 188, 801–810. [Google Scholar] [CrossRef] [PubMed]
- McCausland, M.M.; Benhnia, M.R.; Crickard, L.; Laudenslager, J.; Granger, S.W.; Tahara, T.; Kubo, R.; Koriazova, L.; Kato, S.; Crotty, S. Combination therapy of vaccinia virus infection with human anti-H3 and anti-B5 monoclonal antibodies in a small animal model. Antivir. Ther. 2010, 15, 661–675. [Google Scholar] [CrossRef]
- Roper, R.L.; Payne, L.G.; Moss, B. Extracellular vaccinia virus envelope glycoprotein encoded by the A33R gene. J. Virol. 1996, 70, 3753–3762. [Google Scholar] [CrossRef]
- Paran, N.; Lustig, S.; Zvi, A.; Erez, N.; Israely, T.; Melamed, S.; Politi, B.; Ben-Nathan, D.; Schneider, P.; Lachmi, B.; et al. Active vaccination with vaccinia virus A33 protects mice against lethal vaccinia and ectromelia viruses but not against cowpoxvirus; elucidation of the specific adaptive immune response. Virol. J. 2013, 10, 229. [Google Scholar] [CrossRef]
- Chen, Z.; Earl, P.; Americo, J.; Damon, I.; Smith, S.K.; Yu, F.; Sebrell, A.; Emerson, S.; Cohen, G.; Eisenberg, R.J.; et al. Characterization of chimpanzee/human monoclonal antibodies to vaccinia virus A33 glycoprotein and its variola virus homolog in vitro and in a vaccinia virus mouse protection model. J. Virol. 2007, 81, 8989–8995. [Google Scholar] [CrossRef]
- Mittler, E.; Serris, A.; Esterman, E.S.; Florez, C.; Polanco, L.C.; O’Brien, C.M.; Slough, M.M.; Tynell, J.; Groning, R.; Sun, Y.; et al. Structural and mechanistic basis of neutralization by a pan-hantavirus protective antibody. Sci. Transl. Med. 2023, 15, eadg1855. [Google Scholar] [CrossRef] [PubMed]
- Mittler, E.; Wec, A.Z.; Tynell, J.; Guardado-Calvo, P.; Wigren-Bystrom, J.; Polanco, L.C.; O’Brien, C.M.; Slough, M.M.; Abelson, D.M.; Serris, A.; et al. Human antibody recognizing a quaternary epitope in the Puumala virus glycoprotein provides broad protection against orthohantaviruses. Sci. Transl. Med. 2022, 14, eabl5399. [Google Scholar] [CrossRef] [PubMed]
- Rouvinski, A.; Guardado-Calvo, P.; Barba-Spaeth, G.; Duquerroy, S.; Vaney, M.C.; Kikuti, C.M.; Navarro Sanchez, M.E.; Dejnirattisai, W.; Wongwiwat, W.; Haouz, A.; et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 2015, 520, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Freyn, A.W.; Atyeo, C.; Earl, P.L.; Americo, J.L.; Chuang, G.Y.; Natarajan, H.; Frey, T.R.; Gall, J.G.; Moliva, J.I.; Hunegnaw, R.; et al. An mpox virus mRNA-lipid nanoparticle vaccine confers protection against lethal orthopoxviral challenge. Sci. Transl. Med. 2023, 15, eadg3540. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.B.; Ovsyannikova, I.G.; Jacobson, R.M.; Poland, G.A. The immunology of smallpox vaccines. Curr. Opin. Immunol. 2009, 21, 314–320. [Google Scholar] [CrossRef]
- Meyer, H.; Ehmann, R.; Smith, G.L. Smallpox in the Post-Eradication Era. Viruses 2020, 12, 138. [Google Scholar] [CrossRef]
- Rodriguez, J.R.; Rodriguez, D.; Esteban, M. Insertional inactivation of the vaccinia virus 32-kilodalton gene is associated with attenuation in mice and reduction of viral gene expression in polarized epithelial cells. J. Virol. 1992, 66, 183–189. [Google Scholar] [CrossRef]
- Matho, M.H.; de Val, N.; Miller, G.M.; Brown, J.; Schlossman, A.; Meng, X.; Crotty, S.; Peters, B.; Xiang, Y.; Hsieh-Wilson, L.C.; et al. Murine anti-vaccinia virus D8 antibodies target different epitopes and differ in their ability to block D8 binding to CS-E. PLoS Pathog. 2014, 10, e1004495. [Google Scholar] [CrossRef]
- Sakhatskyy, P.; Wang, S.; Chou, T.H.; Lu, S. Immunogenicity and protection efficacy of monovalent and polyvalent poxvirus vaccines that include the D8 antigen. Virology 2006, 355, 164–174. [Google Scholar] [CrossRef]
- Meng, X.; Zhong, Y.; Embry, A.; Yan, B.; Lu, S.; Zhong, G.; Xiang, Y. Generation and characterization of a large panel of murine monoclonal antibodies against vaccinia virus. Virology 2011, 409, 271–279. [Google Scholar] [CrossRef]
- Matho, M.H.; Schlossman, A.; Gilchuk, I.M.; Miller, G.; Mikulski, Z.; Hupfer, M.; Wang, J.; Bitra, A.; Meng, X.; Xiang, Y.; et al. Structure-function characterization of three human antibodies targeting the vaccinia virus adhesion molecule D8. J. Biol. Chem. 2018, 293, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Matho, M.H.; Maybeno, M.; Benhnia, M.R.; Becker, D.; Meng, X.; Xiang, Y.; Crotty, S.; Peters, B.; Zajonc, D.M. Structural and biochemical characterization of the vaccinia virus envelope protein D8 and its recognition by the antibody LA5. J. Virol. 2012, 86, 8050–8058. [Google Scholar] [CrossRef] [PubMed]
- Kaever, T.; Matho, M.H.; Meng, X.; Crickard, L.; Schlossman, A.; Xiang, Y.; Crotty, S.; Peters, B.; Zajonc, D.M. Linear Epitopes in Vaccinia Virus A27 Are Targets of Protective Antibodies Induced by Vaccination against Smallpox. J. Virol. 2016, 90, 4334–4345. [Google Scholar] [CrossRef]
- Kaever, T.; Meng, X.; Matho, M.H.; Schlossman, A.; Li, S.; Sela-Culang, I.; Ofran, Y.; Buller, M.; Crump, R.W.; Parker, S.; et al. Potent neutralization of vaccinia virus by divergent murine antibodies targeting a common site of vulnerability in L1 protein. J. Virol. 2014, 88, 11339–11355. [Google Scholar] [CrossRef] [PubMed]
- Matho, M.H.; Schlossman, A.; Meng, X.; Benhnia, M.R.; Kaever, T.; Buller, M.; Doronin, K.; Parker, S.; Peters, B.; Crotty, S.; et al. Structural and Functional Characterization of Anti-A33 Antibodies Reveal a Potent Cross-Species Orthopoxviruses Neutralizer. PLoS Pathog. 2015, 11, e1005148. [Google Scholar] [CrossRef]
- da Fonseca, F.G.; Wolffe, E.J.; Weisberg, A.; Moss, B. Effects of deletion or stringent repression of the H3L envelope gene on vaccinia virus replication. J. Virol. 2000, 74, 7518–7528. [Google Scholar] [CrossRef]
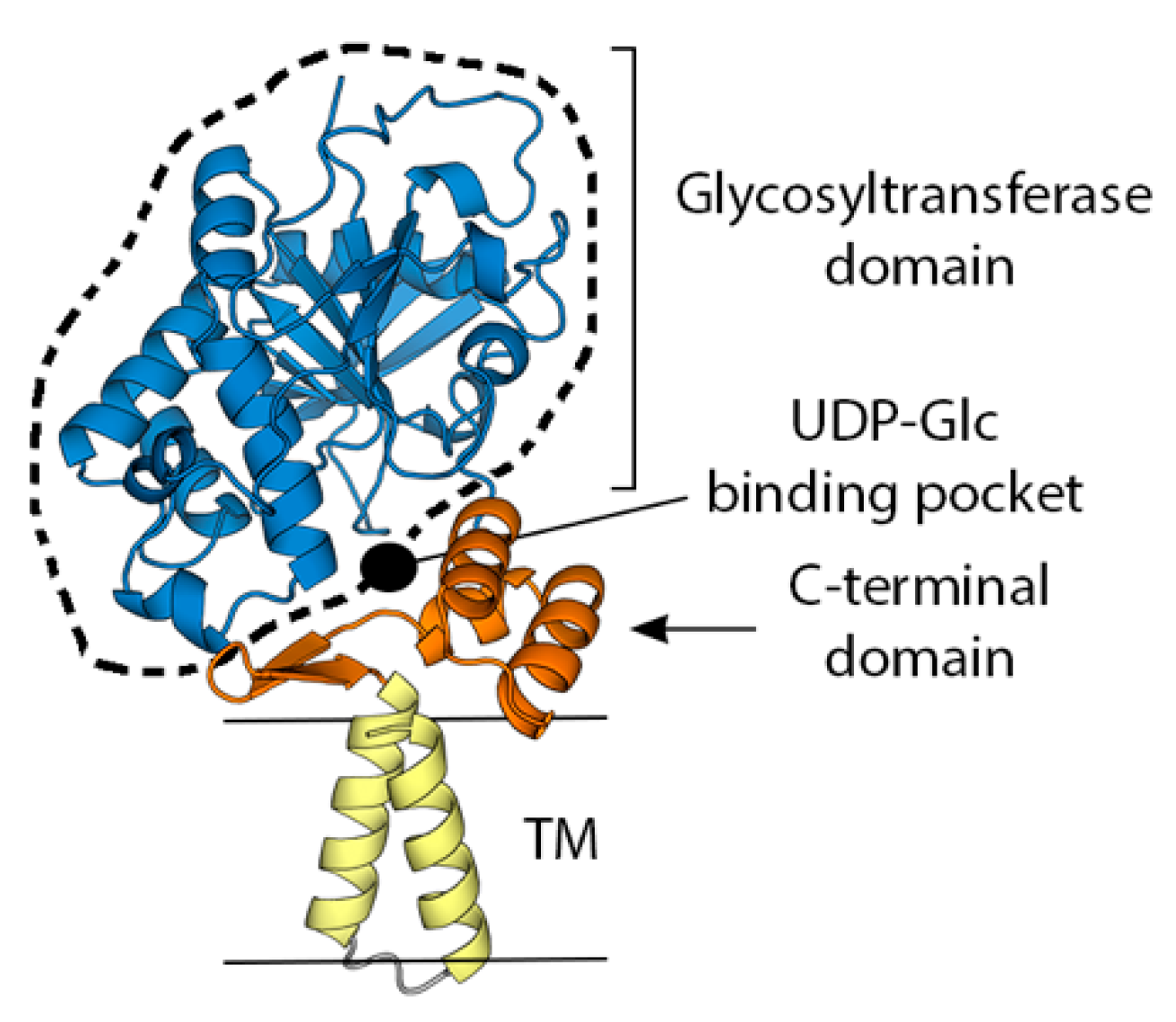
- Singh, K.; Gittis, A.G.; Gitti, R.K.; Ostazeski, S.A.; Su, H.P.; Garboczi, D.N. The Vaccinia Virus H3 Envelope Protein, a Major Target of Neutralizing Antibodies, Exhibits a Glycosyltransferase Fold and Binds UDP-Glucose. J. Virol. 2016, 90, 5020–5030. [Google Scholar] [CrossRef]
- Mirzakhanyan, Y.; Gershon, P. The Vaccinia virion: Filling the gap between atomic and ultrastructure. PLoS Pathog. 2019, 15, e1007508. [Google Scholar] [CrossRef]
- Davies, D.H.; Molina, D.M.; Wrammert, J.; Miller, J.; Hirst, S.; Mu, Y.; Pablo, J.; Unal, B.; Nakajima-Sasaki, R.; Liang, X.; et al. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics 2007, 7, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.B.; Keckler, M.S.; Patel, N.; Davies, D.H.; Felgner, P.; Damon, I.K.; Karem, K.L. Humoral immunity to smallpox vaccines and monkeypox virus challenge: Proteomic assessment and clinical correlations. J. Virol. 2013, 87, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Benhnia, M.R.; McCausland, M.M.; Su, H.P.; Singh, K.; Hoffmann, J.; Davies, D.H.; Felgner, P.L.; Head, S.; Sette, A.; Garboczi, D.N.; et al. Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. J. Virol. 2008, 82, 3751–3768. [Google Scholar] [CrossRef]
- Chang, T.H.; Chang, S.J.; Hsieh, F.L.; Ko, T.P.; Lin, C.T.; Ho, M.R.; Wang, I.; Hsu, S.T.; Guo, R.T.; Chang, W.; et al. Crystal structure of vaccinia viral A27 protein reveals a novel structure critical for its function and complex formation with A26 protein. PLoS Pathog. 2013, 9, e1003563. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.F.; Paez, E.; Esteban, M. A 14,000-Mr envelope protein of vaccinia virus is involved in cell fusion and forms covalently linked trimers. J. Virol. 1987, 61, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Kochan, G.; Escors, D.; Gonzalez, J.M.; Casasnovas, J.M.; Esteban, M. Membrane cell fusion activity of the vaccinia virus A17-A27 protein complex. Cell Microbiol. 2008, 10, 149–164. [Google Scholar] [CrossRef][Green Version]
- Cohn, H.; Bloom, N.; Cai, G.Y.; Clark, J.J.; Tarke, A.; Bermudez-Gonzalez, M.C.; Altman, D.R.; Lugo, L.A.; Lobo, F.P.; Marquez, S.; et al. Mpox vaccine and infection-driven human immune signatures: An immunological analysis of an observational study. Lancet Infect. Dis. 2023, 23, 1302–1312. [Google Scholar] [CrossRef]
- Fogg, C.N.; Americo, J.L.; Earl, P.L.; Resch, W.; Aldaz-Carroll, L.; Eisenberg, R.J.; Cohen, G.H.; Moss, B. Disparity between levels of in vitro neutralization of vaccinia virus by antibody to the A27 protein and protection of mice against intranasal challenge. J. Virol. 2008, 82, 8022–8029. [Google Scholar] [CrossRef]
- Schin, A.M.; Diesterbeck, U.S.; Moss, B. Insights into the Organization of the Poxvirus Multicomponent Entry-Fusion Complex from Proximity Analyses in Living Infected Cells. J. Virol. 2021, 95, e0085221. [Google Scholar] [CrossRef]
- Ichihashi, Y.; Takahashi, T.; Oie, M. Identification of a vaccinia virus penetration protein. Virology 1994, 202, 834–843. [Google Scholar] [CrossRef]
- Foo, C.H.; Lou, H.; Whitbeck, J.C.; Ponce-de-Leon, M.; Atanasiu, D.; Eisenberg, R.J.; Cohen, G.H. Vaccinia virus L1 binds to cell surfaces and blocks virus entry independently of glycosaminoglycans. Virology 2009, 385, 368–382. [Google Scholar] [CrossRef]
- Ravanello, M.P.; Hruby, D.E. Conditional lethal expression of the vaccinia virus L1R myristylated protein reveals a role in virion assembly. J. Virol. 1994, 68, 6401–6410. [Google Scholar] [CrossRef] [PubMed]
- Su, H.P.; Garman, S.C.; Allison, T.J.; Fogg, C.; Moss, B.; Garboczi, D.N. The 1.51-Angstrom structure of the poxvirus L1 protein, a target of potent neutralizing antibodies. Proc. Natl. Acad. Sci. USA 2005, 102, 4240–4245. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Kallemeijn, W.W.; Faronato, M.; Wilkins, K.; Goldsmith, C.S.; Cotter, C.A.; Ojeda, S.; Solari, R.; Moss, B.; Tate, E.W.; et al. Inhibition of vaccinia virus L1 N-myristoylation by the host N-myristoyltransferase inhibitor IMP-1088 generates non-infectious virions defective in cell entry. PLoS Pathog. 2022, 18, e1010662. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.H.; Whitbeck, J.C.; Ponce-de-Leon, M.; Saw, W.T.; Cohen, G.H.; Eisenberg, R.J. The myristate moiety and amino terminus of vaccinia virus l1 constitute a bipartite functional region needed for entry. J. Virol. 2012, 86, 5437–5451. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.W.; Thompson, E.; Wilhelmsen, C.; Zimmerman, M.; Ichou, M.A.; Steffen, S.E.; Schmaljohn, C.S.; Schmaljohn, A.L.; Jahrling, P.B. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 2004, 78, 4433–4443. [Google Scholar] [CrossRef] [PubMed]
- Otter, A.D.; Jones, S.; Hicks, B.; Bailey, D.; Callaby, H.; Houlihan, C.; Rampling, T.; Gordon, N.C.; Selman, H.; Satheshkumar, P.S.; et al. Monkeypox virus-infected individuals mount comparable humoral immune responses as Smallpox-vaccinated individuals. Nat. Commun. 2023, 14, 5948. [Google Scholar] [CrossRef] [PubMed]
- Su, H.P.; Golden, J.W.; Gittis, A.G.; Hooper, J.W.; Garboczi, D.N. Structural basis for the binding of the neutralizing antibody, 7D11, to the poxvirus L1 protein. Virology 2007, 368, 331–341. [Google Scholar] [CrossRef]
- Galmiche, M.C.; Goenaga, J.; Wittek, R.; Rindisbacher, L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 1999, 254, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Aldaz-Carroll, L.; Xiao, Y.; Whitbeck, J.C.; de Leon, M.P.; Lou, H.; Kim, M.; Yu, J.; Reinherz, E.L.; Isaacs, S.N.; Eisenberg, R.J.; et al. Major neutralizing sites on vaccinia virus glycoprotein B5 are exposed differently on variola virus ortholog B6. J. Virol. 2007, 81, 8131–8139. [Google Scholar] [CrossRef]
- Paran, N.; Lustig, S. Complement-bound human antibodies to vaccinia virus B5 antigen protect mice from virus challenge. Expert Rev. Vaccines 2010, 9, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, S.R.; Earley, A.K.; Stone, R.; Norbury, C.C.; Ward, B.M. Vaccinia Virus Glycoproteins A33, A34, and B5 Form a Complex for Efficient Endoplasmic Reticulum to trans-Golgi Network Transport. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef]
- Chan, W.M.; Ward, B.M. There is an A33-dependent mechanism for the incorporation of B5-GFP into vaccinia virus extracellular enveloped virions. Virology 2010, 402, 83–93. [Google Scholar] [CrossRef]
- Roper, R.L.; Wolffe, E.J.; Weisberg, A.; Moss, B. The envelope protein encoded by the A33R gene is required for formation of actin-containing microvilli and efficient cell-to-cell spread of vaccinia virus. J. Virol. 1998, 72, 4192–4204. [Google Scholar] [CrossRef]
- Katz, E.; Wolffe, E.; Moss, B. Identification of second-site mutations that enhance release and spread of vaccinia virus. J. Virol. 2002, 76, 11637–11644. [Google Scholar] [CrossRef] [PubMed]
- Su, H.P.; Singh, K.; Gittis, A.G.; Garboczi, D.N. The structure of the poxvirus A33 protein reveals a dimer of unique C-type lectin-like domains. J. Virol. 2010, 84, 2502–2510. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, A.; Neher, R.A.; Ndodo, N.; Borges, V.; Gannon, B.; Gomes, J.P.; Groves, N.; King, D.J.; Maloney, D.; Lemey, P.; et al. APOBEC3 deaminase editing in mpox virus as evidence for sustained human transmission since at least 2016. Science 2023, 382, 595–600. [Google Scholar] [CrossRef]
- Duffy, J.; Marquez, P.; Moro, P.; Weintraub, E.; Yu, Y.; Boersma, P.; Donahue, J.G.; Glanz, J.M.; Goddard, K.; Hambidge, S.J.; et al. Safety Monitoring of JYNNEOS Vaccine During the 2022 Mpox Outbreak—United States, May 22–October 21, 2022. MMWR Morb. Mortal. Wkly Rep. 2022, 71, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Carson, W.C.; Ortega, E.; Navarra, T.; Tran, S.; Smith, T.G.; Pukuta, E.; Muyamuna, E.; Kabamba, J.; Nguete, B.U.; et al. Serological responses to the MVA-based JYNNEOS monkeypox vaccine in a cohort of participants from the Democratic Republic of Congo. Vaccine 2022, 40, 7321–7327. [Google Scholar] [CrossRef]
- Hooper, J.W.; Custer, D.M.; Schmaljohn, C.S.; Schmaljohn, A.L. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 2000, 266, 329–339. [Google Scholar] [CrossRef]
- Fogg, C.N.; Americo, J.L.; Lustig, S.; Huggins, J.W.; Smith, S.K.; Damon, I.; Resch, W.; Earl, P.L.; Klinman, D.M.; Moss, B. Adjuvant-enhanced antibody responses to recombinant proteins correlates with protection of mice and monkeys to orthopoxvirus challenges. Vaccine 2007, 25, 2787–2799. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Aldaz-Carroll, L.; Ortiz, A.M.; Whitbeck, J.C.; Alexander, E.; Lou, H.; Davis, H.L.; Braciale, T.J.; Eisenberg, R.J.; Cohen, G.H.; et al. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine 2007, 25, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, N.J.; Ray, C.A.; Collier, M.L.; Liao, H.X.; Pickup, D.J.; Johnston, R.E. Vaccination with Venezuelan equine encephalitis replicons encoding cowpox virus structural proteins protects mice from intranasal cowpox virus challenge. Virology 2007, 362, 441–452. [Google Scholar] [CrossRef][Green Version]
- Hooper, J.W.; Custer, D.M.; Thompson, E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 2003, 306, 181–195. [Google Scholar] [CrossRef]
- Buchman, G.W.; Cohen, M.E.; Xiao, Y.; Richardson-Harman, N.; Silvera, P.; DeTolla, L.J.; Davis, H.L.; Eisenberg, R.J.; Cohen, G.H.; Isaacs, S.N. A protein-based smallpox vaccine protects non-human primates from a lethal monkeypox virus challenge. Vaccine 2010, 28, 6627–6636. [Google Scholar] [CrossRef]
- Heraud, J.M.; Edghill-Smith, Y.; Ayala, V.; Kalisz, I.; Parrino, J.; Kalyanaraman, V.S.; Manischewitz, J.; King, L.R.; Hryniewicz, A.; Trindade, C.J.; et al. Subunit recombinant vaccine protects against monkeypox. J. Immunol. 2006, 177, 2552–2564. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.W.; Ferro, A.M.; Golden, J.W.; Silvera, P.; Dudek, J.; Alterson, K.; Custer, M.; Rivers, B.; Morris, J.; Owens, G.; et al. Molecular smallpox vaccine delivered by alphavirus replicons elicits protective immunity in mice and non-human primates. Vaccine 2009, 28, 494–511. [Google Scholar] [CrossRef] [PubMed]
| Antibody Name (PDB 1) | Species | Target and role in OPXV replication | VACV Neutralization IC50 2 (Emax) 3 | MPOX Neutralization IC50 (Emax) | Ref. | ||
|---|---|---|---|---|---|---|---|
| −C 4 | +C | −C | +C | ||||
| MV-neutralizing antibodies | |||||||
| LA5 (4EBQ) | Mouse | D8 (attachment) | < 5 | <10 (80) | ND 6 | ND | [61] |
| VACV-304 (5USL) | Human | < | 0.02 (79) | < | < | [35] | |
| VACV-138 (6B9J) | Human | < | 0.3 (80) | < | < | ||
| VACV-66 (5USH) | Human | < | 0.1 (85) | < | < | ||
| VACV-249 | Human | < | 0.2 (70) | < | < | ||
| MV-33 | Macaque | < | <0.01 (90) | < | 0.03 (60) | [31] | |
| MV-49 | Macaque | < | 1 (>90) | ND | ND | ||
| VACV-314 | Human | H3 (attachment) | < | 0.1 (74) | < | 0.8 (84) | [35] |
| MPXV-72 | Human | < | 11.4 (66) | < | 6.2 (64) | ||
| MV-7 | Macaque | < | 2 (70) | ND | ND | [31] | |
| MV-26 | Macaque | < | 0.1 (80) | ND | ND | ||
| MV-31 | Macaque | < | 0.01 (80) | ND | ND | ||
| MV-32 | Macaque | < | 0.06 (80) | < | 1.0 (60) | ||
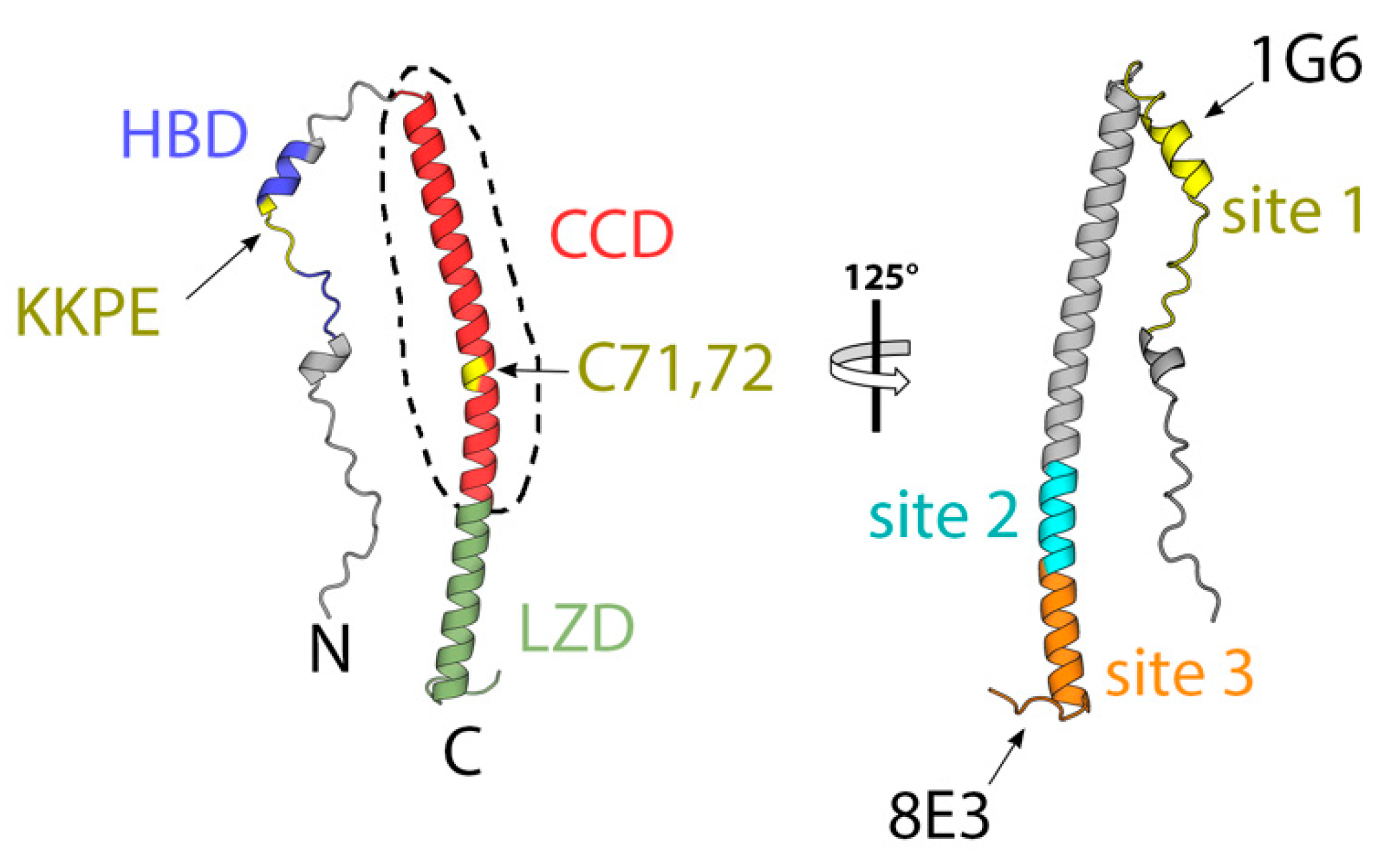
| 1G6 (5EOQ) | Mouse | A27 (attachment) | < | <20 (90) | ND | ND | [65] |
| VACV-301 | Human | 0.5 (61) | 0.1 (77) | 1.6 (84) | 0.8 (92) | [35] | |
| VACV-302 | Human | 12 (81) | 0.1 (53) | 0.1 (88) | 6.3 (82) | ||
| MPXV-26 | Human | L1 (attachment, fusion) | 0.3 (95) | 0.7 (71) | 3 (96) | 6.2 (97) | [35] |
| M12B9 (4U6H) | Mouse | 0.8 (100) | 0.032 (100) | ND | ND | [66] | |
| EV-neutralizing antibodies | |||||||
| VACV-59 | Human | B5 (spread, non-fusogenic EV membrane dissolution) | < | 0.2 (72) | < | < | [35] |
| VACV-283 | Human | < | 0.7 (76) | < | < | ||
| MPXV-13 | Human | < | 0.01 (80) | < | < | ||
| MPXV-25 | Human | < | 0.02 (77) | < | < | ||
| MPXV-51 | Human | A33 (spread) | < | 0.1 (50) | < | 0.8 (77) | [35] |
| MPXV-56 | Human | < | 0.1 (56) | < | 12.5 (75) | ||
| A27D7 (4M1G) | Mouse | < | <10 | ND | ND | [67] | |
| A20G2 (4LU5) | Mouse | < | <10 | ND | ND | ||
| A2C7 (4LQF) | Mouse | < | <10 | ND | ND | ||
| Vaccine Technology | MV Antigens | EV Antigens | Reference |
|---|---|---|---|
| DNA 1 | L1 | A33 | [100] |
| Protein b,1 | A27 | B5 | [41] |
| Protein a,1 | L1 | A33 | [101] |
| Protein b,1 | A27, D8 | B5 | [41] |
| Protein c,1 | L1 | A33, B5 | [36] |
| Protein d,1 | L1 | A33, B5 | [102] |
| VEEV replicon 2 | A27 | A33, B5 | [103] |
| mRNA 3 | L1, A27 | A33, B5 | [14,15,55] |
| DNA 1 | L1, A27 | A33, B5 | [86,104] |
| Protein d,1 | L1, A27 | A33, B5 | [105] |
| DNA 3 | L1, A27 | A33, B5 | [106] |
| VEEV replicon 1 | L1, A27 | A33, B5 | [107] |
| DNA 1 | L1, A27, D8 | A33, B5 | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccardo, V.; Pablo, G.-C. Neutralization Determinants on Poxviruses. Viruses 2023, 15, 2396. https://doi.org/10.3390/v15122396
Riccardo V, Pablo G-C. Neutralization Determinants on Poxviruses. Viruses. 2023; 15(12):2396. https://doi.org/10.3390/v15122396
Chicago/Turabian StyleRiccardo, Vernuccio, and Guardado-Calvo Pablo. 2023. "Neutralization Determinants on Poxviruses" Viruses 15, no. 12: 2396. https://doi.org/10.3390/v15122396
APA StyleRiccardo, V., & Pablo, G.-C. (2023). Neutralization Determinants on Poxviruses. Viruses, 15(12), 2396. https://doi.org/10.3390/v15122396







