Emerging Zoonotic Diseases among Pastoral Communities of Caia and Búzi Districts, Sofala, Mozambique: Evidence of Antibodies against Brucella, Leptospira, Rickettsia, and Crimean-Congo Hemorrhagic Fever Virus
Abstract
1. Introduction
2. Material and Methods
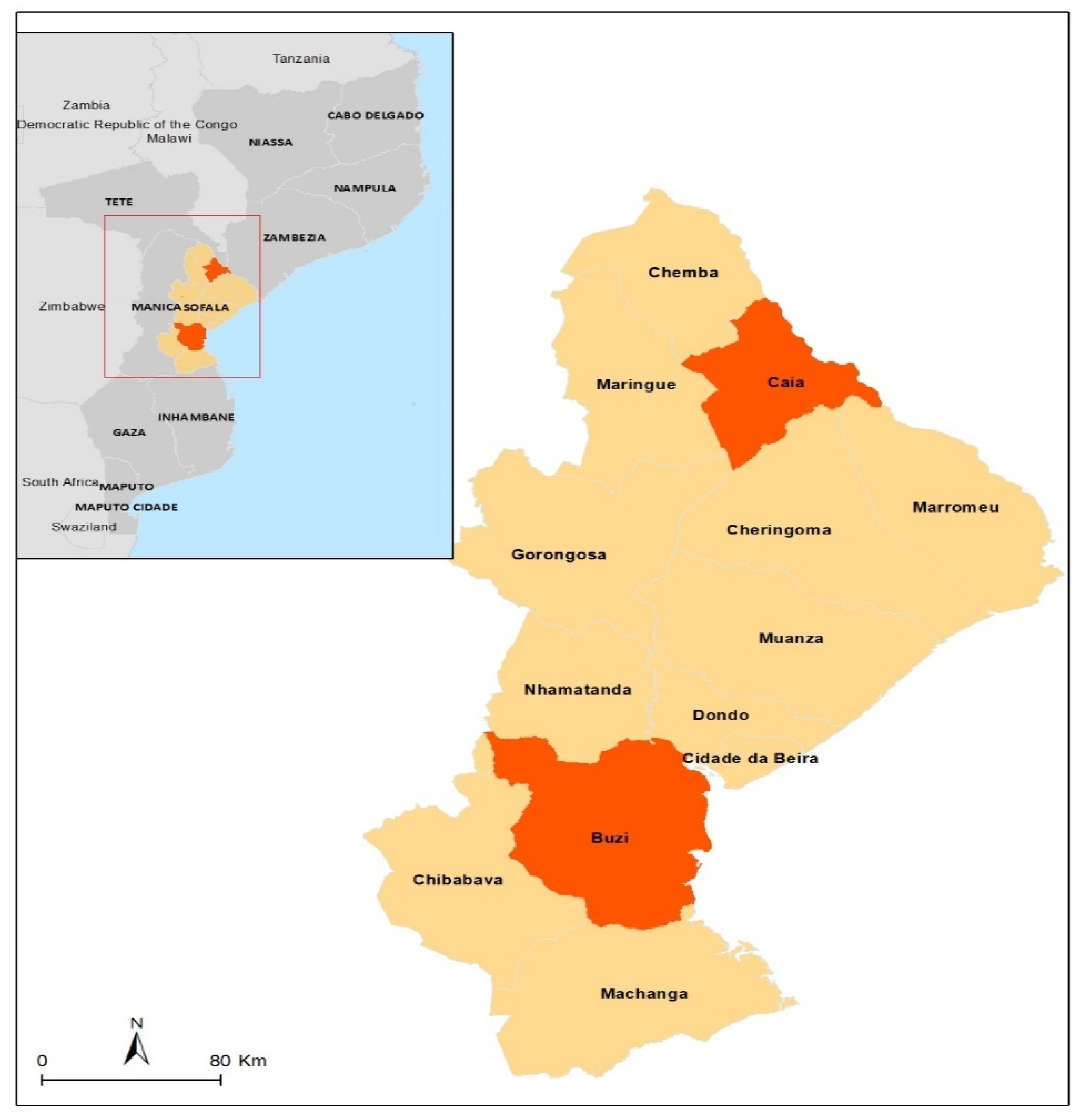
2.1. The Study Area and Population
2.2. Study Design and Sample Size Estimation
2.3. Blood Sample Collection and Laboratory Analysis
2.4. Data Entry and Statistical Analysis
3. Results
3.1. Socio-Demographic Characterization
3.2. Frequency of Antibodies against Brucella, Leptospira, Rickettsia, and CCHFV in Caia and Búzi Districts
4. Discussion
4.1. Seroprevalence of Antibodies against Brucella
4.2. Seroprevalence of Antibodies against Leptospira
4.3. Seroprevalence of Antibodies against Rickettsia
4.4. Seroprevalence of Antibodies against CCHFV
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Búzi District (n = 123) | Caia District (n = 95) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | OR | IC | p-Value | Frequency | OR | IC | p-Value | ||
| Gender | Female | 0 | 1.00 | - | Ref | 1 (1.1%) | 1.00 | - | Ref |
| Male | 3 (2.4%) | >100 | - | 0.996 | 1 (1.1%) | 0.11 | 0.00; 3.07 | 0.138 | |
| Age group | 5–14 years | 0 | 1.00 | 0.00; Inf | 1.000 | 0 | 1.00 | 0.00; Inf | 1.000 |
| 15–29 years | 2 (1.6%) | >100 | - | 0.996 | 1 (1.1%) | >100 | - | 0.997 | |
| 30–59 years | 1 (0.8%) | >100 | - | 0.997 | 1 (1.1%) | >100 | - | 0.997 | |
| ≥60 years | 0 | 1.00 | - | Ref | 0 | 1.00 | - | Ref | |
| Education | None | 0 | 0.00 | - | 0.997 | 1 (1.1%) | 2.83 | 0.10; 79.7 | 0.485 |
| Primary | 2 (1.6%) | 0.65 | 0.06; 14.6 | 0.735 | 1 (1.1%) | 0.00 | - | 0.997 | |
| Basic | 1 (0.8%) | 1.00 | - | Ref | 0 | 1.00 | - | Ref | |
| Medium | 0 | 0.00 | - | 0.997 | 0 | 0.00 | - | 0.999 | |
| Professional technician | 0 | 0.00 | - | 0.999 | 0 | 0.00 | - | 0.999 | |
| Higher | 0 | - | - | - | 0 | 0.00 | - | 0.999 | |
| Occupation | Domestic worker | 0 | 1.00 | 0.00; Inf | 1.000 | 0 | 0.00 | - | 0.999 |
| Student | 0 | 1.00 | 0.00; Inf | 1.000 | 1 (1.1%) | 12.00 | 0.41; 364 | 0.106 | |
| Education professional | 0 | 1.00 | 0.00; Inf | 1.000 | 0 | 0.00 | - | 0.999 | |
| Health professional | 0 | 1.00 | 0.00; Inf | 1.000 | 0 | 0.00 | - | - | |
| Veterinarian | 0 | 1.00 | 0.00; Inf | 1.000 | 0 | 0.00 | - | 0.999 | |
| Slaughterhouse worker | 0 | - | - | - | 0 | 0.00 | - | 0.999 | |
| Cattle owner | 0 | 1.00 | - | Ref | 1 (1.1%) | 1.00 | - | Ref | |
| Cattle herder | 3 (2.4%) | 1.00 | 0.00; Inf | 0.997 | 0 | 0.00 | - | 0.997 | |
| Others | 0 | 1.00 | 0.00; Inf | 1.000 | 0 | 0.00 | - | 0.998 | |
| None | 0 | 1.00 | 0.00; Inf | 1.000 | 0 | - | - | - | |
| Búzi District (n = 123) | Caia District (n = 95) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | OR | IC | p-Value | Frequency | OR | IC | p-Value | ||
| Gender | Female | 4 (3.3%) | 1.00 | - | Ref | 5 (5.3%) | 1.00 | - | Ref |
| Male | 20 (16.3%) | 1.87 | 0.64; 6.89 | 0.289 | 38 (40.0%) | 1.18 | 0.35; 4.28 | 0.789 | |
| Age group | 5–14 years | 0 | 0.00 | - | 0.991 | 0 | 0.00 | - | 0.988 |
| 15–29 years | 8 (6.5%) | 0.52 | - | 0.402 | 19 (20.0%) | 0.86 | 0.14; 5.15 | 0.867 | |
| 30–59 years | 13 (10.6%) | 0.81 | - | 0.773 | 21 (22.1%) | 0.84 | 0.14; 4.96 | 0.841 | |
| ≥60 years | 3 (2.4%) | 1.00 | - | Ref | 3 (3.2%) | 1.00 | - | Ref | |
| Education | None | 4 (3.3%) | 1.52 | 0.29; 8.88 | 0.619 | 6 (6.3%) | 5.60 | 1.07; 35.9 | 0.049 |
| Primary | 17 (13.8%) | 1.65 | 0.48; 7.69 | 0.467 | 5 (5.3%) | 2.26 | 0.75; 7.77 | 0.165 | |
| Basic | 3 (2.4%) | 1.00 | - | Ref | 25 (26.3%) | 1.00 | - | Ref | |
| Medium | 0 | 0.00 | - | 0.989 | 6 (6.3%) | 5.60 | 1.07; 35.9 | 0.049 | |
| Professional techician | 0 | 0.00 | - | 0.996 | 0 | 0.00 | - | 0.992 | |
| Higher | 0 | - | - | - | 1 (1.1%) | >100 | - | 0.991 | |
| Occupation | Domestic worker | 3 (2.4%) | 2.40 | 0.42; 12.1 | 0.291 | 1 (1.1%) | 0.34 | 0.02; 2.55 | 0.354 |
| Students | 1 (0.8%) | 0.49 | 0.02; 3.46 | 0.535 | 3 (3.2%) | 4.11 | 0.48; 86.6 | 0.237 | |
| Education professional | 0 | 0.00 | - | 0.995 | 2 (2.1%) | 1.37 | 0.15; 12.3 | 0.764 | |
| Health professional | 1 (0.8%) | 1.60 | 0.07; 14.0 | 0.699 | 0 | - | - | - | |
| Veterinarian | 1 (0.8%) | >100 | - | 0.993 | 1 (1.1%) | 1.37 | 0.05; 36.1 | 0.828 | |
| Slaughterhouse worker | 0 | - | - | - | 2 (2.1%) | 0.91 | 0.11; 6.03 | 0.924 | |
| Cattle owner | 5 (4.1%) | 1.00 | - | Ref | 19 (20.0%) | 1.00 | - | Ref | |
| Cattle herder | 12 (9.8%) | 2.48 | 0.82; 8.55 | 0.123 | 13 (13.7%) | 1.48 | 0.55; 4.01 | 0.432 | |
| Others | 1 (0.8%) | 0.71 | 0.03; 5.21 | 0.768 | 2 (2.1%) | 0.91 | 0.11; 6.03 | 0.924 | |
| None | 0 | 0.00 | - | 0.995 | 0 | - | - | - | |
| Búzi District (n = 123) | Caia District (n = 95) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | OR | IC | p-Value | Frequency | OR | IC | p-Value | ||
| Gender | Female | 4 (3.3%) | 1.00 | - | Ref | 1 (1.1%) | 1.00 | - | Ref |
| Male | 13 (10.6%) | 1.11 | 0.36; 4.20 | 0.995 | 5 (5.3%) | 0.71 | 0.10; 14.2 | 0.864 | |
| Age group | 5–14 years | 0 | 1.00 | 0.00; Inf | 1.000 | 0 | 0.00 | - | 0.995 |
| 15–29 years | 9 (7.3%) | >100 | - | 0.993 | 3 (3.2%) | 0.39 | 0.04; 8.82 | 0.457 | |
| 30–59 years | 8 (6.5%) | >100 | - | 0.993 | 2 (2.1%) | 0.23 | 0.02; 5.39 | 0.259 | |
| ≥60 years | 0 | 1.00 | - | Ref | 1 (1.1%) | 1.00 | - | Ref | |
| Education | None | 2 (1.6%) | 1.06 | 0.12; 9.73 | 0.954 | 3 (3.2%) | 9.00 | 0.95; 203 | 0.078 |
| Primary | 10 (8.1%) | 1.37 | 0.32; 9.47 | 0.701 | 1 (1.1%) | 0.67 | 0.06; 14.8 | 0.747 | |
| Basic | 2 (1.6%) | - | - | Ref | 2 (2.1%) | 1.00 | - | Ref | |
| Medium | 2 (1.6%) | 1.70 | 0.18; 16.1 | 0.622 | 0 | 0.00 | - | 0.994 | |
| Professional techician | 1 (0.8%) | 8.50 | 0.27; 290 | 0.181 | 0 | 0.00 | - | 0.998 | |
| Higher | 0 | - | - | - | 0 | - | - | 0.998 | |
| Occupation | Domestic worker | 0 | 0.00 | - | 0.993 | 0 | 0.00 | - | 0.997 |
| Student | 2 (1.6%) | 1.38 | 0.17; 8.04 | 0.732 | 0 | 0.00 | - | 0.997 | |
| Education professional | 1 (0.8%) | - | - | 0.997 | 0 | 0.00 | - | 0.997 | |
| Health professional | 2 (1.6%) | 5.00 | 0.59; 45.2 | 0.106 | 0 | - | - | - | |
| Veterinarian | 0 | 0.00 | - | 0.998 | 0 | 0.00 | - | 0.9983 | |
| Slaughterhouse worker | 0 | - | - | - | 0 | 0.00 | - | 0.997 | |
| Cattle owner | 4 (3.3%) | 1.00 | - | Ref | 2 (2.1%) | 1.00 | - | Ref | |
| Cattle herder | 7 (5.7%) | 1.60 | 0.44; 6.59 | 0.482 | 2 (2.1%) | 1.87 | 0.21; 16.4 | 0.544 | |
| Others | 1 (0.8%) | 0.92 | 0.04; 7.23 | 0.941 | 2 (2.1%) | 14.3 | 1.36; 165 | 0.022 | |
| None | 0 | 0.00 | - | 0.998 | 0 | - | - | - | |
| Búzi District (n = 123) | Caia District (n = 95) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | OR | IC | p-Value | Frequency | OR | IC | p-Value | ||
| Gender | Female | 0 | 1.00 | - | Ref | 0 | 1.00 | - | Ref |
| Male | 4 (3.3%) | >100 | - | 0.993 | 5 (5.3%) | >100 | - | 0.993 | |
| Age group | 5–14 years | 1 (0.8%) | 1.67 | 0.06; 47.7 | 0.734 | 1 (1.1%) | >100 | - | 0.994 |
| 15–29 years | 2 (1.6%) | 0.49 | 0.04; 11.1 | 0.573 | 1 (1.1%) | >100 | - | 0.996 | |
| 30–59 years | 0 | 0.00 | - | 0.994 | 3 (3.2%) | >100 | - | 0.995 | |
| ≥60 years | 1 (0.8%) | 1.00 | - | Ref | 0 | 1.00 | - | Ref | |
| Education | None | 1 (0.8%) | >100 | - | 0.997 | 0 | 1.00 | - | 1.000 |
| Primary | 3 (2.4%) | >100 | - | 0.997 | 0 | >100 | - | 0.996 | |
| Basic | 0 | - | - | Ref | 5 (5.3%) | - | - | Ref | |
| Medium | 0 | 1.00 | - | 1.000 | 0 | 1.00 | - | 1.000 | |
| Professional technician | 0 | 1.00 | 0.00; Inf | 1.000 | 0 | 1.00 | - | 1.000 | |
| Higher | 0 | - | - | - | 0 | 1.00 | - | 1.000 | |
| Occupation | Domestic worker | 0 | 0.00 | - | 0.997 | 0 | 0.00 | - | 0.997 |
| Student | 0 | 0.00 | - | 0.997 | 0 | 0.00 | - | 0.998 | |
| Education professional | 0 | 0.00 | - | 0.999 | 0 | 0.00 | - | 0.998 | |
| Health Professional | 0 | 0.00 | - | 0.998 | 0 | 0.00 | - | - | |
| Veterinarian | 0 | 0.00 | - | 0.999 | 0 | 0.00 | - | 0.998 | |
| Slaughterhouse worker | 0 | - | - | - | 1 (1.1%) | 5.38 | 0.22; 70.4 | 0.200 | |
| Cattle owner | 1 (0.8%) | - | - | Ref | 2 (2.1%) | - | - | Ref | |
| Cattle herder | 3 (2.4%) | 2.58 | 0.31; 53.7 | 0.421 | 2 (2.1%) | 1.95 | 0.22; 17.2 | 0.517 | |
| Others | 0 | 0.00 | - | 0.997 | 0 | 0.00 | - | 0.997 | |
| None | 0 | 0.00 | - | 0.999 | 0 | - | - | - | |

References
- Zoonoses. Available online: https://www.who.int/news-room/fact-sheets/detail/zoonoses (accessed on 10 July 2021).
- Zoonotic Diseases. Available online: http://www.emro.who.int/fr/about-who/rc61/zoonotic-diseases.html (accessed on 10 July 2021).
- Woolhouse, M.E.; Gowtage-Sequeria, S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005, 11, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Gebreyes, W.A.; Dupouy-Camet, J.; Newport, M.J.; Oliveira, C.J.; Schlesinger, L.S.; Saif, Y.M.; Kariuki, S.; Saif, L.J.; Saville, W.; Wittum, T.; et al. The global One Health paradigm: Challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Neglected Trop. Dis. 2014, 8, e3257. [Google Scholar] [CrossRef] [PubMed]
- Brucellosis. Available online: https://www.who.int/news-room/fact-sheets/detail/brucellosis (accessed on 23 August 2021).
- Workalemahu, B.; Sewunet, T.; Astatkie, A. Seroepidemiology of Human Brucellosis among Blood Donors in Southern Ethiopia: Calling Attention to a Neglected Zoonotic Disease. Am. J. Trop. Med. Hyg. 2017, 96, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.; Inlameia, O.; Michel, A.; Maxlhuza, G.; Pondja, A.; Fafetine, J.; Macucule, B.; Zacarias, M.; Manguele, J.; Moiane, I.; et al. Bovine tuberculosis and brucellosis in cattle and African buffalo in the Limpopo National Park, Mozambique. Transbound. Emerg. Dis. 2015, 62, 632–638. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control; World Health Organization: Geneva, Switzerland, 2003; Available online: https://apps.who.int/iris/handle/10665/42667 (accessed on 20 July 2021).
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Neglected Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef]
- Bharti, A.R.; Nally, J.E.; Ricaldi, J.N.; Matthias, M.A.; Diaz, M.M.; Lovett, M.A.; Levett, P.N.; Gilman, R.H.; Willig, M.R.; Gotuzzo, E.; et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Hartskeerl, R.A.; Collares-Pereira, M.; Ellis, W.A. Emergence, control, and re-emerging leptospirosis: Dynamics of infection in the changing world. Clin. Microbiol. Infect. 2011, 17, 494–501. [Google Scholar] [CrossRef]
- Ribeiro, P.; Bhatt, N.; Ali, S.; Monteiro, V.; da Silva, E.; Balassiano, I.T.; Aquino, C.; de Deus, N.; Guiliche, O.; Muianga, A.F.; et al. Seroepidemiology of leptospirosis among febrile patients in a rapidly growing suburban slum and a flood-vulnerable rural district in Mozambique, 2012–2014: Implications for the management of fever. Int. J. Infect. Dis. 2017, 64, 50–57. [Google Scholar] [CrossRef]
- Mugabe, V.A.; Inlamea, O.F.; Ali, S.; Maholela, P.; Melchior, B.; Muianga, A.F.; Oludele, J.; Sumail, A.; Antonio, V.; Monteiro, V.O.; et al. Surveillance for arboviruses and leptospirosis among non-malarial acute febrile illness outpatients in areas affected by Cyclones Idai and Kenneth in Mozambique. Front. Trop. Dis. 2023, 4, 1091545. [Google Scholar] [CrossRef]
- Comia, I.; Madureira, A.C.; Schooley, R.T.; Vieira, M.L.; Noormahomed, E.V. Molecular Detection of Leptospira spp. in Rodents Trapped in the Mozambique Island City, Nampula Province, Mozambique. EC Microbiol. 2018, 14, 813–821. [Google Scholar] [PubMed]
- Nicholson, W.; Paddock, C. Rickettsial Diseases. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/rickettsial-including-spotted-fever-and-typhus-fever-rickettsioses-scrub-typhus-anaplasmosis-and-ehr (accessed on 20 July 2021).
- Perlman, S.J.; Hunter, M.S.; Zchori-Fein, E. The emerging diversity of Rickettsia. Proc. Biol. Sci. 2006, 273, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Magaia, V.; Taviani, E.; Cangi, N.; Neves, L. Molecular detection of Rickettsia africae in Amblyomma ticks collected in cattle from Southern and Central Mozambique. J. Infect. Dev. Ctries. 2020, 14, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Matsimbe, A.M.; Magaia, V.; Sanches, G.S.; Antunes, S.; Domingos, A. Molecular detection of pathogens in ticks infesting cattle in Nampula province, Mozambique. Exp. Appl. Acarol. 2017, 73, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Crimean-Congo Hemorrhagic Fever. 9 May 2014. Available online: http://www.cdc.gov/vhf/crimean-congo/ (accessed on 20 July 2021).
- World Health Organization. Crimean-Congo Haemorrhagic Fever: WHO. January 2013. Available online: http://www.who.int/mediacentre/factsheets/fs208/en/ (accessed on 20 July 2021).
- Muianga, A.F.; Watsonb, R.; Vargheseb, A.; Chongo, I.S.; Alia, S.; Monteiroa, V.; Inalda, F.; Chelene, I.; António, V.; Hewsonb, R.; et al. First serological evidence of Crimean-Congo haemorrhagic fever in febrile patients in Mozambique. Int. J. Infect. Dis. 2017, 62, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, M.; Halpin, K. Emerging and endemic zoonotic diseases: Surveillance and diagnostics. Rev. Sci. Tech. 2021, 40, 119–129. [Google Scholar] [PubMed]
- IFAD. Mozambique. Available online: https://www.ifad.org/en/web/operations/w/country/mozambique (accessed on 20 July 2021).
- Instituto Nacional de Estatistical. Available online: http://www.ine.gov.mz (accessed on 20 July 2021).
- Daniel, W.W.; Cross, C.L. Biostatistics: A Foundation for Analysis in the Health Sciences; Wiley: Hoboken, NJ, USA, 2013; ISBN 1118302796/9781118302798. [Google Scholar]
- Mendonça, J.P.; Nhavoto, V.M.; Escrivão, R.J.A. Prevalence of Brucellosis in 2 Slaughterhouses of Maputo City and Province (Mozambique) in 2015. Acta Sci. Nutr. Health 2020, 4, 2–7. [Google Scholar]
- Simpson, G.J.G.; Quan, V.; Frean, J.; Knobel, D.L.; Rossouw, J.; Weyer, J.; Marcotty, T.; Godfroid, J.; Blumberg, L.H. Prevalence of Selected Zoonotic Diseases and Risk Factors at a Human-Wildlife-Livestock Interface in Mpumalanga Province, South Africa. Vector-Borne Zoonotic Dis. 2018, 18, 303–310. [Google Scholar] [CrossRef]
- Omer, M.K.; Assefaw, T.; Skjerve, E.; Tekleghiorghis, T.; Woldehiwet, Z. Prevalence of Antibodies to Brucella spp. and Risk Factors Related to High-Risk Occupational Groups in Eritrea. Epidemiol. Infect. 2002, 129, 85–91. [Google Scholar] [CrossRef]
- Schelling, E.; Diguimbaye, C.; Daoud, S.; Nicolet, J.; Boerlin, P.; Tanner, M.; Zinsstag, J. Brucellosis, Q-fever seroprevalences of nomadic pastoralists and their livestock in Chad. Prev. Vet. Med. 2003, 61, 279–293. [Google Scholar] [CrossRef]
- Bouley, A.J.; Biggs, H.M.; Stoddard, R.A.; Morrissey, A.B.; Bartlett, J.A.; Afwamba, I.A.; Maro, V.P.; Kinabo, G.D.; Saganda, W.; Cleaveland, S.; et al. Brucellosis among Hospitalized Febrile Patients in Northern Tanzania. Am. J. Trop. Med. Hyg. 2012, 87, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Migisha, R.; Nyehangane, D.; Boum, Y.; Page, A.-L.; Zúñiga-Ripa, A.; Conde-Álvarez, R.; Bagenda, F.; Bonnet, M. Prevalence and risk factors of brucellosis among febrile patients attending a community hospital in southwestern Uganda. Sci. Rep. 2018, 8, 15465. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, A.; Dyal, J.W.; Pearson, R.; Kankya, C.; Kajura, C.; Alinaitwe, L.; Pelican, S.K.K.M.; Travis, D.A.; Mahero, M.; Boulware, D.R.; et al. Leptospira Seroprevalence and Risk Factors in Health Centre Patients in Hoima District, Western Uganda. PLoS Neglected Trop. Dis. 2016, 10, e0004858. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.K.; Asenga, L.F.; Matemba, L.; Misinzo, G.; Kazwala, R. Seroprevalence of leptospira infection from agro pastoralist communities in Katavi ecosystem, Tanzania. Int. J. Infect. Dis. 2014, 21, 180. [Google Scholar] [CrossRef][Green Version]
- Collares-Pereira, M.; Gomes, A.C.; Prassad, M.; Vaz, R.G.; Ferrinho, P.; Stanek, G.; Rosario, V.E. Preliminary survey of leptospirosis and Lyme disease amongst febrile patients attending community hospital ambulatory care in Maputo, Mozambique. Cent. Afr. J. Med. 1997, 43, 234–238. [Google Scholar]
- Xu, G.; Walker, D.H.; Jupiter, D.; Melby, P.C.; Arcari, C.M. A review of the global epidemiology of scrub typhus. PLoS Neglected Trop. Dis. 2017, 11, e0006062. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Richards, A.L. Scrub Typhus: No Longer Restricted to the Tsutsugamushi Triangle. Trop. Med. Infect. Dis. 2018, 3, 11. [Google Scholar] [CrossRef]
- Dill, T.; Dobler, G.; Saathoff, E.; Clowes, P.; Kroidl, I.; Ntinginya, E.; Machibya, H.; Maboko, L.; Löscher, T.; Hoelscher, M.; et al. High Seroprevalence for Typhus Group Rickettsiae, Southwestern Tanzania. Emerg. Infect. Dis. 2013, 19, 317–320. [Google Scholar] [CrossRef]
- Barradas, P.F.; Neto, Z.; Teodoro, A.C.; Duarte, L.; Gonçalves, H.; Gartner, F.; Sousa, R.; Amorim, I. Serological Evidence of Rickettsia Exposure among Patients with Unknown Fever Origin in Angola, 2016–2017. Interdiscip. Perspect. Infect. Dis. 2020, 2020, 4905783. [Google Scholar] [CrossRef]
- Lwande, O.W.; Irura, Z.; Tigoi, C.; Chepkorir, E.; Orindi, B.; Musila, L.; Venter, M.; Fischer, A.; Sang, R. Seroprevalence of Crimean Congo Hemorrhagic in Ijara District, Kenya. Vector-Borne Zoonotic Dis. 2012, 12, 727–732. [Google Scholar] [CrossRef]
| Variables | Búzi District (N = 123) | Caia District (N = 95) | |||
|---|---|---|---|---|---|
| Frequency | % | Frequency | % | ||
| Gender | Female | 31 | 25.2 | 12 | 12.6 |
| Male | 92 | 74.7 | 83 | 87.3 | |
| Age | 5–14 years | 7 | 5.6 | 2 | 2.1 |
| 15–29 years | 49 | 39.8 | 41 | 43.1 | |
| 30–59 years | 56 | 45.5 | 46 | 48.4 | |
| ≥60 years | 11 | 8.9 | 6 | 6.3 | |
| Education status | None | 18 | 14.6 | 9 | 9.4 |
| Primary | 72 | 58.5 | 56 | 58.9 | |
| Basic | 19 | 15.4 | 19 | 20 | |
| Medium | 12 | 9.7 | 9 | 9.4 | |
| Professional technician | 2 | 1.6 | 1 | 1 | |
| Higher | 0 | 0.0 | 1 | 1 | |
| Occupation | Domestic worker | 11 | 8.9 | 5.0 | 5.2 |
| Student | 14 | 11.3 | 4.0 | 4.2 | |
| Education professional | 1 | 0.8 | 4.0 | 4.2 | |
| Health professional | 5 | 4.0 | 0.0 | 0.0 | |
| Veterinarian | 1 | 0.8 | 2.0 | 2.1 | |
| Slaughterhouse worker | 0 | 0.0 | 5.0 | 5.2 | |
| Cattle owner | 37 | 30.0 | 45.0 | 47.3 | |
| Cattle herder | 43 | 34.9 | 25.0 | 26.3 | |
| Others | 10 | 8.1 | 5.0 | 5.3 | |
| None | 1 | 0.8 | 0.0 | 0.0 | |
| Búzi (n = 123) | Caia (n = 95) | |||
|---|---|---|---|---|
| N | % | N | % | |
| Brucellosis | 3 | 2.4 | 2 | 2.1 |
| Leptospirosis | 24 | 19.5 | 43 | 45.3 |
| Rickettsiosis | 17 | 13.8 | 6 | 6.3 |
| Crimean-Congo Hemorrhagic Fever | 4 | 3.3 | 5 | 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oludele, J.; Alho, P.; Chongo, I.; Maholela, P.; Magaia, V.; Muianga, A.; Melchior, B.; Isaías, T.; Gatambire, A.; Zimba, E.; et al. Emerging Zoonotic Diseases among Pastoral Communities of Caia and Búzi Districts, Sofala, Mozambique: Evidence of Antibodies against Brucella, Leptospira, Rickettsia, and Crimean-Congo Hemorrhagic Fever Virus. Viruses 2023, 15, 2379. https://doi.org/10.3390/v15122379
Oludele J, Alho P, Chongo I, Maholela P, Magaia V, Muianga A, Melchior B, Isaías T, Gatambire A, Zimba E, et al. Emerging Zoonotic Diseases among Pastoral Communities of Caia and Búzi Districts, Sofala, Mozambique: Evidence of Antibodies against Brucella, Leptospira, Rickettsia, and Crimean-Congo Hemorrhagic Fever Virus. Viruses. 2023; 15(12):2379. https://doi.org/10.3390/v15122379
Chicago/Turabian StyleOludele, John, Pascoal Alho, Inocêncio Chongo, Plácida Maholela, Vlademiro Magaia, Argentina Muianga, Bibiana Melchior, Telma Isaías, Aline Gatambire, Edna Zimba, and et al. 2023. "Emerging Zoonotic Diseases among Pastoral Communities of Caia and Búzi Districts, Sofala, Mozambique: Evidence of Antibodies against Brucella, Leptospira, Rickettsia, and Crimean-Congo Hemorrhagic Fever Virus" Viruses 15, no. 12: 2379. https://doi.org/10.3390/v15122379
APA StyleOludele, J., Alho, P., Chongo, I., Maholela, P., Magaia, V., Muianga, A., Melchior, B., Isaías, T., Gatambire, A., Zimba, E., Nhavoto, E., Notiço, P., Inguana, P., Cantoria, J., António, V., Monteiro, V., Ali, S., Inlamea, O., & Samo Gudo, E. (2023). Emerging Zoonotic Diseases among Pastoral Communities of Caia and Búzi Districts, Sofala, Mozambique: Evidence of Antibodies against Brucella, Leptospira, Rickettsia, and Crimean-Congo Hemorrhagic Fever Virus. Viruses, 15(12), 2379. https://doi.org/10.3390/v15122379






