326K at E Protein Is Critical for Mammalian Adaption of TMUV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Viral Infection
2.3. Sequence Analysis
2.4. Generation of Mutant Viruses
2.5. Virus Titration
2.6. TaqMan Probe-Based Quantitative Real-Time RT-PCR (qRT-PCR)
2.7. Measurement of Proinflammatory Cytokines and IFN-α/β Level
2.8. Mouse Experiments
2.8.1. Neuroinvasiveness Assays
2.8.2. Olfactory Epithelium Assays
2.8.3. Determination of Key Amino Acid Responsible for the Virulence of TMUV HB
2.9. Statistical Analysis
3. Results
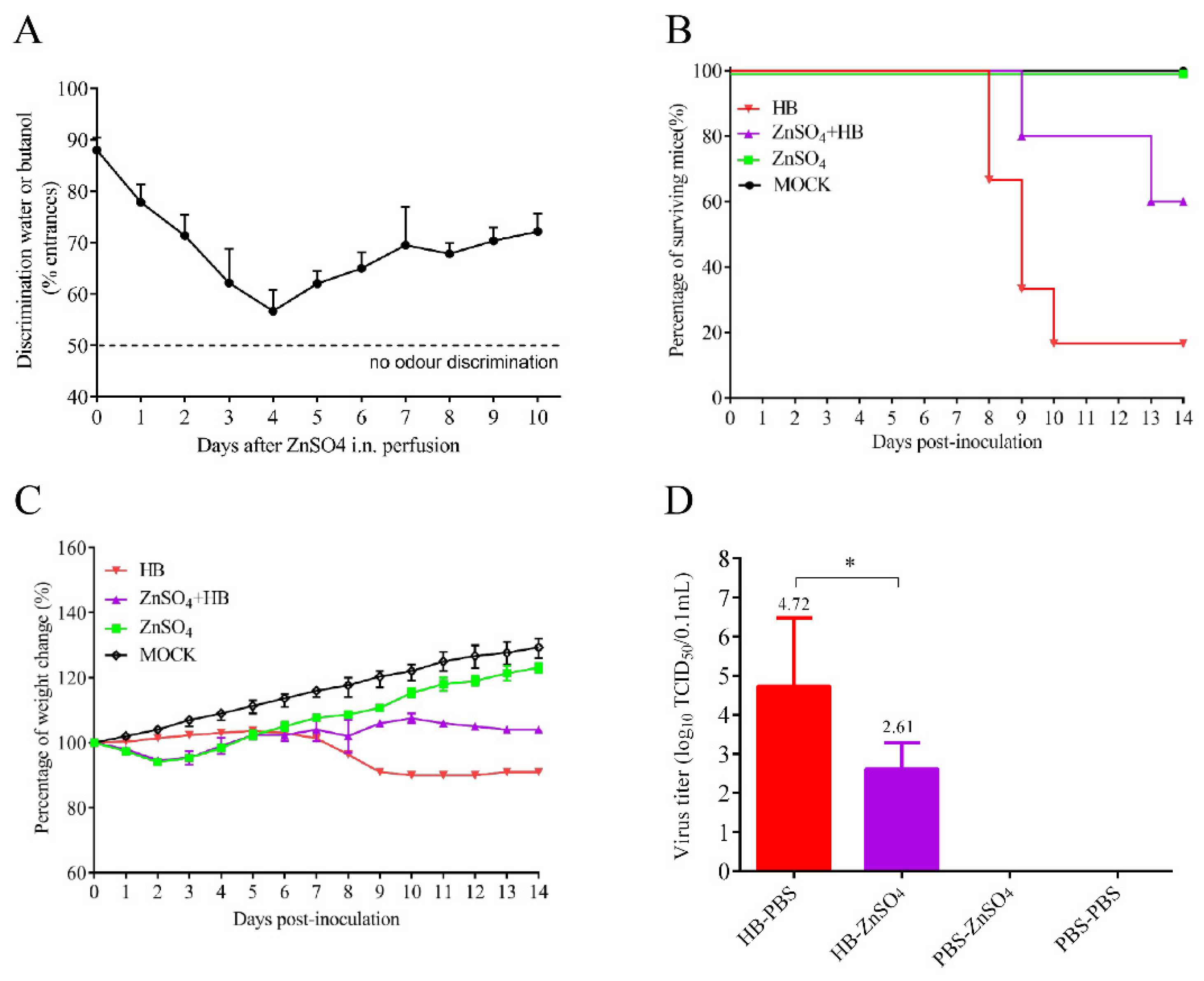
3.1. A TMUV HB Strain Is Neuroinvasive to Mice through Intranasal Infection
3.2. Olfactory Neurons Are the Bridge for TMUV HB to Invade the Mouse CNS
3.3. Both E 326K and NS3 519T Residues Contribute to the Neuroinvasiveness of TMUV HB
3.4. The 326K Residue of E Protein Plays a Major Role in the Neurovirulence of TMUV
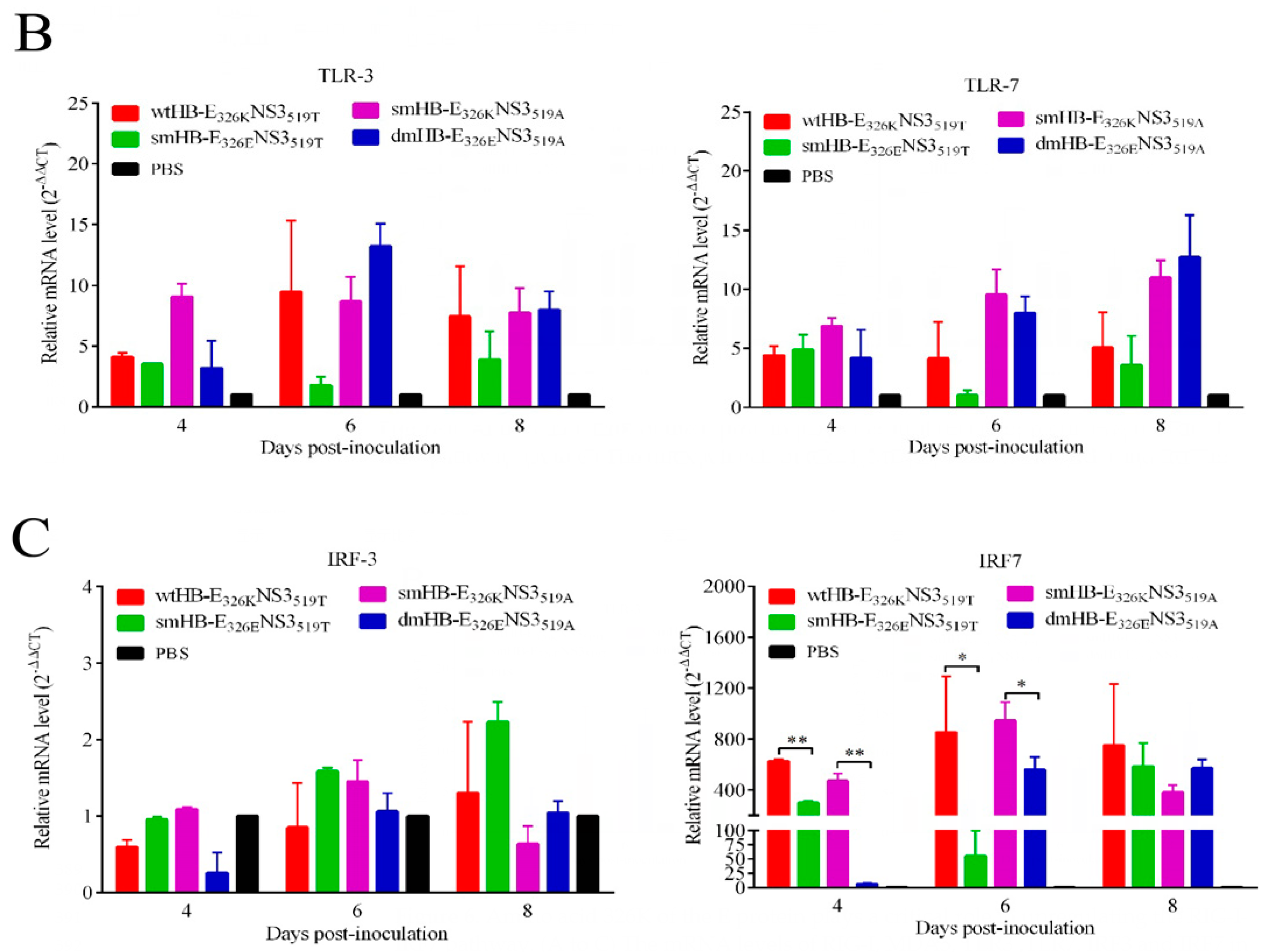
3.5. The TMUV HB Strain Induced Higher Levels of Inflammatory Response Than the Mutant HB with K326E Mutation in Mouse Brain
3.6. TMUV HB Significantly Upregulated the RIG-I-IRF7 Signaling Pathway in Mouse Brains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webster, L.T. Japanese B Encephalitis Virus: Its Differentiation from St. Louis Encephalitis Virus and Relationship to Louping-Ill Virus. Science 1937, 86, 402–403. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.; Phanitchat, T.; Wichit, S.; Morales Vargas, R.E.; Jaroenpool, J.; Diagne, C.T.; Pompon, J.; Misse, D. New Insights into the Biology of the Emerging Tembusu Virus. Pathogens 2021, 10, 1010. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the Flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Hall, R.A.; Lobigs, M. Common E protein determinants for attenuation of glycosaminoglycan-binding variants of Japanese encephalitis and West Nile viruses. J. Virol. 2004, 78, 8271–8280. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Zhou, T.F.; Lai, Z.T.; Zhang, Z.H.; Jia, Z.R.; Zhou, G.F.; Williams, T.; Xu, J.B.; Gu, J.B.; Zhou, X.H.; et al. Competence of Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus Mosquitoes as Zika Virus Vectors, China. Emerg. Infect. Dis. 2017, 23, 1085–1091. [Google Scholar] [CrossRef]
- Zhang, S.L.; Li, L.; Woodson, S.E.; Huang, C.Y.H.; Kinney, R.M.; Barrett, A.D.T.; Beasley, D.W.C. A mutation in the envelope protein fusion loop attenuates mouse neuroinvasiveness of the NY99 strain of West Nile virus. Virology 2006, 353, 35–40. [Google Scholar] [CrossRef]
- Platt, G.S.; Way, H.J.; Bowen, E.T.; Simpson, D.I.; Hill, M.N.; Kamath, S.; Bendell, P.J.; Heathcote, O.H. Arbovirus infections in Sarawak, October 1968–February 1970 Tembusu and Sindbis virus isolations from mosquitoes. Ann. Trop. Med. Parasitol. 1975, 69, 65–71. [Google Scholar] [CrossRef]
- Thontiravong, A.; Ninvilai, P.; Tunterak, W.; Nonthabenjawan, N.; Chaiyavong, S.; Angkabkingkaew, K.; Mungkundar, C.; Phuengpho, W.; Oraveerakul, K.; Amonsin, A. Tembusu-Related Flavivirus in Ducks, Thailand. Emerg. Infect. Dis. 2015, 21, 2164–2167. [Google Scholar] [CrossRef]
- Tang, Y.; Diao, Y.; Yu, C.; Gao, X.; Ju, X.; Xue, C.; Liu, X.; Ge, P.; Qu, J.; Zhang, D. Characterization of a Tembusu virus isolated from naturally infected house sparrows (Passer domesticus) in Northern China. Transbound Emerg. Dis. 2013, 60, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Gao, X.; Diao, Y.; Feng, Q.; Chen, H.; Liu, X.; Ge, P.; Yu, C. Tembusu virus in human, China. Transbound Emerg. Dis. 2013, 60, 193–196. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S.; Li, Z.; Lin, F.; Cheng, X.; Zhu, X.; Wang, J.; Chen, S.; Huang, M.; Zheng, M. Isolation and characterization of a Chinese strain of Tembusu virus from Hy-Line Brown layers with acute egg-drop syndrome in Fujian, China. Arch. Virol. 2014, 159, 1099–1107. [Google Scholar] [CrossRef]
- Liu, M.; Chen, S.; Chen, Y.; Liu, C.; Chen, S.; Yin, X.; Li, G.; Zhang, Y. Adapted Tembusu-like virus in chickens and geese in China. J. Clin. Microbiol. 2012, 50, 2807–2809. [Google Scholar] [CrossRef] [PubMed]
- Ruangrung, K.; Chakritbudsabong, W.; Rungarunlert, S.; Smith, D.R.; Hongeng, S.; Sirinonthanawech, N.; Boonarkart, C.; Pulmanausahakul, R.; Suptawiwat, O.; Auewarakul, P. Analysis of Tembusu virus infection of human cell lines and human induced pluripotent stem cell derived hepatocytes. Virus Res. 2021, 292, 198252. [Google Scholar] [CrossRef] [PubMed]
- Pulmanausahakul, R.; Ketsuwan, K.; Jaimipuk, T.; Smith, D.R.; Auewarakul, P.; Songserm, T. Detection of antibodies to duck tembusu virus in human population with or without the history of contact with ducks. Transbound Emerg. Dis. 2022, 69, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, Y.; Liu, Q.; Wang, Y.; Li, G.; Teng, Q.; Zhang, Y.; Liu, S.; Li, Z. Airborne Transmission of a Novel Tembusu Virus in Ducks. J. Clin. Microbiol. 2015, 53, 2734–2736. [Google Scholar] [CrossRef]
- Yang, L.X.; Liang, T.; Lv, J.F.; Qu, S.H.; Meng, R.Z.; Yang, B.L.; Feng, C.L.; Dai, W.Q.; Wang, X.Y.; Zhang, B.; et al. Substantial Attenuation of Virulence of Tembusu Virus Strain PS Is Determined by an Arginine at Residue 304 of the Envelope Protein. J. Virol. 2021, 95, 10–128. [Google Scholar] [CrossRef]
- Wang, H.J.; Li, X.F.; Liu, L.; Xu, Y.P.; Ye, Q.; Deng, Y.Q.; Huang, X.Y.; Zhao, H.; Qin, E.D.; Shi, P.Y.; et al. The Emerging Duck Flavivirus Is Not Pathogenic for Primates and Is Highly Sensitive to Mammalian Interferon Antiviral Signaling. J. Virol. 2016, 90, 6538–6548. [Google Scholar] [CrossRef]
- Sun, M.X.; Zhang, L.J.; Cao, Y.X.; Wang, J.; Yu, Z.D.; Sun, X.; Liu, F.L.; Li, Z.L.; Liu, P.H.; Su, J.L. Basic Amino Acid Substitution at Residue 367 of the Envelope Protein of Tembusu Virus Plays a Critical Role in Pathogenesis. J. Virol. 2020, 94, 10–128. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.; Shi, Y.; Bai, X.; Yang, P.; Chen, Q. Tembusu Virus entering the central nervous system caused nonsuppurative encephalitis without disrupting the blood-brain barrier. J. Virol. 2021, 95, 10–128. [Google Scholar] [CrossRef]
- Yurayart, N.; Ninvilai, P.; Chareonviriyaphap, T.; Kaewamatawong, T.; Thontiravong, A.; Tiawsirisup, S. Pathogenesis of Thai duck Tembusu virus in BALB/c mice: Descending infection and neuroinvasive virulence. Transbound Emerg. Dis. 2021, 68, 3529–3540. [Google Scholar] [CrossRef]
- Ti, J.; Zhang, M.; Li, Z.; Li, X.; Diao, Y. Duck Tembusu Virus Exhibits Pathogenicity to Kunming Mice by Intracerebral Inoculation. Front. Microbiol. 2016, 7, 190. [Google Scholar] [CrossRef]
- Yan, D.W.; Shi, Y.; Wang, H.W.; Li, G.X.; Li, X.S.; Wang, B.B.; Su, X.; Wang, J.H.; Teng, Q.Y.; Yang, J.M.; et al. A Single Mutation at Position 156 in the Envelope Protein of Tembusu Virus Is Responsible for Virus Tissue Tropism and Transmissibility in Ducks. J. Virol. 2018, 92, 10–128. [Google Scholar] [CrossRef]
- Wu, X.; Shi, Y.; Yan, D.; Li, X.; Yan, P.; Gao, X.; Zhang, Y.; Yu, L.; Ren, C.; Li, G.; et al. Development of a PCR-Based Reverse Genetics System for an Attenuated Duck Tembusu Virus Strain. PLoS ONE 2016, 11, e0156579. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.P.; Peng, S.; Yan, P.X.; Zhou, J.W.; Teng, Q.Y.; Li, G.X.; Li, X.S.; Li, Z.J. Comparison of real-time reverse transcription loop-mediated isothermal amplification and real-time reverse transcription polymerase chain reaction for duck Tembusu virus. J. Virol. Methods 2012, 182, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ducray, A.; Bondier, J.R.; Michel, G.; Bon, K.; Millot, J.L.; Propper, A.; Kastner, A. Recovery following peripheral destruction of olfactory neurons in young and adult mice. Eur. J. Neurosci. 2002, 15, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Deiss, V.; Baudoin, C. Hyposmia for butanol and vanillin in mutant staggerer male mice. Physiol. Behav. 1997, 61, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus infections in the nervous system. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef]
- Burd, G.D. Morphological study of the effects of intranasal zinc sulfate irrigation on the mouse olfactory epithelium and olfactory bulb. Microsc. Res. Tech. 1993, 24, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Lin, Y.; Tang, Y.; Diao, Y. Evolution of Tembusu Virus in Ducks, Chickens, Geese, Sparrows, and Mosquitoes in Northern China. Viruses 2018, 10, 485. [Google Scholar] [CrossRef]
- Swanson, P.A., 2nd; McGavern, D.B. Viral diseases of the central nervous system. Curr. Opin. Virol. 2015, 11, 44–54. [Google Scholar] [CrossRef]
- Beers, D.R.; Henkel, J.S.; Schaefer, D.C.; Rose, J.W.; Stroop, W.G. Neuropathology of herpes simplex virus encephalitis in a rat seizure model. J. Neuropathol. Exp. Neurol. 1993, 52, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Cropp, C.B.; Harrison, A.K. Mode of entry of a neurotropic arbovirus into the central nervous system. Reinvestigation of an old controversy. Lab. Investig. 1983, 48, 399–410. [Google Scholar] [PubMed]
- Ni, H.; Barrett, A.D. Attenuation of Japanese encephalitis virus by selection of its mouse brain membrane receptor preparation escape variants. Virology 1998, 241, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.; Crotta, S.; McCabe, T.M.; Wack, A. Pathogenic potential of interferon alphabeta in acute influenza infection. Nat. Commun. 2014, 5, 3864. [Google Scholar] [CrossRef]
- Davidson, S.; Maini, M.K.; Wack, A. Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. J. Interferon. Cytokine Res. 2015, 35, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef]
- Wu, M.; Skaug, B.; Bi, X.; Mills, T.; Salazar, G.; Zhou, X.; Reveille, J.; Agarwal, S.K.; Blackburn, M.R.; Mayes, M.D.; et al. Interferon regulatory factor 7 (IRF7) represents a link between inflammation and fibrosis in the pathogenesis of systemic sclerosis. Ann. Rheum. Dis. 2019, 78, 1583–1591. [Google Scholar] [CrossRef]
- Yasuda, K.; Richez, C.; Maciaszek, J.W.; Agrawal, N.; Akira, S.; Marshak-Rothstein, A.; Rifkin, I.R. Murine dendritic cell type I IFN production induced by human IgG-RNA immune complexes is IFN regulatory factor (IRF)5 and IRF7 dependent and is required for IL-6 production. J. Immunol. 2007, 178, 6876–6885. [Google Scholar] [CrossRef]
- Ning, S.; Pagano, J.S.; Barber, G.N. IRF7: Activation, regulation, modification and function. Genes Immun. 2011, 12, 399–414. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yan, D.; Peng, S.; Zhang, Y.; Xu, B.; Li, L.; Shi, X.; Ma, T.; Li, X.; Teng, Q.; et al. 326K at E Protein Is Critical for Mammalian Adaption of TMUV. Viruses 2023, 15, 2376. https://doi.org/10.3390/v15122376
Liu X, Yan D, Peng S, Zhang Y, Xu B, Li L, Shi X, Ma T, Li X, Teng Q, et al. 326K at E Protein Is Critical for Mammalian Adaption of TMUV. Viruses. 2023; 15(12):2376. https://doi.org/10.3390/v15122376
Chicago/Turabian StyleLiu, Xingpo, Dawei Yan, Shan Peng, Yuee Zhang, Bangfeng Xu, Luzhao Li, Xiaona Shi, Tianxin Ma, Xuesong Li, Qiaoyang Teng, and et al. 2023. "326K at E Protein Is Critical for Mammalian Adaption of TMUV" Viruses 15, no. 12: 2376. https://doi.org/10.3390/v15122376
APA StyleLiu, X., Yan, D., Peng, S., Zhang, Y., Xu, B., Li, L., Shi, X., Ma, T., Li, X., Teng, Q., Yuan, C., Liu, Q., & Li, Z. (2023). 326K at E Protein Is Critical for Mammalian Adaption of TMUV. Viruses, 15(12), 2376. https://doi.org/10.3390/v15122376






