First Report of Low Pathogenic Avian Influenza Subtype H9N2 in African Houbara Bustards (Chlamydotis undulata undulata) and Gamebirds in Morocco: Clinico-Pathological Findings, Molecular Characterization, and Associated Coinfections
Abstract
1. Introduction
2. Materials and Methods
2.1. Bird Species and Specimen Collection
2.2. Sample Processing
2.2.1. Pathological Examination
2.2.2. RNA Extraction and Real-Time RT-PCR
2.2.3. Virus Isolation
2.2.4. HA Gene Sequencing
2.2.5. Phylogenetic Analysis
3. Results
3.1. Case History and Pathological Findings
3.1.1. Case History and Seasonal Distribution of Outbreaks
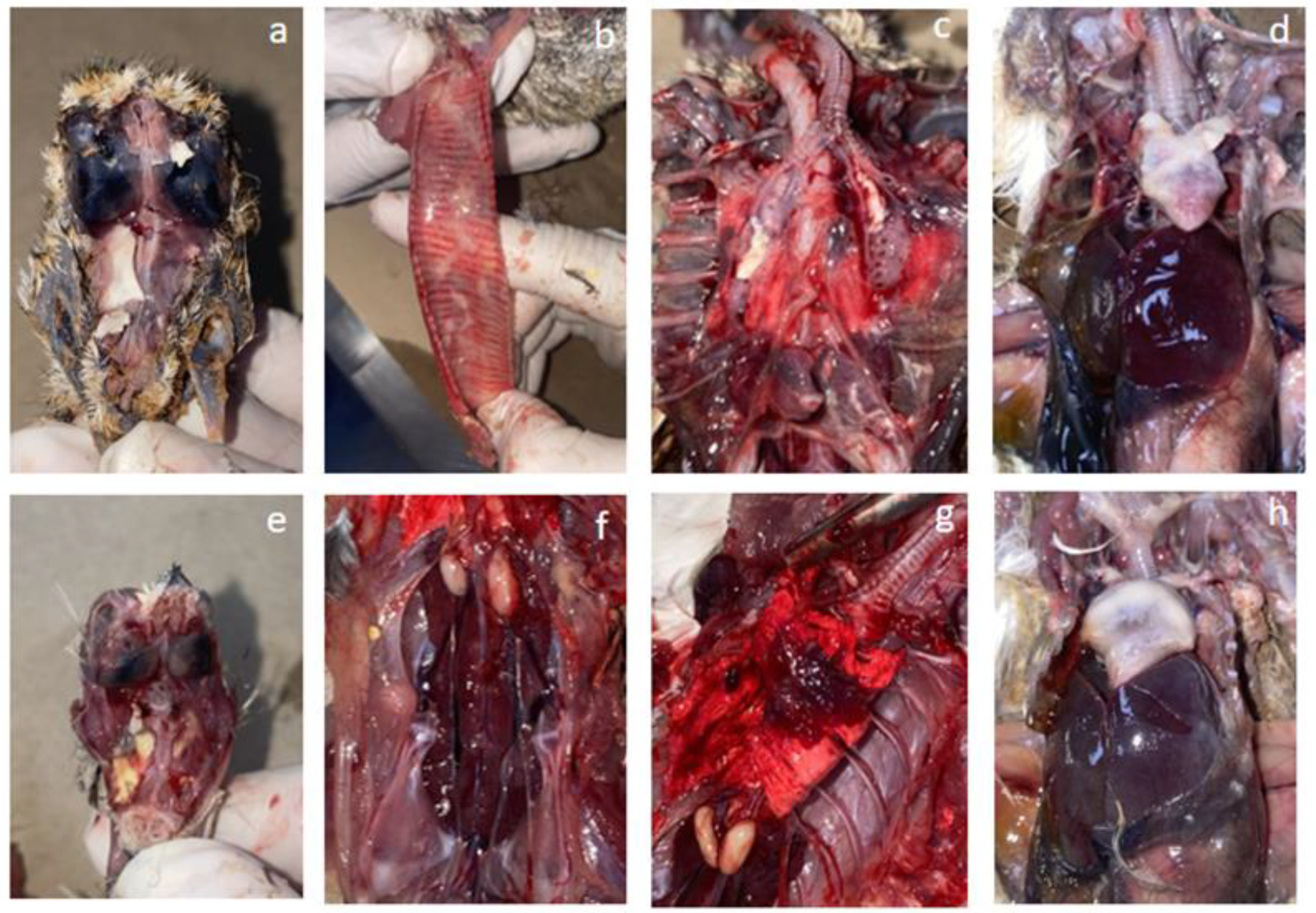
3.1.2. Gross Pathological Findings
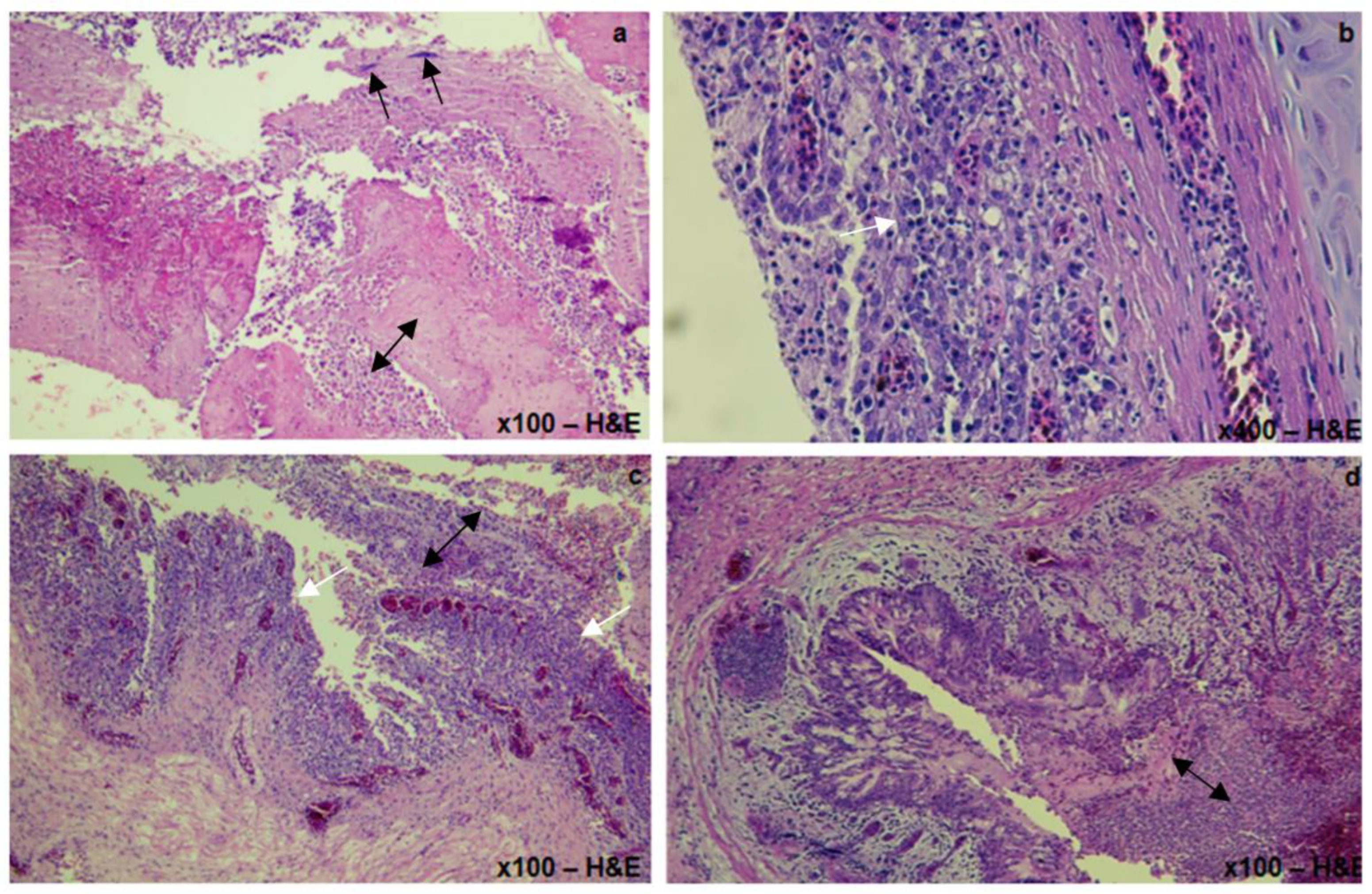
3.1.3. Histopathological Lesions
3.2. H9N2 Identification and Phylogenetic Analysis
3.2.1. H9 Detection by Real Time RT-PCR
3.2.2. Virus Isolation and the Partial HA Gene Amplification
3.2.3. BLAST Search and Phylogenetic Analyses
3.3. Differential Bacteriological and Molecular Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Cases | Bird Species | Bird Population | Age (d/w) | H9 Vaccinationstatus (V/NV) | RT-PCR H9 (Ct) | Clinical Signs | Mortality Rate (%) | Pathological Lesions | Coinfecting Agents |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Quail | ND | ND | NV | 38.34 | Mild rales | None | conjoncitivitis | Negative |
| 2 | Pheasant | ND | ND | NV | 37.47 | Rales, Head swelling | None | Fibrinous sinusitis; catarrhal enteritis | Mycoplasma Gallisepticum |
| 3 | Houbarabustard | ND | 8 w | NV | 33.97 | Coughing, tracheal rales, respiratory distress, anorexia, | NA | Purulent arthritis; Airsacculitis; fibrinous pericarditis; hepatomegaly; splenomegaly; catarrhal enteritis; kidney congestion and hypertrophy; congested lungs | E.Coli Enterococcussp |
| 4 | Partridge | 23,000 | 3 w | V | 32.72 | Tracheal rales, diarrhea, lethargy | 0.33% | Fibrinous sinusitis; fibrinous tracheitis; airsacculitis; kidney hypertrophy; catarrhal enteritis | E.Coli |
| 5 | Houbarabustard | ND | 200 d | NV | 36.69 | Coughing, sneezing, tracheal rales, dyspnea, diarrhea | NA | Purulent sinusitis; fibrinous tracheitis; fibrinous airsacculitis and perihepatitis; enteritis; ulcerative stomatitis | E.Coli Staphylococcus Aureus Pseudomonas sp Candida Alibcans |
| 6 | Houbarabustard | ND | 33 d | NV | 25.89 | Coughing, sneezing, tracheal rales, dyspnea, diarrhea | NA | Intrabronchial fibrin cast; airsacculitis; fibrinous pericarditis and perihepatitis; catarrhal enteritis | Avian Coronavirus E.Coli Enterococcussp |
| 7 | Houbarabustard | ND | 25 d | NV | 37.69 | Coughing, sneezing, tracheal rales, dyspnea, diarrhea, lethargy | NA | airsacculitis; fibrinous pericarditis and perihepatitis; catarrhal enteritis; kidney hypertrophy and congestion | E.Coli Pseudomonas Aerogenosa Enterococcussp |
| 8 | Houbarabustard | ND | 17 d | V | 26.79 | Coughing, sneezing, tracheal rales, dyspnea, diarrhea | NA | Congestive lungs; airsacculitis; fibrinous pericarditis and perihepatitis; catarrhal enteritis; kidney hypertrophy and congestion | E.Coli |
| 9 | Houbarabustard | ND | 36 d | V | 23.54 | Coughing, sneezing, tracheal rales, dyspnea, diarrhea, lethargy | NA | Intrabronchial fibrin cast; fibrinous airsacculitis; catarrhal enteritis Congestive liver | Avian coronavirus E.coli Pseudomonas Aeroginosa |
| 10 | Houbarabustard | ND | 63 d | V | 22.32 | Coughing, sneezing, tracheal rales | NA | Congestive lungs; fibrinous airsacculitis; catarrhal enteritis congestive liver | Avian coronavirus E.Coli |
| 11 | Houbarabustard | ND | 26 d | V | 34.7 | Tracheal rales, coughing | NA | tracheal congestion; lung congestion; liver congestion; thickened air sacs | Negative |
| 12 | Houbarabustard | ND | 33 d | V | 26.27 | Tracheal rales, coughing | NA | Tracheal congestion; lung congestion; liver congestion; thickened air sacs | Negative |
| 13 | Houbarabustard | ND | 43 d | V | 30.31 | Tracheal rales, coughing | NA | Tracheal congestion; lung congestion; liver congestion; thickened air sacs | Negative |
| 14 | Houbarabustard | ND | 71 d | V | 33.1 | Tracheal rales, coughing | NA | Tracheal congestion; lung congestion; liver congestion; thickened air sacs | Negative |
| 15 | Houbarabustard | ND | 76 d | V | 36.94 | Coughing, sneezing, tracheal rales, dyspnea | NA | Fibrrinous sinusitis; lung congestion; fibrinous pericarditis; fibrinous airsacculitis | E.Coli Enterococcussp. |
| 16 | Houbarabustard | ND | 55 d | V | 36.36 | Coughing, sneezing, tracheal rales | NA | Lung congestion; fibrinous pericarditis; fibrinous airsacculitis | E.Coli |
| 17 * | Partridge | 9365 | 32 w | V | 26.19 * | Tracheal rales, diarrhea, cachexia | 0.5% | Fibrinous sinusitis and congestive tracheitis; Intrabronchial fibrin cast; fibrinous pericarditis and perihepatitis; splenomegaly | E.Coli |
| 18 | Houbarabustard | ND | 201 d | V | 35.17 | Dyspnea, diarrhea, anorexia, lethargy | NA | Exsudativetracheitis with intrabronchial fibrin cast; airsacculitis; perihepatitis with hepatomegaly; splenomegaly; catarrhal enteritis; ulcerative oesophagitis, pancreas hypertrophy | Poxvirus MycoplasmaGalliseptcium Candida Albicans |
| 19 | Houbarabustard | ND | 237 d | V | 38.32 | Diarrhea, anorexia, diarrhea, lethargy | NA | Purulent arthritis; aisacculitis; hepatomegaly with perihepatitis; ulcerative esophagitis; catarrhal enteritis; pancreas hypertrophy | Poxvirus E.Coli Staphylococcus sp Candida Albicans |
| 20 | Houbarabustard | ND | 235 d | V | 36.27 | Diarrhea, anorexia | NA | Exsudative tracheitis; fibrinous pericarditis and perihepatitis with hepatomegaly; catarrhal enteritis; pancreas hypertrophy; ulcerative oesophagitis and stomatitis, pancreas hypertrophy | Poxvirus Staphylococcus Aureus Candida Albicans |
| 21 | Houbarabustard | ND | 220 d | V | 37.34 | Diarrhea, anorexia | NA | Airsacculitis; fibrinous pericarditis and perihepatitis with hepatomegaly; catarrhal enteritis; kidneyshy pertrophy and congestion; pancreashy pertrophy | Candida Albicans |
| 22 * | Partridge | 4000 | 105 w | V | 35.23 | Head swelling, tracheal rales, dyspnea, paralysis, overcrowding, diarrhea | 0.5% | Fibrinous sinusitis; fibrinous otitis media and interna; intrabronchial fibrin cast; catarrhal enteritis; kidney hypertrophy and congestion | E.Coli serotype O2K1 Aspergillus Fumigatus |
| 23 | Houbarabustard | ND | 35 w | V | 30.54 | Coughing, tracheal rales, dyspnea, diarrhea, anorexia | NA | Intrabronchial fibrin cast; airsacculitis; fibrinous pericarditis and perihepatitis; enteritis; kidney hypertrophy; ulcerative stomatitis and stomatitis, pancreas hypertrophy | Poxvirus E.Coli |
| 24 | Houbarabustard | ND | 35 w | V | 27.16 | Coughing, sneezing, tracheal rales, dyspnea, diarrhea, anorexia | NA | Intrabronchial fibrin cast; airsacculitis; fibrinous pericarditis and perihepatitis; enteritis; kidney hypertrophy and congestion; ulcerative stomatitis, pancreas hypertrophy | Poxvirus Klebsiellasp |
| 25 | Houbarabustard | 2500 | 15 d | V | 32.1 | Coughing, sneezing, tracheal rales, dyspnea, diarrhea | NA | Fibrinous sinusitis; intrabronchial fibrin cast; airsacculitis; fibrinous pericarditis; liver congestion; enteritis; kidney hypertrophy and congestion | E.Coli Enterococcussp |
| 26 | Houbarabustard | ND | 26 d | V | 33.26 | Coughing, tracheal rales, dyspnea, diarrhea | NA | Fibrinous sinusitis; intrabronchial fibrin cast; fibrinous airsacculitis; enteritis; kidney congestion and hypertrophy | E.Coli |
| 27 | Houbarabustard | ND | 38 d | V | 31.45 | Coughing, tracheal rales, dyspnea, diarrhea | NA | Intrabronchial fibrin cast; liver congestion; enteritis; kidney congestion hypertrophy | E.Coli |
| 28 | Houbarabustard | ND | 23 d | V | 37.19 | Coughing, tracheal rales, dyspnea, diarrhea | NA | Fibrinous sinusitis; intrabronchial fibrin cast; airsacculitis; pericarditis; enteritis; kidney hypertrophy and congestion | E.coli |
| 29 | Partridge | 18,600 | 2 w | V | 33.21 | Dyspnea, rales, diarrhea, lethargy | 0.3% | Fibrinous sinusitis; lung congestion with nodules; fibrinous pericarditis; kidney congestion; enteirits | E. Coli Aspergillus Fumigatus |
| 30 | Quail | ND | ND | V | 33.6 | Respiratory distress | <0.01% | Lung congestion; airsacculitis; liver congestion; kidney congestion and hypertrophy | Mycoplasma Galliseptcium Aspergillus Fumigatus |
References
- Goriup, P.D. The world status of the houbara bustard Chlamydotis undulata. Bird Conserv. Int. 1997, 7, 373–397. [Google Scholar] [CrossRef]
- Bailey, T.A. Diseases and Medical Management of Houbara Bustards and Other Otididae, 1st ed.; Environment Agency: Abu Dhabi, United Arab Emirates, 2008.
- Dolman, P.M.; Scotland, K.M.; Burnside, R.J.; Collar, N.J. Sustainable hunting and the conservation of the threatened houbara bustards. J. Nat. Conserv. 2021, 61, 126000. [Google Scholar] [CrossRef]
- Bailey, T.A.; Nicholls, P.K.; Wernery, U.; Samour, J.; Cooper, J.E.; O’Leary, M.T. Avian paramyxovirus type 1 infection in Houbara bustards (Chlamydotis undulata macqueenii): Clinical and pathologic findings. J. Zoo Wildl. Med. 1997, 28, 325–330. [Google Scholar]
- Bailey, T.A.; Wernery, U.; Gough, R.E.; Manvell, R.; Samour, J.H. Serological survey for avian viruses in houbara bustards (Chlamydotis undulata macqueenii). Vet. Rec. 1996, 139, 238–239. [Google Scholar] [CrossRef]
- Hirschinger, J.; Vergne, T.; Corre, T.; Hingrat, Y.; Guerin, J.L.; Le Loc’h, G. Exposure assessment for avian influenza and Newcastle disease viruses from peridomestic wild birds in a conservation breeding site in the United Arab Emirates. Transbound. Emerg. Dis. 2022, 69, 2361–2372. [Google Scholar] [CrossRef]
- El Houadfi, M.; Fellahi, S.; Nassik, S.; Guérin, J.L.; Ducatez, M.F. First outbreaks and phylogenetic analyses of avian influenza H9N2 viruses isolated from poultry flocks in Morocco. J. Virol. 2016, 13, 1–7. [Google Scholar] [CrossRef]
- Amal, E.B.; Saâdi, N.; Asma, F.; Moncef, B.; Ouafae, F.F. Characterization and phylogenetic analysis of the hemagglutinin gene in H9 influenza viruses from chickens in Morocco from 2017 to 2019. Avian Dis. 2020, 64, 310–314. [Google Scholar] [CrossRef] [PubMed]
- El Mellouli, F.; Mouahid, M.; Fusaro, A.; Zecchin, B.; Zekhnini, H.; El Khantour, A.; Giussani, E.; Palumbo, E.; Rguibi Idrissi, H.; Monne, I.; et al. Spatiotemporal Dynamics, Evolutionary History and Zoonotic Potential of Moroccan H9N2 Avian Influenza Viruses from 2016 to 2021. Viruses 2022, 14, 509. [Google Scholar] [CrossRef]
- Sikht, F.Z.; Ducatez, M.; Touzani, C.D.; Rubrum, A.; Webby, R.; El Houadfi, M.; Tligui, N.; Camus, C.; Fellahi, S. Avian Influenza a H9N2 Viruses in Morocco, 2018–2019. Viruses 2022, 14, 529. [Google Scholar] [CrossRef]
- El Mellouli, F.; Abouchoaib, N.; Zekhnini, H.; Khayli, M.; Fusaro, A.; Idrissi, H.R.; Benhoussa, A. Molecular detection of avian influenza virus in wild birds in Morocco, 2016–2019. Avian Dis. 2022, 66, 29–38. [Google Scholar] [CrossRef]
- Ross, C.S.; Sutton, D.; Skinner, P.; Mahmood, S.; Wynne, F.; Londt, B.; Nunez, A.; Hicks, D.J.; Brookes, S.M.; Banyard, A.C.; et al. Comparative pathogenesis of two genotype VI. 2 avian paramyxovirus type-1 viruses (APMV-1) in pheasants, partridges and chickens. Avian Pathol. 2023, 52, 36–50. [Google Scholar] [CrossRef]
- Bertran, K.; Dolz, R.; Majó, N. Pathobiology of avian influenza virus infection in minor gallinaceous species: A review. Avian Pathol. 2014, 43, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Humberd, J.; Guan, Y.; Webster, R.G. Comparison of the replication of influenza A viruses in Chinese ring-necked pheasants and chukar partridges. J. Virol. 2006, 80, 2151–2161. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier Health Sciences: Edinburgh, UK, 2018. [Google Scholar]
- Monne, I.; Ormelli, S.; Salviato, A.; De Battisti, C.; Bettini, F.; Salomoni, A.; Drago, A.; Zecchin, B.; Capua, I.; Cattoli, G. Development and validation of a one-step real-time PCR assay for simultaneous detection of subtype H5, H7, and H9 avian influenza viruses. J. Clin. Microbiol. 2008, 46, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Fereidouni, S.R.; Starick, E.; Grund, C.; Globig, A.; Mettenleiter, T.C.; Beer, M.; Harder, T. Rapid molecular subtyping by reverse transcription polymerase chain reaction of the neuraminidase gene of avian influenza A viruses. Vet. Microbial. 2009, 135, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Stech, J.; Guan, Y.; Webster, R.G.; Perez, D.R. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 2001, 146, 2275–2289. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Boil. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J. H9N2 influenza virus in China: A cause of concern. Protein Cell 2015, 6, 18–25. [Google Scholar] [CrossRef]
- Kent, J.; Bailey, T.; Silvanose, C.D.; McKeown, S.; Wernery, U.; Kinne, J.; Manvell, R. An outbreak of low pathogenic avian influenza in a mixed-species aviculture unit in Dubai in 2005. Vet. Clin. Exot. Anim. Pract. 2006, 9, 523–531. [Google Scholar] [CrossRef]
- Khan, O.A.; Shuaib, M.A.; Abdel Rhman, S.S.; Ismail, M.M.; Hammad, Y.A.; Abdel Baky, M.H.; Fusaro, A.; Salviato, A.; Cattoli, G. Isolation and identification of highly pathogenic avian influenza H5N1 virus from Houbara bustards (Chlamydotis undulata macqueenii) and contact falcons. Avian Pathol. 2009, 38, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Wernery, U.; Molnar, L.; Manvell, R. Influenza infection in houbara bustards (Chlamydotis undulata macqueenii) in the United Arab Emirates. In Proceedings of the European Association of Avian Veterinarians, Munich, Germany, 6–10 March 2001; pp. 271–276. [Google Scholar]
- Jöstl, N.; Weidinger, P.; Lussy, H.; Bailey, T.A.; Joseph, S.; McKeown, S.; O’Donovan, D.; Li, X.; Nowotny, N. Antibody prevalence to avian influenza virus subtypes H5, H7 and H9 in falcons, captive and wild birds, United Arab Emirates, 2003–2006. Vet. Med. Sci. 2023, 9, 1890–1900. [Google Scholar] [CrossRef]
- Perez, D.R.; Lim, W.; Seiler, J.P.; Yi, G.; Peiris, M.; Shortridge, K.F.; Webster, R.G. Role of quail in the interspecies transmission of H9 influenza A viruses: Molecular changes on HA that correspond to adaptation from ducks to chickens. J. Virol. 2003, 77, 3148–3156. [Google Scholar] [CrossRef]
- El-Zoghby, E.F.; Arafa, A.S.; Hassan, M.K.; Aly, M.M.; Selim, A.; Kilany, W.H.; Hafez, H.M. Isolation of H9N2 avian influenza virus from bobwhite quail (Colinus virginianus) in Egypt. Arch. Virol. 2012, 157, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Świętoń, E.; Tarasiuk, K.; Olszewska-Tomczyk, M.; Iwan, E.; Śmietanka, K. A turkey-origin H9N2 avian influenza virus shows low pathogenicity but different within-host diversity in experimentally infected turkeys, quail and ducks. Viruses 2020, 12, 319. [Google Scholar] [CrossRef]
- Nili, H.; Asasi, K.; Dadras, H.; Ebrahimi, M. Pathobiology of H9N2 avian influenza virus in Japanese quail (Coturnix coturnix japonica). Avian Dis. 2007, 51, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Mettenleiter, T.C.; Abdelwhab, E.M. A brief summary of the epidemiology and genetic relatedness of avian influenza H9N2 virus in birds and mammals in the Middle East and North Africa. Epidemiol. Infect. 2017, 145, 3320–3333. [Google Scholar] [CrossRef]
- Aamir, U.B.; Wernery, U.; Ilyushina, N.; Webster, R.G. Characterization of avian H9N2 influenza viruses from United Arab Emirates 2000 to 2003. Virology 2007, 361, 45–55. [Google Scholar] [CrossRef]
- Yu, J.E.; Yoon, H.; Lee, H.J.; Lee, J.H.; Chang, B.J.; Song, C.S.; Nahm, S.S. Expression patterns of influenza virus receptors in the respiratory tracts of four species of poultry. J. Vet. Sci. 2011, 12, 7–13. [Google Scholar] [CrossRef][Green Version]
- Costa, T.; Chaves, A.J.; Valle, R.; Darji, A.; van Riel, D.; Kuiken, T.; Majo, N.; Ramis, A. Distribution patterns of influenza virus receptors and viral attachment patterns in the respiratory and intestinal tracts of seven avian species. Vet. Res. 2012, 43, 1–13. [Google Scholar] [CrossRef]
- Hadi, T.; Saeedeh, M.; Samaneh, N. Heterologous H9N2 avian influenza viral shedding pattern in Alectoris chukar. Online J. Vet. Res. 2013, 17, 566–570. [Google Scholar]
- Nili, H.; Mohammadi, A.; Habibi, H.; Firouzi, S. Pathogenesis of H9N2 virus in Chukar partridges. Avian Pathol. 2013, 42, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Mosleh, N.; Nazifi, S.; Nili, H.; Habibi, H. Effect of H9N2 virus infection on the acute phase response in chukar partridges (Alectoris chukar). Bulg. J. Vet. Med. 2013, 16, 20–28. [Google Scholar]
- Alyas, K.; Wajid, A.; Dundon, W.G.; Ather, S.; Batool, T.; Babar, M.E. Isolation and characterization of avian influenza H9N2 viruses from different avian species in Pakistan 2016–17. Avian Dis. 2019, 63, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Jin, S.; Wang, T.; Sun, W.; Zhang, Y.; Li, F.; Zhao, M.; Sun, L.; Hu, X.; et al. H9N2 influenza virus spillover into wild birds from poultry in China bind to human-type receptors and transmit in mammals via respiratory droplets. Transbound. Emerg. Dis. 2022, 69, 669–684. [Google Scholar] [CrossRef]
- Nili, H.; Asasi, K. Natural cases and an experimental study of H9N2 avian influenza in commercial broiler chickens of Iran. Avian Pathol. 2002, 31, 247–252. [Google Scholar] [CrossRef]
- Stipkovits, L.; Glavits, R.; Palfi, V.; Beres, A.; Egyed, L.; Denes, B.; Somogyi, M.; Szathmary, S. Pathologic lesions caused by coinfection of Mycoplasma gallisepticum and H3N8 low pathogenic avian influenza virus in chickens. Vet. Pathol. 2012, 49, 273–283. [Google Scholar] [CrossRef]
- Umar, S.; Guerin, J.L.; Ducatez, M.F. Low pathogenic avian influenza and coinfecting pathogens: A review of experimental infections in avian models. Avian Dis. 2017, 61, 3–15. [Google Scholar] [CrossRef]
- Amin, F.; Mukhtar, N.; Aslam, A.; Sheikh, A.A.; Sultan, B.; Hussain, M.; Shehzad, R.; Muzaffar, A.; Shahid, F.M.; Aziz, M.; et al. Rate of Multiple Viral and Bacterial CoInfection (s) in Influenza A/H9N2–Infected Broiler Flocks. Avian Dis. 2022, 66, 197–204. [Google Scholar] [CrossRef]
- Nili, H.; Asasi, K. Avian influenza (H9N2) outbreak in Iran. Avian Dis. 2003, 47, 828–831. [Google Scholar] [CrossRef]
- Sid, H.; Hartmann, S.; Petersen, H.; Ryll, M.; Rautenschlein, S. Mycoplasma gallisepticum modifies the pathogenesis of influenza A virus in the avian tracheal epithelium. Int. J. Med. Microbiol. 2016, 306, 174–186. [Google Scholar] [CrossRef]
- Haghighat-Jahromi, M.; Asasi, K.; Nili, H.; Dadras, H.; Shooshtari, A.H. Coinfection of avian influenza virus (H9N2 subtype) with infectious bronchitis live vaccine. Arch. Virol. 2008, 153, 651–655. [Google Scholar] [CrossRef]
- Hassan, K.E.; Ali, A.; Shany, S.A.; El-Kady, M.F. Experimental co-infection of infectious bronchitis and low pathogenic avian influenza H9N2 viruses in commercial broiler chickens. Res. Vet. Sci. 2017, 115, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Belkasmi, S.F.; Fellahi, S.; Touzani, C.D.; Faraji, F.Z.; Maaroufi, I.; Delverdier, M.; Guérin, J.L.; Fihri, O.F.; El Houadfi, M.; Ducatez, M.F. Co-infections of chickens with avian influenza virus H9N2 and Moroccan Italy 02 infectious bronchitis virus: Effect on pathogenesis and protection conferred by different vaccination programmes. Avian Pathol. 2020, 49, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; You, R.; Zhang, D.; Yuan, Q.; Xiang, B.; Liang, J.; Lin, Q.; Ding, C.; Liao, M.; Chen, L.; et al. Infectious bronchitis virus infection increases pathogenicity of H9N2 avian influenza virus by inducing severe inflammatory response. Front. Vet. Sci. 2022, 8, 824179. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, D.; Mawditt, K.; Sharma, M.; Drury, S.E.; Ainsworth, H.L.; Britton, P.; Gough, R.E. Detection of a coronavirus from turkey poults in Europe genetically related to infectious bronchitis virus of chickens. Avian Pathol. 2001, 30, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.; Wong, E.Y.; Tsang, C.C.; Ahmed, S.S.; Au-Yeung, R.K.; Yuen, K.Y.; Werenery, U.; Woo, P.C. Discovery and sequence analysis of four deltacoronaviruses from birds in the Middle East reveal interspecies jumping with recombination as a potential mechanism for avian-to-avian and avian-to-mammalian transmission. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef]
- Wille, M.; Holmes, E.C. Wild birds as reservoirs for diverse and abundant gamma-and deltacoronaviruses. FEMS Microbial. Rev. 2020, 44, 631–644. [Google Scholar] [CrossRef]
- Domańska-Blicharz, K.; Miłek-Krupa, J.; Pikuła, A. Diversity of coronaviruses in wild representatives of the Aves Class in Poland. Viruses 2021, 13, 1497. [Google Scholar] [CrossRef]
- Swayne, D.E. Animal Influenza, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian influenza in wild birds and poultry: Dissemination pathways, monitoring methods, and virus ecology. Pathogens 2021, 10, 630. [Google Scholar] [CrossRef]
- Hirschinger, J.; Munoz, M.C.; Hingrat, Y.; Vergne, T.; Guerin, J.L.; Le Loc’h, G. Exposure to and circulation of avian influenza and Newcastle disease viruses in peridomestic wild birds in the United Arab Emirates. J. Wildl. Dis. 2020, 56, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.S.; Kim, T.S.; Son, J.S.; Park, J.E.; Wang, S.J.; Jheong, W.H.; Mo, I.P. The difference of detection rate of avian influenza virus in the wild bird surveillance using various methods. J. Vet. Sci. 2019, 20, e56. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Cardona, C.; Dao, P.; Crossley, B.; Hietala, S.; Boyce, W. Inability of real-time reverse transcriptase PCR assay to detect subtype H7 avian influenza viruses isolated from wild birds. J. Clin. Microbial. 2008, 46, 1844–1846. [Google Scholar] [CrossRef]
- Suarez, D.L.; Das, A.; Ellis, E. Review of rapid molecular diagnostic tools for avian influenza virus. Avian Dis. 2007, 51, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tang, Y.; Lin, L.; Wolfgang, D.R. Next-generation sequencing confirmation of real-time RT-PCR false positive influenza-A virus detection in waterfowl and swine swab samples. J. Next Gener. Seq. Appl. 2016, 3, 2. [Google Scholar] [CrossRef]


| House Sparrows Sample Number | H9N2 PCR Results (Ct) | Other Viral Pathogens | Parasitic Pathogens | Bacterial Pathogens * |
|---|---|---|---|---|
| 1 | 37.5 | N | Tetrameres sp. Eimeria sp. | E. coli Klebsiella sp. Staphylococcus Aureus Enterococcus sp. |
| 2 | 38.6 | Avian coronavirus | Tetrameres sp. | |
| 3 | N | Avian coronavirus | Eimeria sp. | |
| 4 | N | N | Tetrameres sp. | |
| 5 | 37.5 | Avian coronavirus | N | |
| 6 | N | Avian coronavirus | Eimeria sp. | |
| 7 | N | Avian coronavirus | Eimeria sp. | |
| 8 | N | Avian coronavirus | N | |
| 9 | N | Avian coronavirus | Eimeria sp. | |
| 10 | N | Avian coronavirus | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bidoudan, Y.; Mouahid, M.; Fassi Fihri, O.; Bollo, E.; Arbani, O.; Ducatez, M.; Banni, B.; Tligui, N.; Fellahi, S. First Report of Low Pathogenic Avian Influenza Subtype H9N2 in African Houbara Bustards (Chlamydotis undulata undulata) and Gamebirds in Morocco: Clinico-Pathological Findings, Molecular Characterization, and Associated Coinfections. Viruses 2023, 15, 2374. https://doi.org/10.3390/v15122374
Bidoudan Y, Mouahid M, Fassi Fihri O, Bollo E, Arbani O, Ducatez M, Banni B, Tligui N, Fellahi S. First Report of Low Pathogenic Avian Influenza Subtype H9N2 in African Houbara Bustards (Chlamydotis undulata undulata) and Gamebirds in Morocco: Clinico-Pathological Findings, Molecular Characterization, and Associated Coinfections. Viruses. 2023; 15(12):2374. https://doi.org/10.3390/v15122374
Chicago/Turabian StyleBidoudan, Yassmina, Mohamed Mouahid, Ouafaa Fassi Fihri, Enrico Bollo, Oumayma Arbani, Mariette Ducatez, Brahim Banni, Noursaid Tligui, and Siham Fellahi. 2023. "First Report of Low Pathogenic Avian Influenza Subtype H9N2 in African Houbara Bustards (Chlamydotis undulata undulata) and Gamebirds in Morocco: Clinico-Pathological Findings, Molecular Characterization, and Associated Coinfections" Viruses 15, no. 12: 2374. https://doi.org/10.3390/v15122374
APA StyleBidoudan, Y., Mouahid, M., Fassi Fihri, O., Bollo, E., Arbani, O., Ducatez, M., Banni, B., Tligui, N., & Fellahi, S. (2023). First Report of Low Pathogenic Avian Influenza Subtype H9N2 in African Houbara Bustards (Chlamydotis undulata undulata) and Gamebirds in Morocco: Clinico-Pathological Findings, Molecular Characterization, and Associated Coinfections. Viruses, 15(12), 2374. https://doi.org/10.3390/v15122374







