From Infection to Malignancy: Tracing the Impact of Human Papillomavirus on Uterine Endometrial Cancer in a Nationwide Population-Based Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
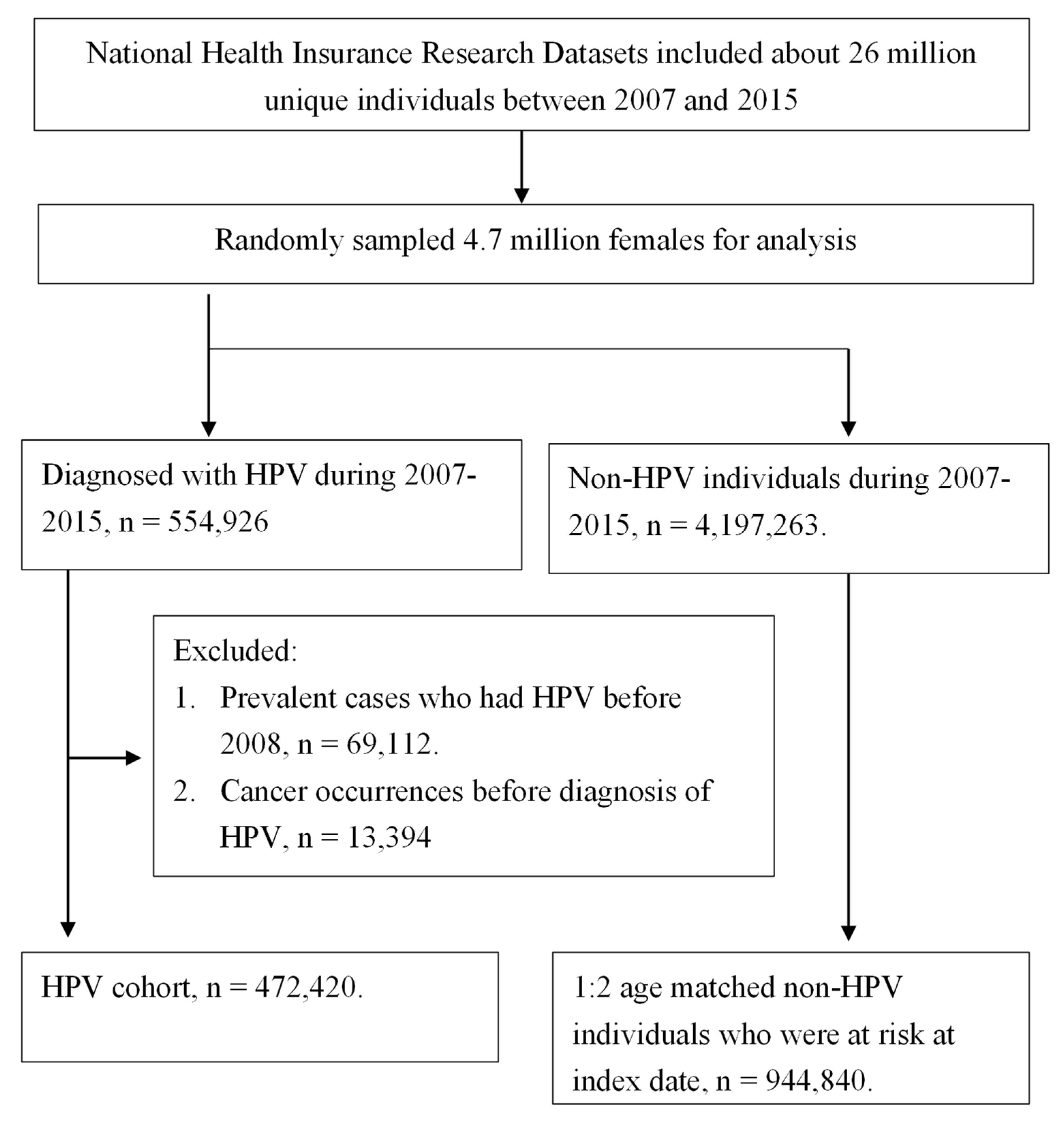
2.2. Identification of Study Cohort and Exclusion Criteria
2.3. Study Events, Covariates, and Primary Outcomes
2.4. Statistical Analysis
3. Results
3.1. Study Population Examining Uterine Body and Cervical Cancer Incidences in Correlation with HPV Infection
3.2. Demographic Characteristics of HPV-Infected and Noninfected Individuals
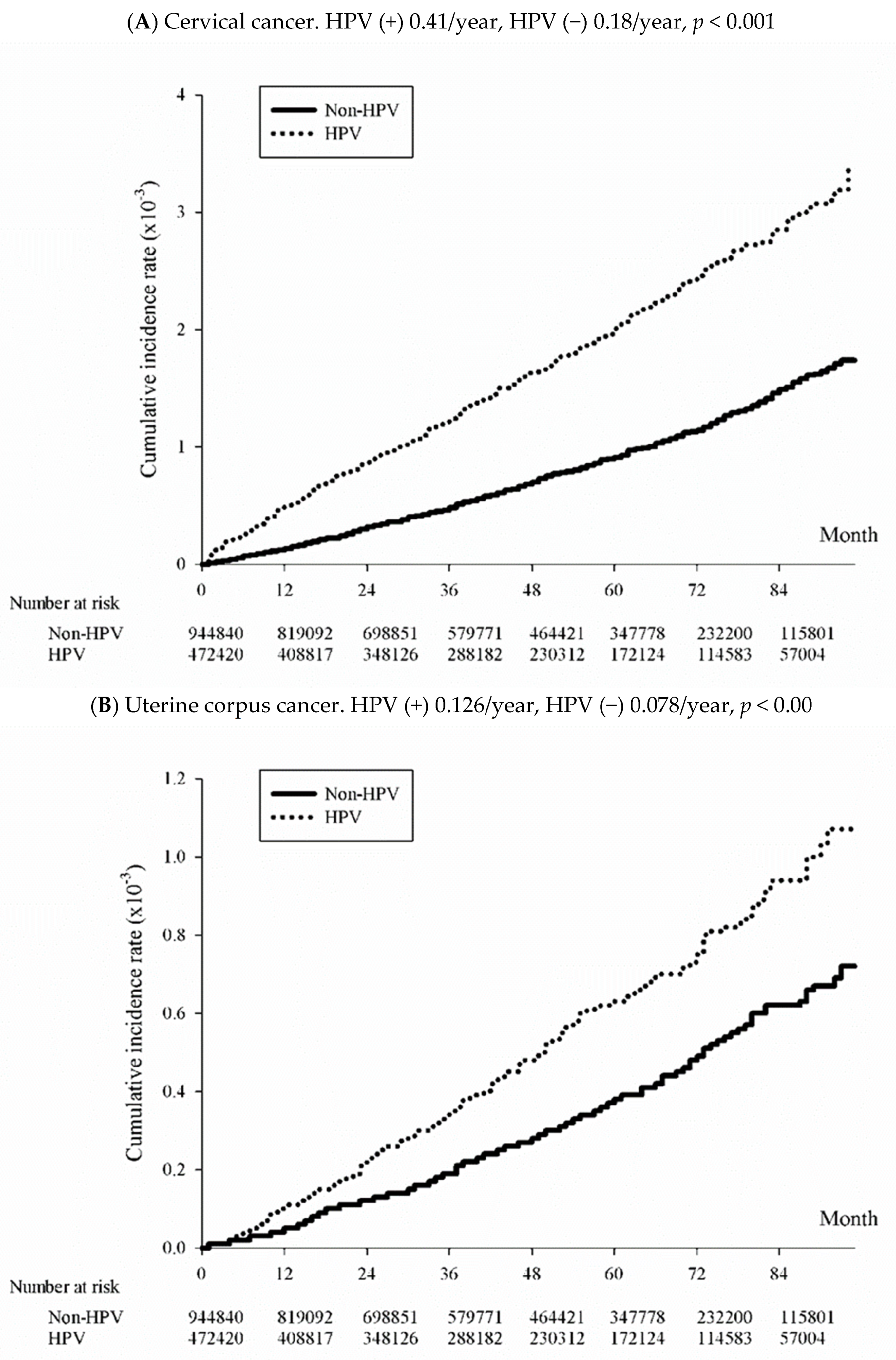
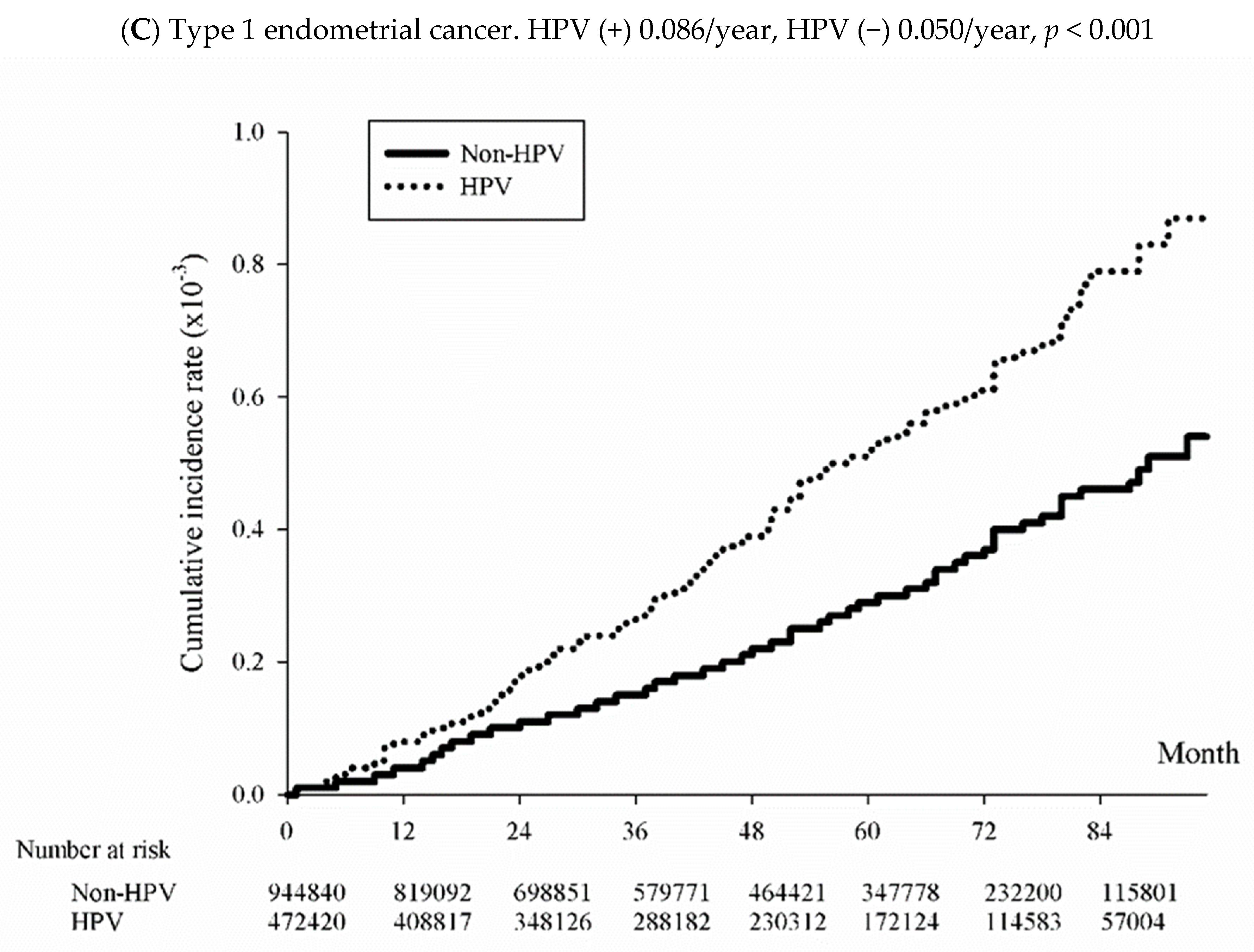
3.3. Incidence of Uterine and Cervical Cancer among the HPV-Exposed and Unexposed Individuals
3.4. Factors Associated with Uterine Endometrial and Cervical Cancers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. 2020. Available online: https://www.who.int/publications-detail/who-report-on-cancer-setting-priorities-investing-wisely-and-providing-care-for-all (accessed on 9 September 2023).
- Ministry of Health and Welfare. 2020 Statistics of Causes of Death, Taiwan. Available online: https://www.mohw.gov.tw/lp-5256-2.html (accessed on 19 October 2023).
- Chesson, H.W.; Dunne, E.F.; Hariri, S.; Markowitz, L.E. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex. Transm. Dis. 2014, 41, 660–664. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Struyf, F.; Del Rosario-Raymundo, M.R.; Hildesheim, A.; Skinner, S.R.; Wacholder, S.; Garland, S.M.; Herrero, R.; David, M.P.; Wheeler, C.M.; et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: Combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol. 2015, 16, 775–786. [Google Scholar] [CrossRef]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Munoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Bertoli, H.K.; Thomsen, L.T.; Iftner, T.; Dehlendorff, C.; Kjær, S.K. Risk of vulvar, vaginal and anal high-grade intraepithelial neoplasia and cancer according to cervical human papillomavirus (HPV) status: A population-based prospective cohort study. Gynecol. Oncol. 2020, 157, 456–462. [Google Scholar] [CrossRef]
- Olesen, T.B.; Svahn, M.F.; Faber, M.T.; Duun-Henriksen, A.K.; Junge, J.; Norrild, B.; Kjaer, S.K. Prevalence of Human Papillomavirus in endometrial cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2014, 134, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Cancer Registry Center, Ministry of Health and Welfare, Taiwan. Incidence and Mortality Rates for the Top 10 Cancer in Taiwan. 2020. Available online: https://twcr.tw/wp-content/uploads/2023/04/Top-10-cancers-in-Taiwan-2020.pdf (accessed on 9 September 2023).
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- de Haan, J.; Verheecke, M.; Van Calsteren, K.; Van Calster, B.; Shmakov, R.G.; Mhallem Gziri, M.; Halaska, M.J.; Fruscio, R.; Lok, C.A.R.; Boere, I.A.; et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: A 20-year international cohort study of 1170 patients. Lancet Oncol. 2018, 19, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Urick, M.E.; Bell, D.W. Clinical actionability of molecular targets in endometrial cancer. Nat. Rev. Cancer 2019, 19, 510–521. [Google Scholar] [CrossRef]
- Brinton, L.A.; Felix, A.S.; McMeekin, D.S.; Creasman, W.T.; Sherman, M.E.; Mutch, D.; Cohn, D.E.; Walker, J.L.; Moore, R.G.; Downs, L.S.; et al. Etiologic heterogeneity in endometrial cancer: Evidence from a Gynecologic Oncology Group trial. Gynecol. Oncol. 2013, 129, 277–284. [Google Scholar] [CrossRef]
- Park, B.; Ahn, K.H.; Choi, Y.; Kim, J.H.; Seong, H.; Kim, Y.J.; Choi, J.Y.; Song, J.Y.; Lee, E.; Jun, Y.H.; et al. Cancer incidence among adults with HIV in a population-based cohort in Korea. JAMA Netw. Open 2022, 5, e2224897, Erratum in JAMA Netw. Open 2022, 5, e2238976. [Google Scholar] [CrossRef]
- Chen, Y.G.; Yang, C.W.; Chung, C.H.; Ho, C.L.; Chen, W.L.; Chien, W.C. The association between metabolic risk factors, nonalcoholic fatty liver disease, and the incidence of liver cancer: A nationwide population-based cohort study. Hepatol. Int. 2022, 16, 807–816, Erratum in Hepatol. Int. 2022, 16, 488. [Google Scholar] [CrossRef]
- Lin, F.; Huang, J.Y. The association between HPV infection and cervical and head & neck cancer, 2007–2105, Taiwan. Mendeley Data 2022, 1. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hung, Y.T.; Chuang, Y.L.; Chen, Y.J.; Weng, W.S.; Liu, J.S.; Liang, K.Y. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J. Health Manag. 2006, 4, 1–22. [Google Scholar]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Cancer Registry Center, Ministry of Health and Welfare, Taiwan. Top 10 Cancer in Taiwan, 2008–2014. 2017. Available online: https://twcr.tw/?page_id=1855&lang=en (accessed on 9 September 2023).
- Huang, J.-Y.; Lin, C.; Tsai, S.C.-S.; Lin, F.C.-F. Human Papillomavirus Is Associated with Adenocarcinoma of Lung: A Population-Based Cohort Study. Front. Med. 2022, 9, 932196. [Google Scholar] [CrossRef]
- Jassim, G.; Obeid, A.; Al Nasheet, H.A. Knowledge, attitudes, and practices regarding cervical cancer and screening among women visiting primary health care Centres in Bahrain. BMC Public Health 2018, 18, 128. [Google Scholar] [CrossRef]
- Kaaks, R.; Lukanova, A.; Kurzer, M.S. Obesity, endogenous hormones, and endometrial cancer risk: A synthetic review. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1531–1543. [Google Scholar]
- Schultz, W.M.; Kelli, H.M.; Lisko, J.C.; Varghese, T.; Shen, J.; Sandesara, P.; Quyyumi, A.A.; Taylor, H.A.; Gulati, M.; Harold, J.G.; et al. Socioeconomic Status and Cardiovascular Outcomes: Challenges and Interventions. Circulation 2018, 137, 2166–2178. [Google Scholar] [CrossRef]
- Gnade, C.M.; Hill, E.K.; Botkin, H.E.; Hefel, A.R.; Hansen, H.E.; Sheets, K.A.; Mott, S.L.; Hardy-Fairbanks, A.J.; Stockdale, C.K. Is the age of cervical cancer diagnosis changing over time? J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102040. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Registration and Analysis Service. Uterine Cancer in the United Kingdom: Overall Trends and Variation by Age. Available online: http://www.ncin.org.uk/publications/data_briefings/uterine_cancer_in_the_united_kingdom_overall_trends_and_variation_by_age (accessed on 5 September 2023).
- Bosch, F.X.; Lorincz, A.; Munoz, N.; Meijer, C.J.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Pim, D.; Banks, L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene 1999, 18, 7690–7700. [Google Scholar] [CrossRef] [PubMed]
- Chiantore, M.V.; Iuliano, M.; Mongiovì, R.M.; Dutta, S.; Tommasino, M.; Di Bonito, P.; Accardi, L.; Mangino, G.; Romeo, G. The E6 and E7 proteins of beta3 human papillomavirus 49 can deregulate both cellular and extracellular vesicles-carried microRNAs. Infect. Agent. Cancer 2022, 17, 29. [Google Scholar] [CrossRef]
- Wentzensen, N.; Vinokurova, S.; von Knebel Doeberitz, M. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 2004, 64, 3878–3884. [Google Scholar] [CrossRef]
- Levine, D.; The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Nero, C.; Ciccarone, F.; Pietragalla, A.; Scambia, G. PTEN and Gynecological Cancers. Cancers 2019, 11, 1458. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Saccone, G.; Viggiani, M.; Giampaolino, P.; Insabato, L.; Mollo, A.; De Placido, G.; Zullo, F. PTEN expression in endometrial hyperplasia and risk of cancer: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2019, 299, 1511–1524. [Google Scholar] [CrossRef]
- Chiosea, S.I.; Grandis, J.R.; Lui, V.W.; Diergaarde, B.; Maxwell, J.H.; Ferris, R.L.; Kim, S.W.; Luvison, A.; Miller, M.; Nikiforova, M.N. PIK3CA, HRAS and PTEN in human papillomavirus positive oropharyngeal squamous cell carcinoma. BMC Cancer 2013, 13, 602. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Vahrenkamp, J.M.; Berrett, K.C.; Clark, K.A.; Guillen, K.P.; Scherer, S.D.; Yang, C.H.; Welm, B.E.; Janát-Amsbury, M.M.; Graves, B.J.; et al. ETV4 Is Necessary for Estrogen Signaling and Growth in Endometrial Cancer Cells. Cancer Res. 2020, 80, 1234–1245. [Google Scholar] [CrossRef]
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Kondo, S.; Wakisaka, N.; Wakae, K.; Aga, M.; Moriyama-Kita, M.; Ishikawa, K.; Ueno, T.; Nakanishi, Y.; Hatano, M.; et al. Expression of estrogen receptor alpha is associated with pathogenesis and prognosis of human papillomavirus-positive oropharyngeal cancer. Int. J. Cancer 2019, 145, 1547–1557. [Google Scholar] [CrossRef]
- Lin, F.C.; Huang, J.Y.; Tsai, S.C.; Nfor, O.N.; Chou, M.C.; Wu, M.F.; Lee, C.T.; Jan, C.F.; Liaw, Y.P. The association between human papillomavirus infection and female lung cancer: A population-based cohort study. Medicine 2016, 95, e3856. [Google Scholar] [CrossRef] [PubMed]
- Abu-Lubad, M.A.; Jarajreh, D.A.; Helaly, G.F.; Alzoubi, H.M.; Haddadin, W.J.; Dabobash, M.D.; Albataineh, E.M.; Aqel, A.A.; Alnawaiseh, N.A. Human papillomavirus as an independent risk factor of invasive cervical and endometrial carcinomas in Jordan. J. Infect. Public Health 2020, 13, 613–618. [Google Scholar] [CrossRef] [PubMed]
| Variables | ICD-9 Codes Identified from Database |
|---|---|
| Exposures | |
| HPV infection | 079.4, 078.1, 795.05, 795.09, 795.15, 795.19, 796.75, 796.79. |
| Study events | |
| Cancer of uterine cervix | ICD-9 codes: 180, ICD-10 code C53 |
| Cancer of uterine corpus | ICD-9 codes: 182, ICD-10 codes C54, C55 |
| Type 1 endometrial cancer * | 814, 856, 801, 838, 848 |
| Type 2 endometrial cancer * | 857, 802, 831, 844, 898, 895, 826, 805, 845, 846 |
| Comorbidities | |
| Ischemic heart disease | 410–414 |
| Abnormal liver function | 273.4, 275.0, 275.1, 453.0, 571, 573, 576.1, 456.0, 456.1, 456.2, 789.5, 789.59, 572.2, 567, 572.4. |
| Renal failure | 585 |
| Chronic kidney disease | 403.11, 403.91, 404.12, 404.13, 404.92, 404.93, 585, 586, 587, 274.1, 403.10, 403.90, 404.10, 404.11, 404.90, 404.91, 581, 582, 583, 590.0, 593.6, 593.9, 753.12, 753.13, 753.14 |
| Hypertension | 401–405 |
| Diabetes mellitus | 250 |
| Lipid dysfunction | 272 |
| Stroke | 430–438 |
| COPD | 490–492, 493–496 |
| Peptic ulcer | 531–533 |
| GI bleeding | 578 |
| Gout | 274 |
| Non-HPV | HPV | p | |
|---|---|---|---|
| Total case numbers | 944,840 | 472,420 | |
| Age | 1.0000 | ||
| <20 | 240,114 (25.41%) | 120,057 (25.41%) | |
| 20–40 | 343,124 (36.32%) | 171,562 (36.32%) | |
| 40–60 | 252,046 (26.68%) | 126,023 (26.68%) | |
| 60–80 | 94,532 (10.01%) | 47,266 (10.01%) | |
| >=80 | 15,024 (1.59%) | 7512 (1.59%) | |
| Residential area | <0.0001 | ||
| Taipei area | 344,248 (36.43%) | 200,491 (42.44%) | |
| Northern | 136,141 (14.41%) | 61,490 (13.02%) | |
| Central | 174,021 (18.42%) | 90,930 (19.25%) | |
| Southern | 130,536 (13.82%) | 53,853 (11.40%) | |
| Kaohsiung area | 138,316 (14.64%) | 57,888 (12.25%) | |
| Eastern | 21,578 (2.28%) | 7768 (1.64%) | |
| Low-income | <0.0001 | ||
| Yes | 10,850 (1.15%) | 4461 (0.94%) | |
| No | 933,990 (98.85%) | 467,959 (99.06%) | |
| Comorbidities | |||
| Ischemic heart disease | 28,420 (3.01%) | 16,171 (3.42%) | <0.0001 |
| Hypertension | 97,281 (10.30%) | 49,718 (10.52%) | <0.0001 |
| Stroke | 17,177 (1.82%) | 8664 (1.83%) | 0.5026 |
| Diabetes mellitus | 45,250 (4.79%) | 20,860 (4.42%) | <0.0001 |
| Abnormal liver function | 37,355 (3.95%) | 17,822 (3.77%) | <0.0001 |
| Renal failure | 6083 (0.64%) | 2892 (0.61%) | 0.0252 |
| Chronic kidney diseases | 10,685 (1.13%) | 5531 (1.17%) | 0.0352 |
| GI bleeding | 4141 (0.44%) | 2195 (0.46%) | 0.0266 |
| Hyperlipidemia | 65,605 (6.94%) | 38,553 (8.16%) | <0.0001 |
| COPD | 11,597 (1.23%) | 6454 (1.37%) | <0.0001 |
| Peptic ulcer | 63,365 (6.71%) | 37,381 (7.91%) | <0.0001 |
| Gout | 9541 (1.01%) | 4979 (1.05%) | 0.0139 |
| HPV | Non-HPV | |||||
|---|---|---|---|---|---|---|
| Event | Incidence Rate † | Event | Incidence Rate † | Crude HR | Adjusted HR | |
| CC | 777 | 41.65 (38.82–44.68) | 712 | 18.98 (17.63–20.42) | 2.195 (1.983–2.430) | 2.225 (2.008–2.464) |
| Uterine EC | 235 | 12.60 (11.08–14.31) | 293 | 7.81 (6.96–8.76) | 1.614 (1.360–1.917) | 1.588 (1.335–1.888) |
| Type 1 EC | 192 | 0.86 (0.74–0.99) | 225 | 0.50 (0.44–0.57) | 1.717 (1.417–2.082) | 1.671 (1.376–2.029) |
| Endometrioid adenocarcinoma | 177 | 0.79 (0.68–0.92) | 206 | 0.46 (0.40–0.52) | 1.729 (1.415–2.114) | 1.686 (1.377–2.065) |
| Adenocarcinoma, others | 14 | 0.06 (0.04–0.11) | 18 | 0.04 (0.03–0.06) | 1.563 (0.777–3.142) | 1.499 (0.742–3.030) |
| Type 2 EC | 18 | 0.08 (0.05–0.13) | 26 | 0.06 (0.04–0.08) | 1.395 (0.765–2.544) | 1.450 (0.791–2.656) |
| Uterine Endometrial Cancer | Uterine Cervical Cancer | |||||
|---|---|---|---|---|---|---|
| aHR | 95% CI | p | aHR | 95% CI | p | |
| HPV infection | 1.588 | 1.335–1.888 | <0.0001 * | 2.225 | 2.008–2.464 | <0.0001 * |
| no infection | Reference | Reference | ||||
| Age | ||||||
| <20 | 0.043 | 0.010–0.174 | <0.0001 * | 0.028 | 0.016–0.048 | <0.0001 * |
| 20–40 | Reference | Reference | ||||
| 40–60 | 6.547 | 5.035–8.514 | <0.0001 * | 1.124 | 1.001–1.262 | 0.0481 * |
| 60–80 | 4.275 | 3.019–6.054 | <0.0001 * | 1.068 | 0.884–1.291 | 0.496 |
| ≥80 | 1.622 | 0.637–4.134 | 0.311 | 0.956 | 0.633–1.443 | 0.829 |
| Low-income | 0.52 | 0.129–2.090 | 0.357 | 1.044 | 0.590–1.848 | 0.883 |
| Yes | ||||||
| No | 1 | - | - | 1 | - | - |
| Comorbidity (ref: without) | ||||||
| Ischemic heart disease | 0.963 | 0.659–1.409 | 0.847 | 0.796 | 0.591–1.070 | 0.131 |
| Hypertension | 1.622 | 1.275–2.063 | <0.0001 * | 1.125 | 0.934–1.355 | 0.214 |
| Stroke | 0.781 | 0.442–1.379 | 0.394 | 0.922 | 0.636–1.336 | 0.667 |
| Diabetes mellitus | 1.175 | 0.859–1.607 | 0.313 | 1.109 | 0.873–1.410 | 0.397 |
| Abnormal liver function | 1.303 | 0.942–1.802 | 0.110 | 1.334 | 1.067–1.669 | 0.011 * |
| Renal failure | 1.107 | 0.351–3.494 | 0.863 | 2.725 | 1.235–6.009 | 0.013 * |
| Chronic kidney diseases | 1.185 | 0.487–2.882 | 0.708 | 1.001 | 0.497–2.016 | 0.998 |
| Hyperlipidemia | 1.096 | 0.833–1.443 | 0.513 | 0.808 | 0.651–1.003 | 0.053 |
| COPD | 0.189 | 0.047–0.761 | 0.019 * | 1.109 | 0.738–1.665 | 0.619 |
| Peptic ulcer | 0.998 | 0.752–1.326 | 0.990 | 1.19 | 0.994–1.425 | 0.058 |
| Gout | 1.823 | 1.117–2.974 | 0.016* | 1.109 | 0.717–1.716 | 0.641 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-J.; Tsai, S.C.-S.; Huang, J.-Y.; Lee, M.-S.; Wang, P.-H.; Lin, F.C.-F. From Infection to Malignancy: Tracing the Impact of Human Papillomavirus on Uterine Endometrial Cancer in a Nationwide Population-Based Cohort Study. Viruses 2023, 15, 2314. https://doi.org/10.3390/v15122314
Wu P-J, Tsai SC-S, Huang J-Y, Lee M-S, Wang P-H, Lin FC-F. From Infection to Malignancy: Tracing the Impact of Human Papillomavirus on Uterine Endometrial Cancer in a Nationwide Population-Based Cohort Study. Viruses. 2023; 15(12):2314. https://doi.org/10.3390/v15122314
Chicago/Turabian StyleWu, Pei-Ju, Stella Chin-Shaw Tsai, Jing-Yang Huang, Maw-Sheng Lee, Po-Hui Wang, and Frank Cheau-Feng Lin. 2023. "From Infection to Malignancy: Tracing the Impact of Human Papillomavirus on Uterine Endometrial Cancer in a Nationwide Population-Based Cohort Study" Viruses 15, no. 12: 2314. https://doi.org/10.3390/v15122314
APA StyleWu, P.-J., Tsai, S. C.-S., Huang, J.-Y., Lee, M.-S., Wang, P.-H., & Lin, F. C.-F. (2023). From Infection to Malignancy: Tracing the Impact of Human Papillomavirus on Uterine Endometrial Cancer in a Nationwide Population-Based Cohort Study. Viruses, 15(12), 2314. https://doi.org/10.3390/v15122314






