Immunogenicity in Mice Immunized with Recombinant Adenoviruses Expressing Varicella-Zoster Virus Envelope Glycoprotein E
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Qubit Fluorometric Quantitation
2.3. Adeno-XTMRapid Titer Procedure
2.4. In Vitro gE Protein Expression
2.5. Animal and Immunization
2.6. Enzyme-Linked Immunosorbent Assay (ELISA) for gE-Specific Antibodies
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
3.1. In Vitro Characterization of rChAd63/gE
3.2. gE-Specific IgG Responses Induced by Immunization with Recombinant Adenoviruses
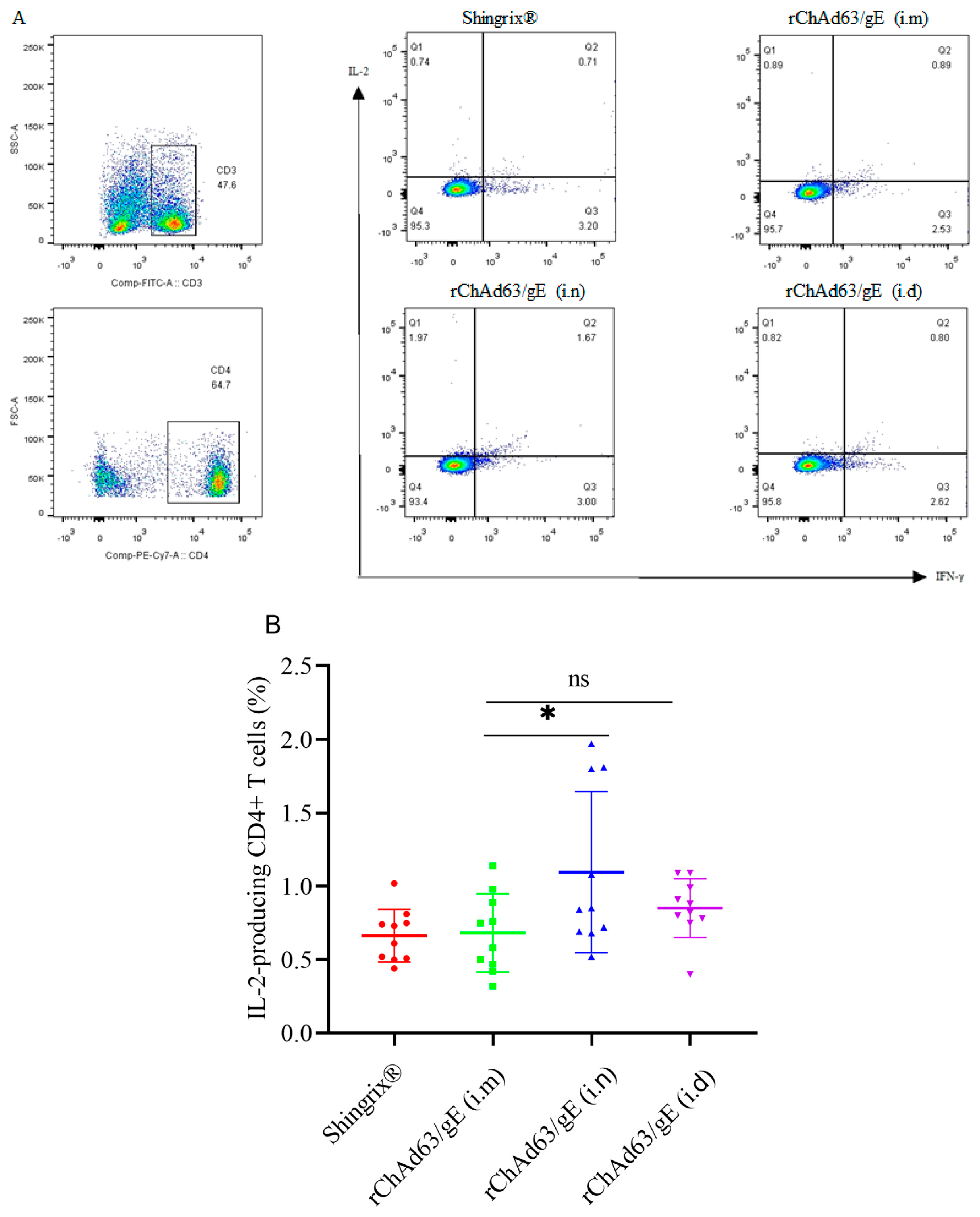
3.3. Efficient Induction of gE-Specific IFN-γ and/or IL-2-Secreting CD4+ T Cells in Mice Immunized with Recombinant Adenoviruses
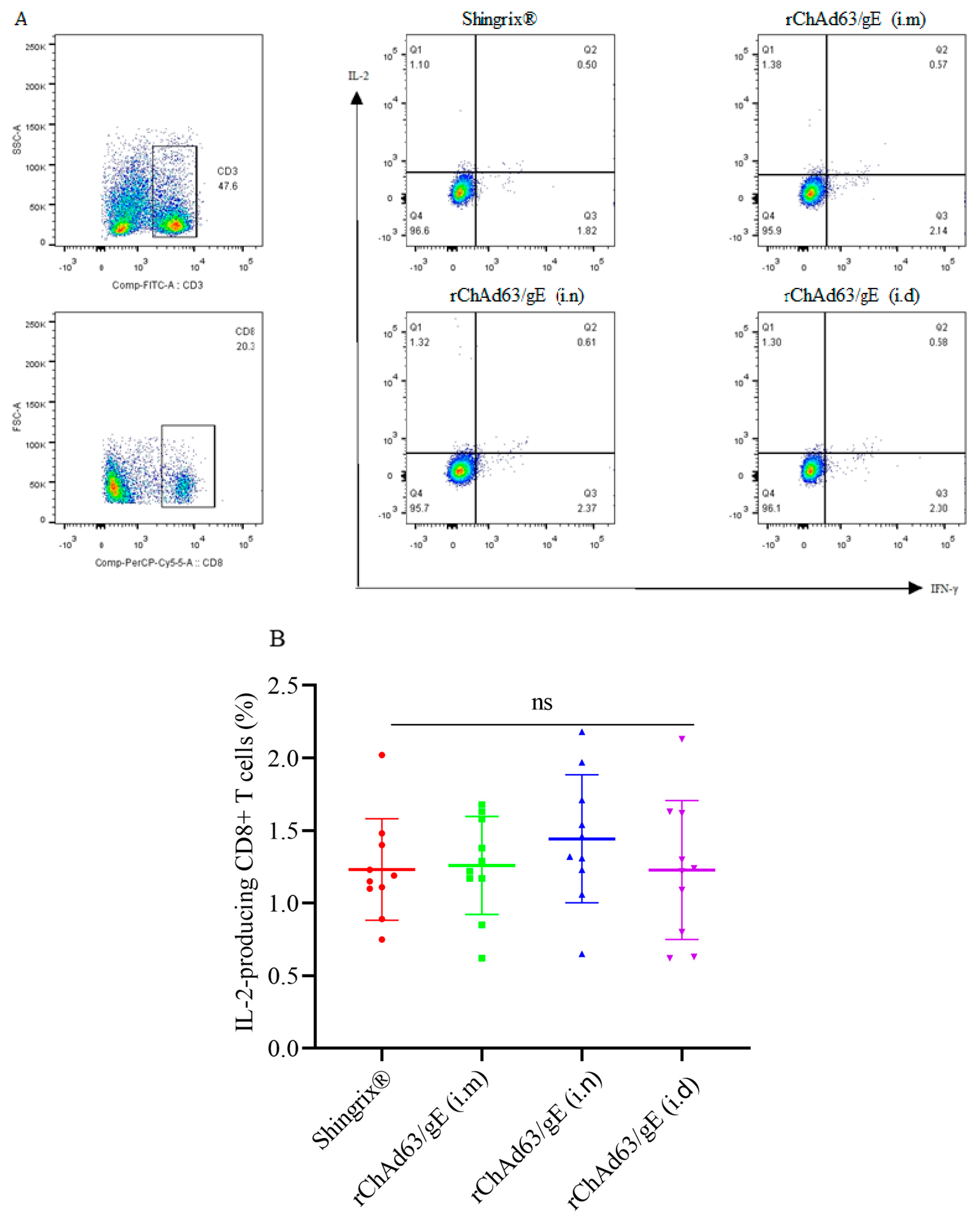
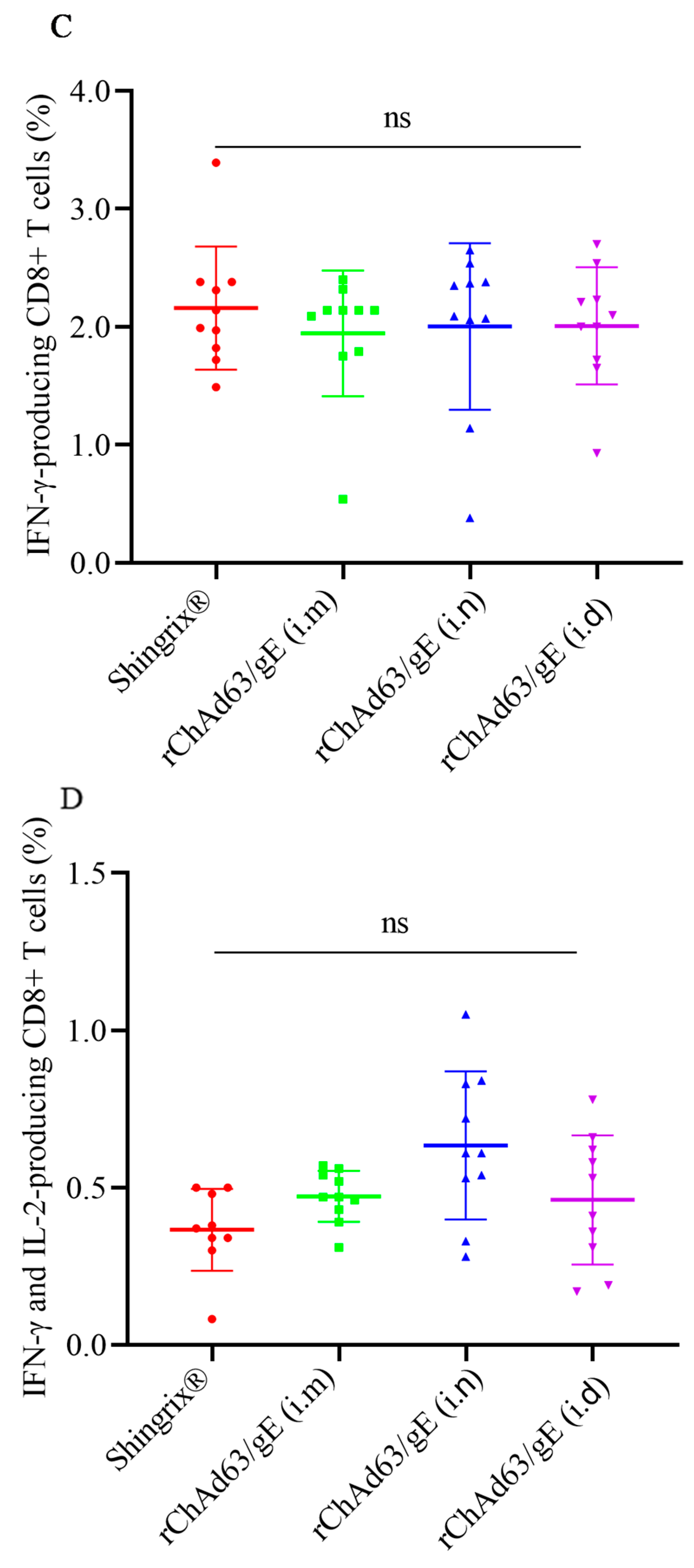
3.4. Efficient Induction of gE-Specific IFN-γ and/or IL-2-Secreting CD8+ T Cells in Mice Immunized with Recombinant Adenoviruses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harbecke, R.; Cohen, J.I.; Oxman, M.N. Herpes Zoster Vaccines. J. Infect. Dis. 2021, 224 (Suppl. S2), S429–S442. [Google Scholar] [CrossRef] [PubMed]
- Berarducci, B.; Ikoma, M.; Stamatis, S.; Sommer, M.; Grose, C.; Arvin, A.M. Essential functions of the unique N-terminal region of the varicella-zoster virus glycoprotein E ectodomain in viral replication and in the pathogenesis of skin infection. J. Virol. 2006, 80, 9481–9496. [Google Scholar] [CrossRef]
- Berarducci, B.; Rajamani, J.; Zerboni, L.; Che, X.; Sommer, M.; Arvin, A.M. Functions of the unique N-terminal region of glycoprotein E in the pathogenesis of varicella-zoster virus infection. Proc. Natl. Acad. Sci. USA 2010, 107, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Fowler, W.J.; Garcia-Valcarcel, M.; Hill-Perkins, M.S.; Murphy, G.; Harper, D.R.; Jeffries, D.J.; Burns, N.R.; Adams, S.E.; Kingsman, A.J.; Layton, G.T. Identification of immunodominant regions and linear B cell epitopes of the gE envelope protein of varicella-zoster virus. Virology 1995, 214, 531–540. [Google Scholar] [CrossRef]
- Malavige, G.N.; Jones, L.; Black, A.P.; Ogg, G.S. Varicella zoster virus glycoprotein E-specific CD4+ T cells show evidence of recent activation and effector differentiation, consistent with frequent exposure to replicative cycle antigens in healthy immune donors. Clin. Exp. Immunol. 2008, 152, 522–531. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014—Recommendations. Vaccine 2016, 34, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.M.; Andriolo, B.N.; Torloni, M.R.; Soares, B.G.; de Oliveira Gomes, J.; Andriolo, R.B.; Canteiro Cruz, E. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst. Rev. 2019, 2019, CD008858. [Google Scholar] [CrossRef] [PubMed]
- Li-Kim-Moy, J.; Phillips, A.; Morgan, A.; Glover, C.; Jayasinghe, S.; Hull, B.; Dey, A.; Beard, F.; Hickie, M.; Macartney, K. Disseminated varicella zoster virus infection following live attenuated herpes zoster vaccine: Descriptive analysis of reports to Australia’s spontaneous vaccine pharmacovigilance system, 2016–2020. BMJ Open 2023, 13, e067287. [Google Scholar] [CrossRef]
- Price, N.B.; Grose, C. Corticosteroids Contribute to Serious Adverse Events Following Live Attenuated Varicella Vaccination and Live Attenuated Zoster Vaccination. Vaccines 2021, 9, 23. [Google Scholar] [CrossRef]
- Breuer, J. Molecular Genetic Insights Into Varicella Zoster Virus (VZV), the vOka Vaccine Strain, and the Pathogenesis of Latency and Reactivation. J. Infect. Dis. 2018, 218 (Suppl. S2), S75–S80. [Google Scholar] [CrossRef]
- Garnett, G.P.; Grenfell, B.T. The epidemiology of varicella-zoster virus infections: The influence of varicella on the prevalence of herpes zoster. Epidemiol. Infect. 1992, 108, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Monslow, M.A.; Elbashir, S.; Sullivan, N.L.; Thiriot, D.S.; Ahl, P.; Smith, J.; Miller, E.; Cook, J.; Cosmi, S.; Thoryk, E.; et al. Immunogenicity generated by mRNA vaccine encoding VZV gE antigen is comparable to adjuvanted subunit vaccine and better than live attenuated vaccine in nonhuman primates. Vaccine 2020, 38, 5793–5802. [Google Scholar] [CrossRef] [PubMed]
- Oxman, M.N.; Levin, M.J.; Johnson, G.R.; Schmader, K.E.; Straus, S.E.; Gelb, L.D.; Arbeit, R.D.; Simberkoff, M.S.; Gershon, A.A.; Davis, L.E.; et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 2005, 352, 2271–2284. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.L.; Lal, H.; Kovac, M.; Chlibek, R.; Hwang, S.J.; Diez-Domingo, J.; Godeaux, O.; Levin, M.J.; McElhaney, J.E.; Puig-Barbera, J.; et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N. Engl. J. Med. 2016, 375, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Heineman, T.C.; Cunningham, A.; Levin, M. Understanding the immunology of Shingrix, a recombinant glycoprotein E adjuvanted herpes zoster vaccine. Curr. Opin. Immunol. 2019, 59, 42–48. [Google Scholar] [CrossRef]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barbera, J.; et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef]
- Burke, B.L.; Steele, R.W.; Beard, O.W.; Wood, J.S.; Cain, T.D.; Marmer, D.J. Immune responses to varicella-zoster in the aged. Arch. Intern. Med. 1982, 142, 291–293. [Google Scholar] [CrossRef]
- Asada, H. VZV-specific cell-mediated immunity, but not humoral immunity, correlates inversely with the incidence of herpes zoster and the severity of skin symptoms and zoster-associated pain: The SHEZ study. Vaccine 2019, 37, 6776–6781. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Gabriel, E.E.; Miao, X.; Li, X.; Su, S.C.; Parrino, J.; Chan, I.S. Fold rise in antibody titers by measured by glycoprotein-based enzyme-linked immunosorbent assay is an excellent correlate of protection for a herpes zoster vaccine, demonstrated via the vaccine efficacy curve. J. Infect. Dis. 2014, 210, 1573–1581. [Google Scholar] [CrossRef]
- Haberthur, K.; Engelmann, F.; Park, B.; Barron, A.; Legasse, A.; Dewane, J.; Fischer, M.; Kerns, A.; Brown, M.; Messaoudi, I. CD4 T cell immunity is critical for the control of simian varicella virus infection in a nonhuman primate model of VZV infection. PLoS Pathog. 2011, 7, e1002367. [Google Scholar] [CrossRef]
- Steain, M.; Sutherland, J.P.; Rodriguez, M.; Cunningham, A.L.; Slobedman, B.; Abendroth, A. Analysis of T cell responses during active varicella-zoster virus reactivation in human ganglia. J. Virol. 2014, 88, 2704–2716. [Google Scholar] [CrossRef]
- Weinberg, A.; Levin, M.J. VZV T cell-mediated immunity. Curr. Top. Microbiol. Immunol. 2010, 342, 341–357. [Google Scholar] [PubMed]
- Arvin, A.M.; Koropchak, C.M.; Williams, B.R.; Grumet, F.C.; Foung, S.K. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J. Infect. Dis. 1986, 154, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Malavige, G.N.; Jones, L.; Kamaladasa, S.D.; Wijewickrama, A.; Seneviratne, S.L.; Black, A.P.; Ogg, G.S. Viral load, clinical disease severity and cellular immune responses in primary varicella zoster virus infection in Sri Lanka. PLoS ONE 2008, 3, e3789. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.; Zhang, J.H.; Oxman, M.N.; Johnson, G.R.; Hayward, A.R.; Caulfield, M.J.; Irwin, M.R.; Clair, J.; Smith, J.G.; Stanley, H.; et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J. Infect. Dis. 2009, 200, 1068–1077. [Google Scholar] [CrossRef]
- Gershon, A.A.; Gershon, M.D.; Breuer, J.; Levin, M.J.; Oaklander, A.L.; Griffiths, P.D. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J. Clin. Virol. 2010, 48 (Suppl. S1), S2–S7. [Google Scholar] [CrossRef]
- O’Connor, K.M.; Paauw, D.S. Herpes zoster. Med. Clin. N. Am. 2013, 97, 503–522. [Google Scholar] [CrossRef]
- Hata, A.; Zerboni, L.; Sommer, M.; Kaspar, A.A.; Clayberger, C.; Krensky, A.M.; Arvin, A.M. Granulysin blocks replication of varicella-zoster virus and triggers apoptosis of infected cells. Viral Immunol. 2001, 14, 125–133. [Google Scholar] [CrossRef]
- Ihara, T.; Kamiya, H.; Starr, S.E.; Arbeter, A.M.; Lange, B. Natural killing of varicella-zoster virus (VZV)-infected fibroblasts in normal children, children with VZV infections, and children with Hodgkin’s disease. Acta Paediatr. Jpn. 1989, 31, 523–528. [Google Scholar] [CrossRef]
- Laing, K.J.; Ouwendijk, W.J.D.; Koelle, D.M.; Verjans, G. Immunobiology of Varicella-Zoster Virus Infection. J. Infect. Dis. 2018, 218 (Suppl. S2), S68–S74. [Google Scholar] [CrossRef]
- Vossen, M.T.; Biezeveld, M.H.; de Jong, M.D.; Gent, M.R.; Baars, P.A.; von Rosenstiel, I.A.; van Lier, R.A.; Kuijpers, T.W. Absence of circulating natural killer and primed CD8+ cells in life-threatening varicella. J. Infect. Dis. 2005, 191, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Tatsis, N.; Ertl, H.C.J. Adenoviruses as vaccine vectors. Mol. Ther. 2004, 10, 616–629. [Google Scholar] [CrossRef]
- Fu, Y.H.; He, J.S.; Zheng, X.X.; Wu, Q.; Zhang, M.; Wang, X.B.; Wang, Y.; Xie, C.; Tang, Q.; Wei, W.; et al. Intranasal immunization with a replication-deficient adenoviral vector expressing the fusion glycoprotein of respiratory syncytial virus elicits protective immunity in BALB/c mice. Biochem. Biophys. Res. Commun. 2009, 381, 528–532. [Google Scholar] [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Galvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef]
- Saeland, E.; van der Fits, L.; Bolder, R.; Heemskerk-van der Meer, M.; Drijver, J.; van Polanen, Y.; Vaneman, C.; Tettero, L.; Serroyen, J.; Schuitemaker, H.; et al. Immunogenicity and protective efficacy of adenoviral and subunit RSV vaccines based on stabilized prefusion F protein in pre-clinical models. Vaccine 2022, 40, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Armour, B.; Chataway, T.; Troelnikov, A.; Colella, A.; Yacoub, O.; Hockley, S.; Tan, C.W.; Gordon, T.P. Vaccine-induced immune thrombotic thrombocytopenia is mediated by a stereotyped clonotypic antibody. Blood 2022, 140, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, S.; D’Agostino, M.R.; Zhang, A.; Stacey, H.D.; Marzok, A.; Kang, A.; Singh, R.; Bavananthasivam, J.; Ye, G.; Luo, X.Q.; et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell 2022, 185, 896–915.e19. [Google Scholar] [CrossRef]
- Criscuolo, E.; Caputo, V.; Diotti, R.A.; Sautto, G.A.; Kirchenbaum, G.A.; Clementi, N. Alternative Methods of Vaccine Delivery: An Overview of Edible and Intradermal Vaccines. J. Immunol. Res. 2019, 2019, 8303648. [Google Scholar] [CrossRef]
- Dendouga, N.; Fochesato, M.; Lockman, L.; Mossman, S.; Giannini, S.L. Cell-mediated immune responses to a varicella-zoster virus glycoprotein E vaccine using both a TLR agonist and QS21 in mice. Vaccine 2012, 30, 3126–3135. [Google Scholar] [CrossRef]
- Goud, R.; Lufkin, B.; Duffy, J.; Whitaker, B.; Wong, H.L.; Liao, J.M.; Lo, A.C.; Parulekar, S.; Agger, P.; Anderson, S.A.; et al. Risk of Guillain-Barre Syndrome Following Recombinant Zoster Vaccine in Medicare Beneficiaries. JAMA Intern. Med. 2021, 181, 1623–1630. [Google Scholar] [CrossRef]
- Nicolai, L.; Leunig, A.; Pekayvaz, K.; Esefeld, M.; Anjum, A.; Rath, J.; Riedlinger, E.; Ehreiser, V.; Mader, M.; Eivers, L.; et al. Thrombocytopenia and splenic platelet-directed immune responses after IV ChAdOx1 nCov-19 administration. Blood 2022, 140, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Laing, K.J.; Russell, R.M.; Dong, L.; Schmid, D.S.; Stern, M.; Magaret, A.; Haas, J.G.; Johnston, C.; Wald, A.; Koelle, D.M. Zoster Vaccination Increases the Breadth of CD4+ T Cells Responsive to Varicella Zoster Virus. J. Infect. Dis. 2015, 212, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Sei, J.J.; Cox, K.S.; Dubey, S.A.; Antonello, J.M.; Krah, D.L.; Casimiro, D.R.; Vora, K.A. Effector and Central Memory Poly-Functional CD4+ and CD8+ T Cells are Boosted upon ZOSTAVAX® Vaccination. Front. Immunol. 2015, 6, 553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, J.; Cao, H.; Liu, C. Immune Responses to Varicella-Zoster Virus Glycoprotein E Formulated with Poly(Lactic-co-Glycolic Acid) Nanoparticles and Nucleic Acid Adjuvants in Mice. Virol. Sin. 2021, 36, 122–132. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Heineman, T.C.; Lal, H.; Godeaux, O.; Chlibek, R.; Hwang, S.J.; McElhaney, J.E.; Vesikari, T.; Andrews, C.; Choi, W.S.; et al. Immune Responses to a Recombinant Glycoprotein E Herpes Zoster Vaccine in Adults Aged 50 Years or Older. J. Infect. Dis. 2018, 217, 1750–1760. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Turner, R.R.; Miller, A.C.; Para, M.F.; Merigan, T.C. Evolution of recurrent herpes simplex lesions. An immunohistologic study. J. Clin. Investig. 1985, 75, 226–233. [Google Scholar] [CrossRef]
- Qi, Q.; Cavanagh, M.M.; Le Saux, S.; Wagar, L.E.; Mackey, S.; Hu, J.; Maecker, H.; Swan, G.E.; Davis, M.M.; Dekker, C.L.; et al. Defective T Memory Cell Differentiation after Varicella Zoster Vaccination in Older Individuals. PLoS Pathog. 2016, 12, e1005892. [Google Scholar] [CrossRef]
- Hayashi, N.; Liu, D.; Min, B.; Ben-Sasson, S.Z.; Paul, W.E. Antigen challenge leads to in vivo activation and elimination of highly polarized TH1 memory T cells. Proc. Natl. Acad. Sci. USA 2002, 99, 6187–6191. [Google Scholar] [CrossRef]
- Younes, S.A.; Yassine-Diab, B.; Dumont, A.R.; Boulassel, M.R.; Grossman, Z.; Routy, J.P.; Sekaly, R.P. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 2003, 198, 1909–1922. [Google Scholar] [CrossRef]
- Abendroth, A.; Lin, I.; Slobedman, B.; Ploegh, H.; Arvin, A.M. Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells. J. Virol. 2001, 75, 4878–4888. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Huang, L.; Ding, H.; Xu, H.; Shu, R.; Yu, J.; Peng, X.; Fu, Y.; He, J. Immunogenicity in Mice Immunized with Recombinant Adenoviruses Expressing Varicella-Zoster Virus Envelope Glycoprotein E. Viruses 2023, 15, 2288. https://doi.org/10.3390/v15122288
Zheng Y, Huang L, Ding H, Xu H, Shu R, Yu J, Peng X, Fu Y, He J. Immunogenicity in Mice Immunized with Recombinant Adenoviruses Expressing Varicella-Zoster Virus Envelope Glycoprotein E. Viruses. 2023; 15(12):2288. https://doi.org/10.3390/v15122288
Chicago/Turabian StyleZheng, Yanpeng, Lei Huang, Huiru Ding, Huawei Xu, Rigan Shu, Jiemei Yu, Xianglei Peng, Yuanhui Fu, and Jinsheng He. 2023. "Immunogenicity in Mice Immunized with Recombinant Adenoviruses Expressing Varicella-Zoster Virus Envelope Glycoprotein E" Viruses 15, no. 12: 2288. https://doi.org/10.3390/v15122288
APA StyleZheng, Y., Huang, L., Ding, H., Xu, H., Shu, R., Yu, J., Peng, X., Fu, Y., & He, J. (2023). Immunogenicity in Mice Immunized with Recombinant Adenoviruses Expressing Varicella-Zoster Virus Envelope Glycoprotein E. Viruses, 15(12), 2288. https://doi.org/10.3390/v15122288





