African Swine Fever: Transmission, Spread, and Control through Biosecurity and Disinfection, Including Polish Trends
Abstract
1. Introduction
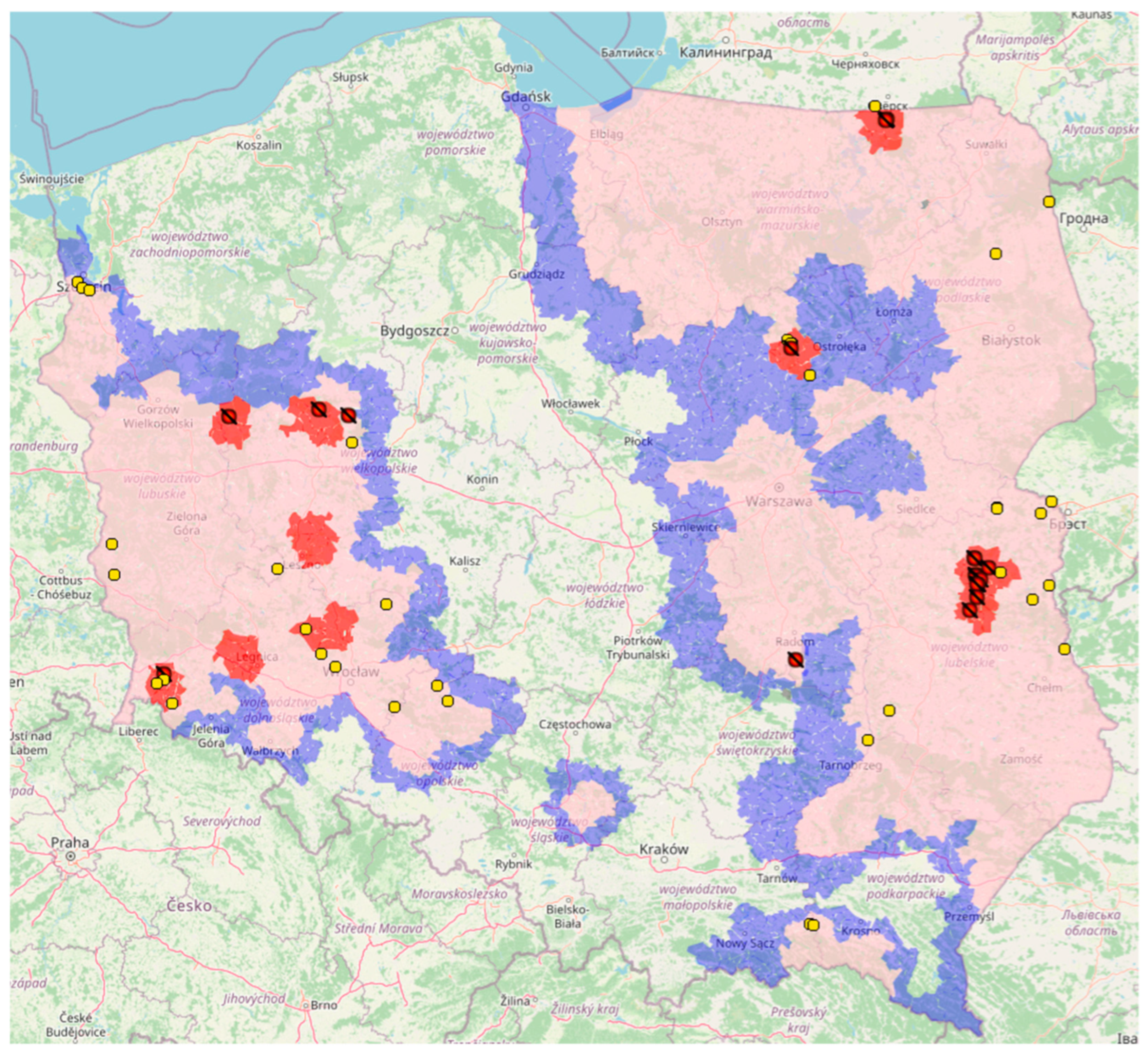
2. Historical Outline and Current Epizootic Situation
3. Etiological Agent
4. Host Range and ASFV Vectors
5. Susceptibility of ASFV to Physical and Chemical Agents
6. Disease Control: Combating ASF through Administrative Methods
7. Prevention—Monitoring and Regulation of Wild Boar Populations
8. Perspectives on Developing a Vaccine against ASF
9. Biosecurity of Swine Herds
10. Disinfection
- −
- 1% formaldehyde;
- −
- sodium hypochlorite (0.0075% to 0.03%);
- −
- 2% caustic soda solution (the most potent virucide);
- −
- glutaraldehyde, formaldehyde;
- −
- 1% sodium or calcium hydroxide (inactivation of the virus in suspension at 4 °C);
- −
- phenols, such as Lysol and creolin;
- −
- lipid solvent-based chemicals;
- −
11. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Główny Urząd Statystyczny. Pogłowie Świń Według Stanu w Grudniu 2017 r.; Główny Urząd Statystyczny: Warsaw, Poland, 2018.
- Beltrán-Alcrudo, D.; Arias, M.; Gallardo, C.; Kramer, S.A.; Penrith, M.L. A Manual for Veterinarians Manual African Swine Fever: Detection and Diagnosis; FAO: Rome, Italy, 2017; ISBN 9789251097526. [Google Scholar]
- Podgórski, T.; Pepin, K.M.; Radko, A.; Podbielska, A.; Łyjak, M.; Woźniakowski, G.; Borowik, T. How do genetic relatedness and spatial proximity shape African swine fever infections in wild boar? Transbound. Emerg. Dis. 2022, 69, 2656–2666. [Google Scholar] [CrossRef] [PubMed]
- Penrith, M.-L. African swine fever. Onderstepoort J. Vet. Res. 2009, 76, 91–95. [Google Scholar] [CrossRef] [PubMed]
- USDA United States Department of Agriculture. Livestock and Poultry: World Markets and Trade China’s Meat and Poultry Import Forecast 2018: Decline and Constrained Growth. Foreign Agric. Serv. 2017, 27. [Google Scholar]
- Sánchez-Cordón, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, K. African swine fever in China. Vet. Rec. 2018, 183, 300–301. [Google Scholar] [CrossRef]
- Główny Urząd Statystyczny. Pogłowie Świń Według Stanu w Czerwcu 2019 r.; Główny Urząd Statystyczny: Warsaw, Poland, 2019.
- Rykaczewski, G. Produkcja Trzody Chlewnej; Santander Bank Polska S.A.: Warsaw, Poland, 2019. [Google Scholar]
- Eustace Montgomery, R. On A Form of Swine Fever Occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- Costard, S.; Wieland, B.; De Glanville, W.; Jori, F.; Rowlands, R.; Vosloo, W.; Roger, F.; Pfeiffer, D.U.; Dixon, L.K. African swine fever: How can global spread be prevented? Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2683–2696. [Google Scholar] [CrossRef]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Martínez-López, B. African swine fever (ASF): Five years around Europe. Vet. Microbiol. 2013, 165, 45–50. [Google Scholar] [CrossRef]
- Sardinia Free of ASF [Internet]. Available online: https://www.sanidadanimal.info/en/712-sardinia-free-asf (accessed on 14 July 2023).
- ASF Italy: First Case Detected in Piedmont Region [Internet]. Available online: https://www.pigprogress.net/health-nutrition/health/asf-italy-first-case-detected-in-piedmont-region/ (accessed on 20 July 2023).
- Regione Autonoma della Sardegna Peste suina, confermato il genotipo due nell’allevamento di Dorgali. L’assessore Doria: “Caso di importazione, reazione tempestiva del sistema sanitario”. Available online: https://www.regione.sardegna.it/ (accessed on 10 October 2023).
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef]
- Guinat, C.; Gogin, A.; Blome, S.; Keil, G.; Pollin, R.; Pfeiffer, D.U.; Dixon, L. Transmission routes of African swine fever virus to domestic pigs: Current knowledge and future research directions. Vet. Rec. 2016, 178, 262–267. [Google Scholar] [CrossRef]
- Arias, M.; Jurado, C.; Gallardo, C.; Fernández-Pinero, J.; Sánchez-Vizcaíno, J.M. Gaps in African swine fever: Analysis and priorities. Transbound. Emerg. Dis. 2018, 65, 235–247. [Google Scholar] [CrossRef]
- Costard, S.; Mur, L.; Lubroth, J.; Sanchez-Vizcaino, J.M.; Pfeiffer, D.U. Epidemiology of African swine fever virus. Virus Res. 2013, 173, 191–197. [Google Scholar] [CrossRef]
- Pejsak, Z.; Truszczyński, M.; Niemczuk, K.; Kozak, E.; Markowska-Daniel, I. Epidemiology of African Swine Fever in Poland since the detection of the first case. Pol. J. Vet. Sci. 2014, 17, 665–672. [Google Scholar] [CrossRef]
- OIE World Organization of Animal Health. African Swine Fever (ASF)—Situation Report; OIE World Organization of Animal Health: Paris, France, 2021.
- Le Potier, M.F. African swine fever in Europe. Bull. Acad. Vet. Fr. 2021, 174, 298–303. [Google Scholar] [CrossRef]
- FAO. African Swine Fever Threatens People’ s Republic of China. Anim. Health Risk Anal. 2018, 1-20, 1–20. [Google Scholar]
- Perrin, L.; Bowen, J. African Swine Fever (ASF) in South East Asia; Department for Environment, Food and Rural Affairs, Animal and Plant Health Agency: Addlestone, UK, 2021; pp. 1–5.
- OIE World Organization of Animal Health. African Swine Fever (ASF)—Situation Report 2 ASF Distribution and the Situation in 2020 and 2021 (Based on INs, FURs and SMRs) African Swine Fever (ASF)—Situation Report 2 Summary of the ASF Situation by World Region (2020–2021); OIE World Organization of Animal Health: Paris, France, 2021; Volume 2021.
- Van Goethem, B. Update on African Swine Fever Situation in the EU. 2021, pp. 1–16. Available online: https://www.europarl.europa.eu/cmsdata/239117/ASF%20EU%20update%201%20Sept%202021%20-%20EP-final.pdf (accessed on 16 November 2023).
- World Animal Health Information System (WAHIS). African Swine Fever (ASF)—Situation Report 32; World Animal Health Information System (WAHIS): Paris, France, 2023. [Google Scholar]
- Śmietanka, K.; Woźniakowski, G.; Kozak, E.; Niemczuk, K.; Frączyk, M.; Bocian, Ł.; Kowalczyk, A.; Pejsak, Z. African Swine Fever Epidemic, Poland, 2014–2015. Emerg. Infect. Dis. 2016, 22, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Woźniakowski, G.; Kozak, E.; Kowalczyk, A.; Łyjak, M.; Pomorska-Mól, M.; Niemczuk, K.; Pejsak, Z. Current status of African swine fever virus in a population of wild boar in eastern Poland (2014–2015). Arch. Virol. 2016, 161, 189–195. [Google Scholar] [CrossRef]
- Woźniakowski, G.; Pejsak, Z.; Jabłoński, A. Emergence of african swine fever in Poland (2014–2021). successes and failures in disease eradication. Agriculture 2021, 11, 738. [Google Scholar] [CrossRef]
- Hawes, P.C.; Netherton, C.L.; Wileman, T.E.; Monaghan, P. The Envelope of Intracellular African Swine Fever Virus Is Composed of a Single Lipid Bilayer. J. Virol. 2008, 82, 7905–7912. [Google Scholar] [CrossRef] [PubMed]
- Przedpełski, K. Effective Biosecurity. Available online: www.krir.pl/2014-01-03-03-24-03/afrykanski-pomor-swin/5585-skuteczna-biosakuracja (accessed on 16 November 2023).
- Konopka, B.; Welz, M.; Bocian, Ł.; Niemczuk, K.; Walczak, M.; Frant, M.; Mazur, N. Analiza przebiegu epizootii afrykańskiego pomoru świń w zachodniej Polsce. Życie Weter. 2020, 95, 468–475. [Google Scholar]
- Pejsak, Z.; Niemczuk, K.; Frant, M.; Mazur, N.; Pomorska-Mól, M.; Ziętek-Barszcz, A.; Bocian, Ł.; Łyjak, M.; Borowska, D.; Woźniakowski, G. Four years of African swine fever in Poland. New insights into epidemiology and prognosis of future disease spread. Pol. J. Vet. Sci. 2018, 21, 835–841. [Google Scholar] [CrossRef]
- ROZPORZĄDZENIE WYKONAWCZE KOMISJI (UE) 2021/605 z dnia 7 kwietnia 2021 r. ustanawiające szczególne środki zwalczania afrykańskiego pomoru świń; Komisja Europejska: Warsaw, Poland, 2021.
- Walczak, M.; Frant, M.; Juszkiewicz, M.; Mazur-Panasiuk, N.; Szymankiewicz, K.; Bruczyńska, M.; Woźniakowski, G. Vertical transmission of anti-ASFV antibodies as one of potential causes of seropositive results among young wild boar population in Poland. Pol. J. Vet. Sci. 2020, 23, 21–25. [Google Scholar] [CrossRef]
- Boklund, A.; Cay, B.; Depner, K.; Földi, Z.; Guberti, V.; Masiulis, M.; Miteva, A.; More, S.; Olsevskis, E.; Šatrán, P.; et al. Epidemiological analyses of African swine fever in the European Union (November 2017 until November 2018). EFSA J. 2018, 16, e05494. [Google Scholar] [CrossRef]
- GIW General Veterinary Inspectorate/Główny Inspektorat Weterynarii. Afrykanski Pomór Świń (ASF). Available online: https://bip.wetgiw.gov.pl/asf/mapa/ (accessed on 8 August 2023).
- Wang, N.; Zhao, D.; Wang, J.; Zhang, Y.; Wang, M.; Gao, Y.; Li, F.; Wang, J.; Bu, Z.; Rao, Z.; et al. Architecture of African swine fever virus and implications for viral assembly. Science 2019, 366, 640–644. [Google Scholar] [CrossRef]
- Beato, M.S.; D’errico, F.; Iscaro, C.; Petrini, S.; Giammarioli, M.; Feliziani, F. Disinfectants against African Swine Fever: An Updated Review. Viruses 2022, 14, 1384. [Google Scholar] [CrossRef]
- Andrés, G.; Charro, D.; Matamoros, T.; Dillard, R.S.; Abrescia, N.G.A. The cryo-EM structure of African swine fever virus unravels a unique architecture comprising two icosahedral protein capsids and two lipoprotein membranes. J. Biol. Chem. 2020, 295, 1–12. [Google Scholar] [CrossRef]
- Dixon, L.K.; Chapman, D.A.G.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kang, W.; Yang, W.; Zhang, J.; Li, D.; Zheng, H. Structure of African Swine Fever Virus and Associated Molecular Mechanisms Underlying Infection and Immunosuppression: A Review. Front. Immunol. 2021, 12, 715582. [Google Scholar] [CrossRef] [PubMed]
- Oberin, M.; Hillman, A.; Ward, M.P.; Holley, C.; Firestone, S.; Cowled, B. The Potential Role of Wild Suids in African Swine Fever Spread in Asia and the Pacific Region. Viruses 2022, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Laddomada, A. The last mile in the eradication of ASF in Sardinia. Bull. l’OIE 2020, 2020, 1–4. [Google Scholar] [CrossRef]
- Laddomada, A.; Rolesu, S.; Loi, F.; Cappai, S.; Oggiano, A.; Madrau, M.P.; Sanna, M.L.; Pilo, G.; Bandino, E.; Brundu, D.; et al. Surveillance and control of African Swine Fever in free-ranging pigs in Sardinia. Transbound. Emerg. Dis. 2019, 66, 1114–1119. [Google Scholar] [CrossRef]
- Walczak, M.; Żmudzki, J.; Mazur-Panasiuk, N.; Juszkiewicz, M.; Woźniakowski, G. Analysis of the Clinical Course of Experimental Infection with Highly Pathogenic African Swine Fever Strain, Isolated from an Outbreak in Poland. Aspects Related to the Disease Suspicion at the Farm Level. Pathogens 2020, 9, 237. [Google Scholar] [CrossRef]
- Zimmerman, J.J.; Karriker, L.A.; Ramirez, A.; Schwartz, K.J.; Stevenson, G.W. Diseases of Swine, 10th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 9780813822679. [Google Scholar]
- Schulz, K.; Oļševskis, E.; Viltrop, A.; Masiulis, M.; Staubach, C.; Nurmoja, I.; Lamberga, K.; Seržants, M.; Malakauskas, A.; Conraths, F.J.; et al. Eight Years of African Swine Fever in the Baltic States: Epidemiological Reflections. Pathogens 2022, 11, 711. [Google Scholar] [CrossRef] [PubMed]
- Penrith, M.-L.; Vosloo, W. Review of African swine fever: Transmission, spread and control: Review article. J. S. Afr. Vet. Assoc. 2009, 80, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Frant, M.; Woźniakowski, G.; Pejsak, Z. African swine fever (ASF) and ticks. No risk of tick-mediated ASF spread in Poland and Baltic states. J. Vet. Res. 2017, 61, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Boinas, F.S.; Wilson, A.J.; Hutchings, G.H.; Martins, C.; Dixon, L.J. The persistence of African swine fever virus in field-infected Ornithodoros erraticus during the ASF endemic period in Portugal. PLoS ONE 2011, 6, e20383. [Google Scholar] [CrossRef] [PubMed]
- Cukor, J.; Linda, R.; Václavek, P.; Mahlerová, K.; Šatrán, P.; Havránek, F. Confirmed cannibalism in wild boar and its possible role in African swine fever transmission. Transbound. Emerg. Dis. 2020, 67, 1068–1073. [Google Scholar] [CrossRef]
- Denstedt, E.; Porco, A.; Hwang, J.; Nga, N.T.T.; Ngoc, P.T.B.; Chea, S.; Khammavong, K.; Milavong, P.; Sours, S.; Osbjer, K.; et al. Detection of African swine fever virus in free-ranging wild boar in Southeast Asia. Transbound. Emerg. Dis. 2021, 68, 2669–2675. [Google Scholar] [CrossRef]
- Olesen, A.S.; Lohse, L.; Boklund, A.; Halasa, T.; Gallardo, C.; Pejsak, Z.; Belsham, G.J.; Rasmussen, T.B.; Bøtner, A. Transmission of African swine fever virus from infected pigs by direct contact and aerosol routes. Vet. Microbiol. 2017, 211, 92–102. [Google Scholar] [CrossRef]
- Fila, M.; Woźniakowski, G. African swine fever virus—The possible role of flies and other insects in virus transmission. J. Vet. Res. 2020, 64, 1–7. [Google Scholar] [CrossRef]
- Olesen, A.S.; Lohse, L.; Frimodt, M.; Anette, H.; Halasa, T.; Belsham, G.J.; Bruun, T.; Anette, R. Infection of pigs with African swine fever virus via ingestion of stable flies (Stomoxys calcitrans). Transbound. Emerg. Dis. 2018, 65(5), 1152–1157. [Google Scholar] [CrossRef]
- de Carvalho Ferreira, H.C.; Tudela Zúquete, S.; Wijnveld, M.; Weesendorp, E.; Jongejan, F.; Stegeman, A.; Loeffen, W.L.A. No evidence of African swine fever virus replication in hard ticks. Ticks Tick Borne Dis. 2014, 5, 582–589. [Google Scholar] [CrossRef]
- Chenais, E.; Depner, K.; Guberti, V.; Dietze, K.; Viltrop, A.; Ståhl, K. Epidemiological considerations on African swine fever in Europe 2014-2018. Porc. Health Manag. 2019, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Flis, M.; Nestorowicz, J. Afrykański pomór świń w Polsce—Drogi i kierunki rozprzestrzeniania się choroby ze szczególnym uwzględnieniem województwa lubelskiego. Życie Weter. 2017, 94, 574–577. [Google Scholar]
- Kalmar, I.D.; Cay, A.B.; Tignon, M. Sensitivity of African swine fever virus (ASFV) to heat, alkalinity and peroxide treatment in presence or absence of porcine plasma. Vet. Microbiol. 2018, 219, 144–149. [Google Scholar] [CrossRef]
- Davies, K.; Goatley, L.C.; Guinat, C.; Netherton, C.L.; Gubbins, S.; Dixon, L.K.; Reis, A.L. Survival of African Swine Fever Virus in Excretions from Pigs Experimentally Infected with the Georgia 2007/1 Isolate. Transbound. Emerg. Dis. 2017, 64, 425–431. [Google Scholar] [CrossRef]
- Mebus, C.A.; House, C.; Gonzalvo, F.R.; Pineda, J.M.; Tapiador, J.; Pire, J.J.; Bergada, J.; Yedloutschnig, R.J.; Sahu, S.; Becerra, V.; et al. Survival of foot-and-mouth disease, African swine fever, and hog cholera viruses in Spanish serrano cured hams and Iberian cured hams, shoulders and loins. Food Microbiol. 1993, 10, 133–143. [Google Scholar] [CrossRef]
- Mebus, C.; Arias, M.; Pineda, J.M.; Tapiador, J.; House, C.; Sánchez-Vizcaíno, J.M. Survival of several porcine viruses in different Spanish dry-cured meat products. Food Chem. 1997, 59, 555–559. [Google Scholar] [CrossRef]
- Plowright, W.; Parker, J. The stability of African swine fever virus with particular reference to heat and pH inactivation. Arch. Gesamte Virusforsch. 1967, 21, 383–402. [Google Scholar] [CrossRef]
- Turner, C.; Williams, S.M. Laboratory-scale inactivation of African swine fever virus and swine vesicular disease virus in pig slurry. J. Appl. Microbiol. 1999, 87, 148–157. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Żmudzki, J.; Woźniakowski, G. African swine fever virus—Persistence in different environmental conditions and the possibility of its indirect transmission. J. Vet. Res. 2019, 63, 303–310. [Google Scholar] [CrossRef]
- Dee, S.A.; Bauermann, F.V.; Niederwerder, M.C.; Singrey, A.; Clement, T.; de Lima, M.; Long, C.; Patterson, G.; Sheahan, M.A.; Stoian, A.M.M.; et al. Survival of viral pathogens in animal feed ingredients under transboundary shipping models. PLoS ONE 2018, 13, e0194509. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.; Martínez-López, B.; Sánchez-Vizcaíno, J.M. Risk of African swine fever introduction into the European Union through transport-associated routes: Returning trucks and waste from international ships and planes. BMC Vet. Res. 2012, 8, 149. [Google Scholar] [CrossRef]
- Zani, L.; Masiulis, M.; Bušauskas, P.; Dietze, K.; Pridotkas, G.; Globig, A.; Blome, S.; Mettenleiter, T.; Depner, K.; Karvelienė, B. African swine fever virus survival in buried wild boar carcasses. Transbound. Emerg. Dis. 2020, 67, tbed.13554. [Google Scholar] [CrossRef] [PubMed]
- Cortiñas Abrahantes, J.; Gogin, A.; Richardson, J.; Gervelmeyer, A.; Depner, K.; Gortazar, C.; Guberti, V.; Masiulis, M.; More, S.; Oļševskis, E.; et al. Epidemiological analyses on African swine fever in the Baltic countries and Poland. EFSA J. 2017, 15, e04732. [Google Scholar] [CrossRef]
- Probst, C.; Gethmann, J.; Amendt, J.; Lutz, L.; Teifke, J.P.; Conraths, F.J. Estimating the Postmortem Interval of Wild Boar Carcasses. Vet. Sci. 2020, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Gethmann, J.; Amler, S.; Globig, A.; Knoll, B.; Conraths, F.J. The potential role of scavengers in spreading African swine fever among wild boar. Sci. Rep. 2019, 9, 11450. [Google Scholar] [CrossRef]
- Frant, M.; Gal, A.; Bocian, Ł.; Ziętek-Barszcz, A.; Niemczuk, K.; Woźniakowski, G. African Swine Fever Virus (ASFV) in Poland in 2019—Wild Boars: Searching Pattern. Agriculture 2021, 11, 45. [Google Scholar] [CrossRef]
- More, S.; Miranda, M.A.; Bicout, D.; Bøtner, A.; Butterworth, A.; Calistri, P.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Michel, V.; et al. African swine fever in wild boar. EFSA J. 2018, 16, e05344. [Google Scholar] [CrossRef]
- Pejsak, Z.; Truszczyński, M. Oporność wirusa afrykańskiego pomoru świń na warunki środowiska oraz czynniki fizyczne i chemiczne. Życie Weter 2017, 92, 880–882. [Google Scholar]
- Cycle, L. African Swine Fever Aetiology; World Organisation for Animal Health: Paris, France, 2013; pp. 1–500.
- Główny Inspektorat Weterynarii (GIW). Przepisy Prawne Dotyczące ASF Prawo Unijne [Internet]. Available online: https://www.wetgiw.gov.pl/nadzor-weterynaryjny/przepisy-prawne (accessed on 23 July 2023).
- Juszkiewicz, M.; Walczak, M.; Mazur-Panasiuk, N.; Woźniakowski, G. Effectiveness of chemical compounds used against african swine fever virus in commercial available disinfectants. Pathogens 2020, 9, 878. [Google Scholar] [CrossRef]
- Główny Inspektorat Weterynarii (GIW). Likwidacja Ognisk ASF. Available online: https://www.wetgiw.gov.pl/nadzor-weterynaryjny/zwalczanie (accessed on 4 September 2023).
- European Commission. Available online: https://food.ec.europa.eu/animals/animal-diseases/diseases-and-control-measures/african-swine-fever_en (accessed on 4 September 2023).
- Główny Inspektorat Weterynarii (GIW). Mapa Ognisk ASF w Polsce Oraz Aktualny Zasięg Obszarów Objętych Ograniczeniami (Zgodnie z Rozporządzeniem Wykonawczym Komisji (UE) 2023/1485 z Dnia 18 Lipca 2023 r. Available online: https://bip.wetgiw.gov.pl/asf/mapa/ (accessed on 8 August 2023).
- Ministerstwo Rolnictwa i Rozwoju Wsi. Likwidacja Ogniska ASF; Ministerstwo Rolnictwa i Rozwoju Wsi: Warsaw, Poland, 2020.
- Ministerstwo Rolnictwa i Rozwoju Wsi. Afrykański Pomór Świń: Ponowne Zasiedlanie Gospodarstw po Wystąpieniu Ogniska Choroby; Ministerstwo Rolnictwa i Rozwoju Wsi: Warsaw, Poland, 2019.
- Krzysztof Śmietanka, Z.P. Epidemiologia afrykańskiego pomoru świń ze szczególnym uwzględnieniem sytuacji w Polsce. In Afrykański Pomór Świń; PIWet-PIB: Puławy, Poland, 2016; p. 197. [Google Scholar]
- Desmecht, D.; Gerbier, G.; Gortázar Schmidt, C.; Grigaliuniene, V.; Helyes, G.; Kantere, M.; Korytarova, D.; Linden, A.; Miteva, A.; Neghirla, I.; et al. Epidemiological analysis of African swine fever in the European Union (September 2019 to August 2020). EFSA J. 2021, 19, e06572. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a Highly Effective African Swine Fever Virus Vaccine by Deletion of the I177L Gene Results in Sterile Immunity against the Current Epidemic Eurasia Strain. J. Virol. 2020, 94, e02017-19. [Google Scholar] [CrossRef]
- Sánchez, E.G.; Pérez-Núñez, D.; Revilla, Y. Development of vaccines against African swine fever virus. Virus Res. 2019, 265, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Kihm, U.; Ackermann, M.; Mueller, H.; Pool, R. Approaches to Vaccination; Springer: Boston, MA, USA, 1987; pp. 127–144. [Google Scholar]
- Stone, S.S.; Hess, W.R. Antibody response to inactivated preparations of African swine fever virus in pigs. Am. J. Vet. Res. 1967, 28, 475–481. [Google Scholar]
- Blome, S.; Gabriel, C.; Beer, M. Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine 2014, 32, 3879–3882. [Google Scholar] [CrossRef]
- Argilaguet, J.M.; Pérez-Martín, E.; Nofrarías, M.; Gallardo, C.; Accensi, F.; Lacasta, A.; Mora, M.; Ballester, M.; Galindo-Cardiel, I.; López-Soria, S.; et al. DNA Vaccination Partially Protects against African Swine Fever Virus Lethal Challenge in the Absence of Antibodies. PLoS ONE 2012, 7, e40942. [Google Scholar] [CrossRef]
- Arias, M.; De Torre, A.; Dixon, L.; Gallardo, C.; Jori, F.; Laddomada, A.; Martins, C.; Parkhouse, R.M.; Revilla, Y.; Rodriguez, F. African Swine Fever Virus Vaccines. Vaccines 2017, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Salguero, F.J.; Gil, S.; Revilla, Y.; Gallardo, C.; Arias, M.; Martins, C. Cytokine mRNA expression and pathological findings in pigs inoculated with African swine fever virus (E-70) deleted on A238L. Vet. Immunol. Immunopathol. 2008, 124, 107–119. [Google Scholar] [CrossRef]
- Li, G.; Liu, X.; Yang, M.; Zhang, G.; Wang, Z.; Guo, K.; Gao, Y.; Jiao, P.; Sun, J.; Chen, C.; et al. Crystal structure of the African swine fever virus pS273R protease and implications for inhibitor design. J. Virol. 2020, 94, e02125-19. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Mao, R.; Zhou, Y.; Yin, J.; Sun, Y.; Yin, X. Research progress on live attenuated vaccine against African swine fever virus. Microb. Pathog. 2021, 158, 105024. [Google Scholar] [CrossRef]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Espinoza, N.; Velazquez-Salinas, L.; Gay, C.G.; Gladue, D.P. ASFV-G-∆I177L as an Effective Oral Nasal Vaccine against the Eurasia Strain of Africa Swine Fever. Viruses 2021, 13, 765. [Google Scholar] [CrossRef]
- Deutschmann, P.; Carrau, T.; Sehl-Ewert, J.; Forth, J.H.; Viaplana, E.; Mancera, J.C.; Urniza, A.; Beer, M.; Blome, S. Taking a Promising Vaccine Candidate Further: Efficacy of ASFV-G-ΔMGF after Intramuscular Vaccination of Domestic Pigs and Oral Vaccination of Wild Boar. Pathogens 2022, 11, 996. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci. 2020, 63, 623–634. [Google Scholar] [CrossRef]
- Reuters Vietnam Approves Commercial Use of First African Swine Fever Vaccines. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/vietnam-approves-commercial-use-first-african-swine-fever-vaccines-2023-07-24/ (accessed on 5 September 2023).
- Zhao, D.; Sun, E.; Huang, L.; Ding, L.; Zhu, Y.; Zhang, J.; Shen, D.; Zhang, X.; Zhang, Z.; Ren, T.; et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat. Commun. 2023, 14, 3096. [Google Scholar] [CrossRef] [PubMed]
- Pejsak, Z.; Truszczyński, M. Szczepionka przeciwko afrykańskiemu pomorowi świń. Życie Weter. 2020, 95, 358–361. [Google Scholar]
- Walczak, M.; Szczotka-Bochniarz, A.; Żmudzki, J.; Juszkiewicz, M.; Szymankiewicz, K.; Niemczuk, K.; Pérez-Núñez, D.; Liu, L.; Revilla, Y. Non-Invasive Sampling in the Aspect of African Swine Fever Detection—A Risk to Accurate Diagnosis. Viruses 2022, 14, 1756. [Google Scholar] [CrossRef] [PubMed]
- Bellini, S.; Rutili, D.; Guberti, V. Preventive measures aimed at minimizing the risk of African swine fever virus spread in pig farming systems. Acta Vet. Scand. 2016, 58, 82. [Google Scholar] [CrossRef]
- Ouma, E.; Dione, M.; Birungi, R.; Lule, P.; Mayega, L.; Dizyee, K. African swine fever control and market integration in Ugandan peri-urban smallholder pig value chains: An ex-ante impact assessment of interventions and their interaction. Prev. Vet. Med. 2018, 151, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Barongo, M.B.; Bishop, R.P.; Fèvre, E.M.; Knobel, D.L.; Ssematimba, A. A Mathematical Model that Simulates Control Options for African Swine Fever Virus (ASFV). PLoS ONE 2016, 11, e0158658. [Google Scholar] [CrossRef]
- Główny Inspektorat Weterynarii (GIW). Przestrzeganie Zasad Bioasekuracji w Gospodarstwach Podlegających Wymaganiom Rozporządzenia ws Środków ASF; Główny Inspektorat Weterynarii (GIW): Warszawa, Poland, 2013; pp. 1–8.
- Pejsak, Z.; Truszczyński, M. Bioasekuracja—Podstawowy sposób ochrony zwierząt przed chorobami zakaźnymi. Życie Weter. 2017, 92, 427–430. [Google Scholar]
- Pudenz, C.C.; Schulz, L.L.; Tonsor, G.T. Adoption of Secure Pork Supply Plan Biosecurity by U.S. Swine Producers. Front. Vet. Sci. 2019, 6, 146. [Google Scholar] [CrossRef]
- Mutua, F.; Dione, M. The Context of Application of Biosecurity for Control of African Swine Fever in Smallholder Pig Systems: Current Gaps and Recommendations. Front. Vet. Sci. 2021, 8, 689811. [Google Scholar] [CrossRef] [PubMed]
- FAD-PREP. NAHEMS NAHEMS Guidelines: Biosecurtiy; Center for Food Security and Public Health: Ames, IA, USA; U.S. Department of Agriculture Animal and Plant Health Inspection Service: Washington, DC, USA, 2016; pp. 2–56.
- Dietze, K.; Depner, K. Role of Biosecurity in Protecting Farms against ASF 1st Step: Collecting Our Thoughts Is Biosecurity Important? Biosecurity: EU Animal Health Law. 2019, pp. 1–14. Available online: https://www.fao.org/fileadmin/user_upload/reu/europe/documents/events2019/ASFBalkans/16.pdf (accessed on 16 November 2023).
- Juszkiewicz, M.; Walczak, M.; Mazur-Panasiuk, N.; Woźniakowski, G. Virucidal effect of chosen disinfectants against African swine fever virus (ASFV)—Preliminary studies. Pol. J. Vet. Sci. 2019, 22, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Štukelj, M.; Bellini, S. Understanding and Combatting African Swine Fever; Wageningen Academic Publishers: Noordwijk, The Netherlands, 2021; pp. 283–304. [Google Scholar] [CrossRef]
- De Lorenzi, G.; Borella, L.; Alborali, G.L.; Prodanov-Radulović, J.; Štukelj, M.; Bellini, S. African swine fever: A review of cleaning and disinfection procedures in commercial pig holdings. Res. Vet. Sci. 2020, 132, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Juszkiewicz, M.; Walczak, M.; Woźniakowski, G. Characteristics of selected active substances used in disinfectants and their virucidal activity against ASFV. J. Vet. Res. 2019, 63, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Gallina, L.; Scagliarini, A. Virucidal efficacy of common disinfectants against orf virus. Vet. Rec. 2010, 166, 725. [Google Scholar] [CrossRef]
- Shirai, J.; Kanno, T.; Tsuchiya, Y.; Mitsubayashi, S.; Seki, R. Effects of Chlorine, Iodine, and Quaternary Ammonium Compound Disinfectants on Several Exotic Disease Viruses. J. Vet. Med. Sci. 2000, 62, 85–92. [Google Scholar] [CrossRef]
- Shirai, J.; Kanno, T.; Inoue, T.; Mitsubayashi, S.; Seki, R. Effects of Quaternary Ammonium Compounds with 0.1% Sodium Hydroxide on Swine Vesicular Disease Virus. J. Vet. Med. Sci. 1997, 59, 323–328. [Google Scholar] [CrossRef][Green Version]
- Krug, P.W.; Lee, L.J.; Eslami, A.C.; Larson, C.R.; Rodriguez, L. Chemical disinfection of high-consequence transboundary animal disease viruses on nonporous surfaces. Biologicals 2011, 39, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Krug, P.W.; Larson, C.R.; Eslami, A.C.; Rodriguez, L.L. Disinfection of foot-and-mouth disease and African swine fever viruses with citric acid and sodium hypochlorite on birch wood carriers. Vet. Microbiol. 2012, 156, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Krug, P.W.; Davis, T.; O’Brien, C.; LaRocco, M.; Rodriguez, L.L. Disinfection of transboundary animal disease viruses on surfaces used in pork packing plants. Vet. Microbiol. 2018, 219, 219–225. [Google Scholar] [CrossRef]
- Gabbert, L.R.; Neilan, J.G.; Rasmussen, M. Recovery and chemical disinfection of foot-and-mouth disease and African swine fever viruses from porous concrete surfaces. J. Appl. Microbiol. 2020, 129, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Luo, R.; Wang, T.; Qi, M.; Wang, B.; Sun, M.; Luo, Y.; Ji, C.; Sun, Y.; Qiu, H.J. Efficient inactivation of African swine fever virus by a highly complexed iodine. Vet. Microbiol. 2021, 263, 109245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Sun, Y.; Zhang, F.; Ai, X.; Feng, X.; Hu, W.; Zhang, X.; Zhao, D.; Bu, Z.; He, X. Viricidal activity of several disinfectants against African swine fever virus. J. Integr. Agric. 2021, 20, 3084–3088. [Google Scholar] [CrossRef]
- Paliy, A.P.; Stegniy, B.T.; Kuzminov, A.V.; Buzun, A.I.; Gerilovich, A.P.; Bogach, M.V.; Stegniy, M.Y. Effectiveness of aldehyde disinfectant “DZPT-2” against the African swine fever virus. Ukr. J. Ecol. 2020, 10, 131–138. [Google Scholar]
- World Organisation for Animal Health—WOAH Terrestrial Animal Health Code, General Recommendations on Disinfection and Disinsectisation. World Organ. Anim. Health 2011, 1, 169.
- Juszkiewicz, M.; Walczak, M.; Woźniakowski, G.; Szczotka-Bochniarz, A. Virucidal Activity of Plant Extracts against African Swine Fever Virus. Pathogens 2021, 10, 1357. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Technical Disease Card for African Swine Fever; World Organisation for Animal Health: Paris, France, 2019.
- Fischer, M.; Mohnke, M.; Probst, C.; Pikalo, J.; Conraths, F.J.; Beer, M.; Blome, S. Stability of African swine fever virus on heat-treated field crops. Transbound. Emerg. Dis. 2020, 67, 2318–2323. [Google Scholar] [CrossRef]
- FAO. Preparation of African Swine Fever Contingency Plans; FAO: Rome, Italy, 2009; Volume FAO Animal, ISBN 9789251064269. [Google Scholar]
- Zhang, L.; Luo, Y.; Wang, W.; Sun, Y.; Zhang, J.; Fatima, M.; Jia, X.; Qiu, H.-J. Efficient inactivation of African swine fever virus by ozonized water. Vet. Microbiol. 2020, 247, 108796. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juszkiewicz, M.; Walczak, M.; Woźniakowski, G.; Podgórska, K. African Swine Fever: Transmission, Spread, and Control through Biosecurity and Disinfection, Including Polish Trends. Viruses 2023, 15, 2275. https://doi.org/10.3390/v15112275
Juszkiewicz M, Walczak M, Woźniakowski G, Podgórska K. African Swine Fever: Transmission, Spread, and Control through Biosecurity and Disinfection, Including Polish Trends. Viruses. 2023; 15(11):2275. https://doi.org/10.3390/v15112275
Chicago/Turabian StyleJuszkiewicz, Małgorzata, Marek Walczak, Grzegorz Woźniakowski, and Katarzyna Podgórska. 2023. "African Swine Fever: Transmission, Spread, and Control through Biosecurity and Disinfection, Including Polish Trends" Viruses 15, no. 11: 2275. https://doi.org/10.3390/v15112275
APA StyleJuszkiewicz, M., Walczak, M., Woźniakowski, G., & Podgórska, K. (2023). African Swine Fever: Transmission, Spread, and Control through Biosecurity and Disinfection, Including Polish Trends. Viruses, 15(11), 2275. https://doi.org/10.3390/v15112275







