An Outbreak of Parvovirus B19 in Israel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data
2.1.1. Data Sources
2.1.2. Data Extraction and Study Population
2.2. Study Design and Statistical Analysis
2.3. Ethics and Data Declarations
3. Results
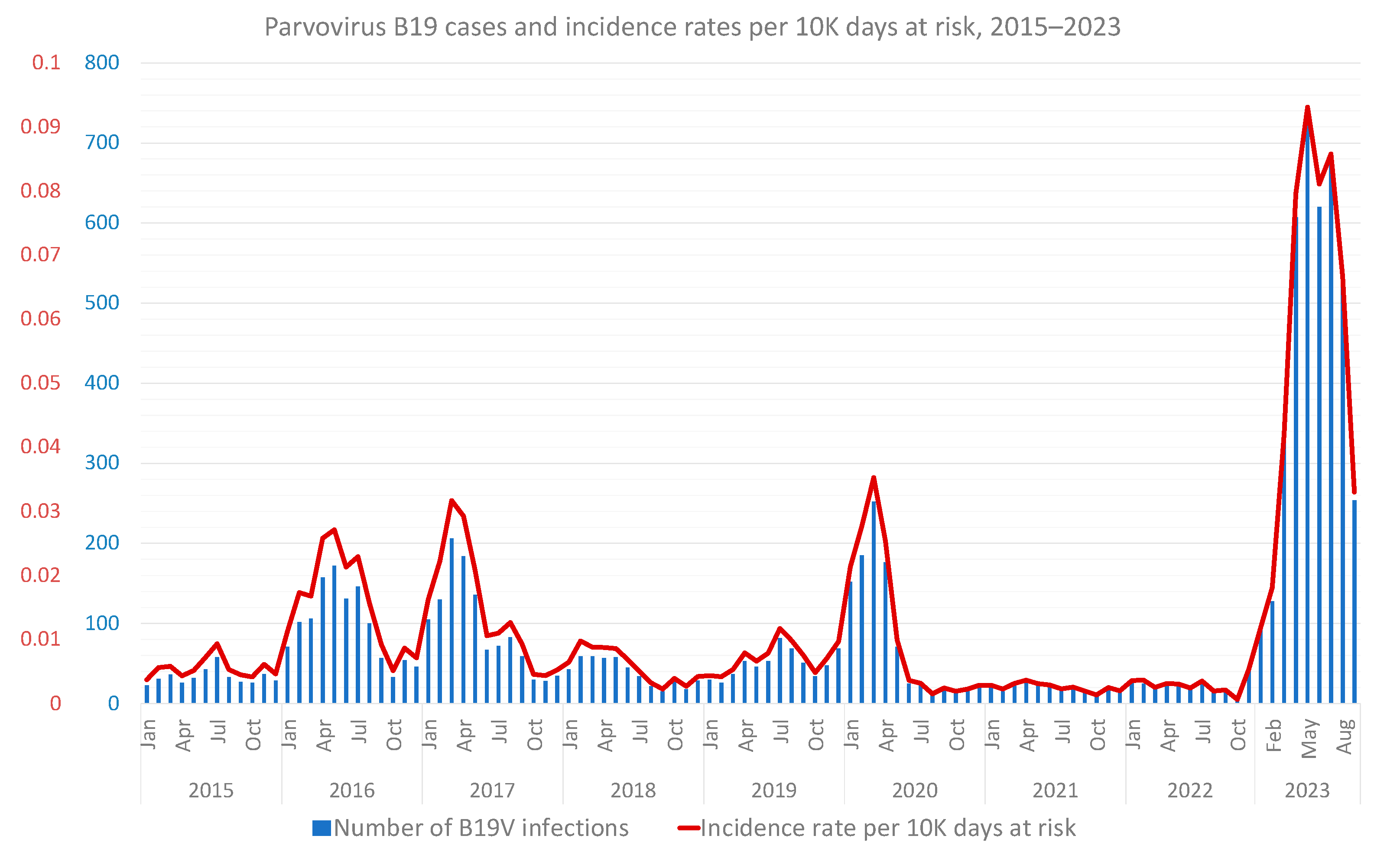
3.1. Primary Analysis: Incidence and Trend in the General Population
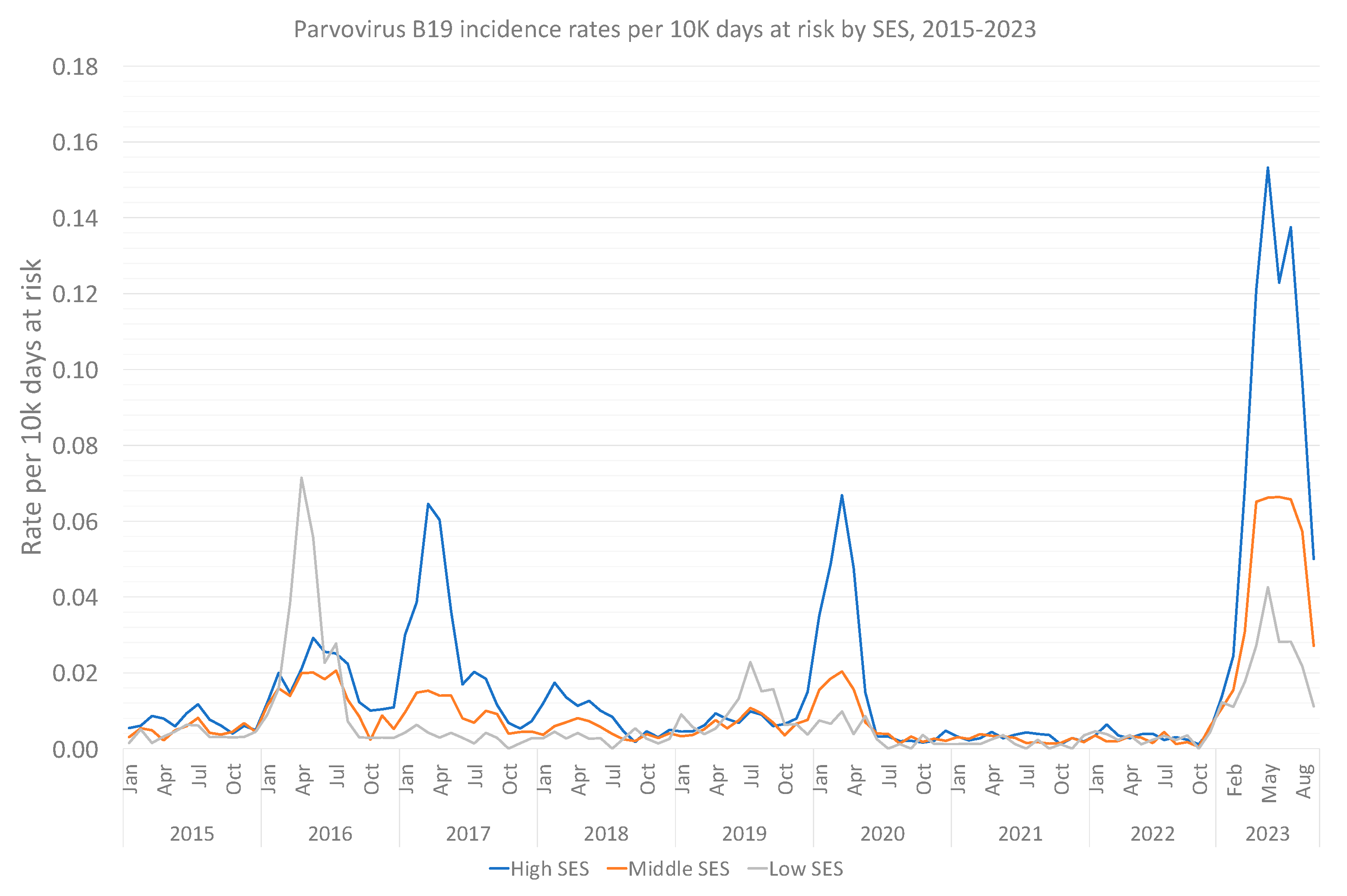
3.2. Sub-Analysis: Children and Pregnant Women
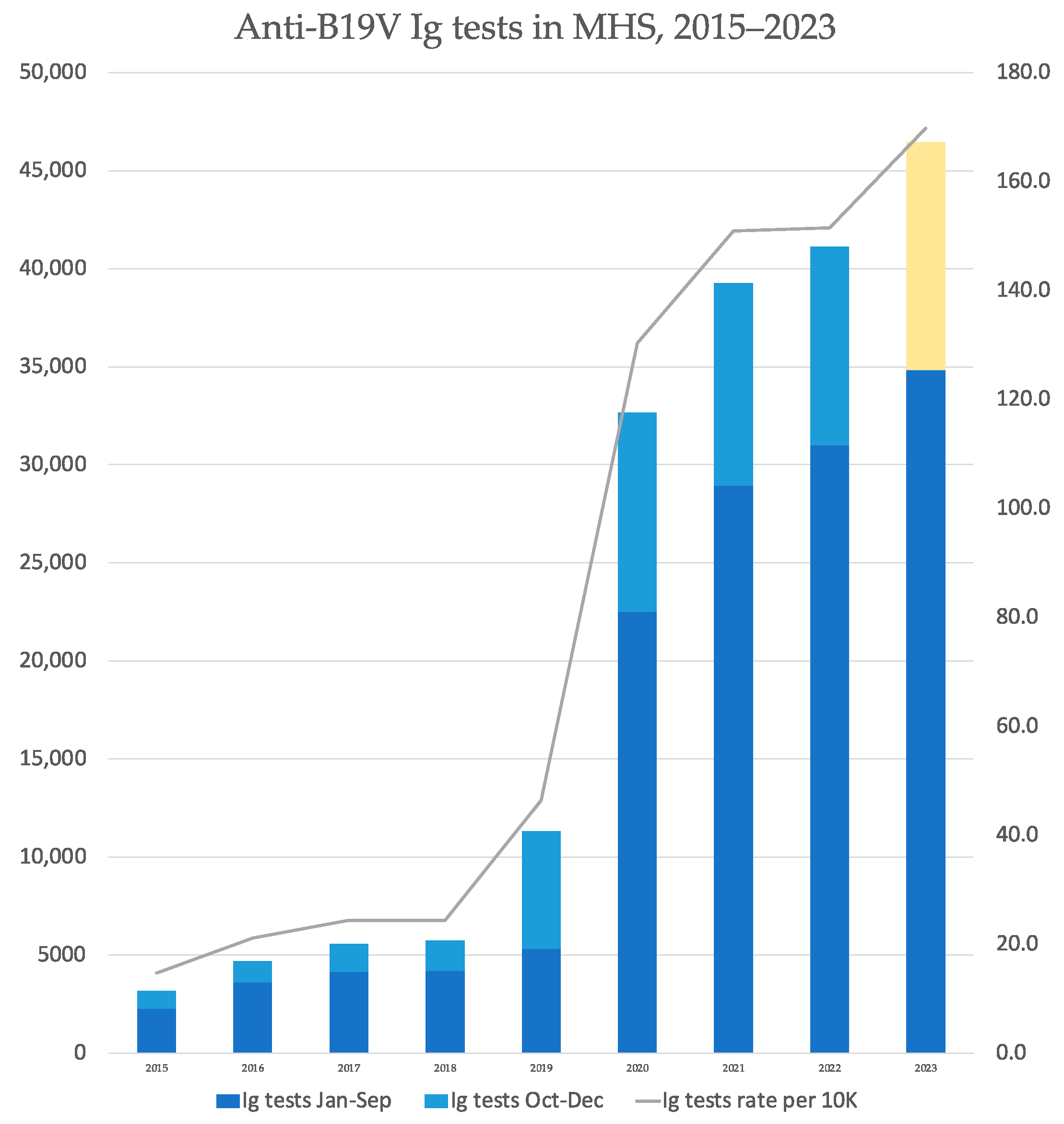
3.3. Supplementary Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Auriti, C.; De Rose, D.U.; Santisi, A.; Martini, L.; Piersigilli, F.; Bersani, I.; Ronchetti, M.P.; Caforio, L. Pregnancy and viral infections: Mechanisms of fetal damage, diagnosis and prevention of neonatal adverse outcomes from cytomegalovirus to SARS-CoV-2 and Zika virus. Biochim. Biophys. Acta Mol. Basis. Dis 2021, 1867, 166198. [Google Scholar] [CrossRef]
- Zakrzewska, K.; Arvia, R.; Bua, G.; Margheri, F.; Gallinella, G. Parvovirus B19: Insights and implication for pathogenesis, prevention and therapy. Asp. Mol. Med. 2023, 1, 100007. [Google Scholar] [CrossRef]
- Attwood, L.O.; Holmes, N.E.; Hui, L. Identification and management of congenital parvovirus B19 infection. Prenat. Diagn. 2020, 40, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Bonvicini, F.; Bua, G.; Gallinella, G. Parvovirus B19 infection in pregnancy—Awareness and opportunities. Curr. Opin. Virol. 2017, 27, 8–14. [Google Scholar] [CrossRef]
- Anderson, L.J. Role of parvovirus B19 in human disease. Pediatr. Infect. Dis. J. 1987, 6, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Young, N.S.; Brown, K.E. Parvovirus B19. N. Engl. J. Med. 2004, 350, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Chorba, T.; Coccia, P.; Holman, R.C.; Tattersall, P.; Anderson, L.J.; Sudman, J.; Young, N.S.; Kurczynski, E.; Saarinen, U.M.; Moir, R.; et al. The role of parvovirus B19 in aplastic crisis and erythema infectiosum (fifth disease). J. Infect. Dis. 1986, 154, 383–393. [Google Scholar] [CrossRef]
- Andrews, M.; Martin, R.W.; Duff, A.R.; Greig, H.D.; Frost, S.A. Fifth disease: Report of an outbreak. J. R. Coll. Gen. Pract. 1984, 34, 573. [Google Scholar]
- Mage, V.; Lipsker, D.; Barbarot, S.; Bessis, D.; Chosidow, O.; Del Giudice, P.; Aractingi, S.; Avouac, J.; Bernier, C.; Descamps, V.; et al. Different patterns of skin manifestations associated with parvovirus B19 primary infection in adults. J. Am. Acad. Dermatol. 2014, 71, 62–69. [Google Scholar] [CrossRef]
- Gutermuth, J.; Nadas, K.; Zirbs, M.; Seifert, F.; Hein, R.; Ring, J.; Brockow, K. Papular-purpuric gloves and socks syndrome. Lancet 2011, 378, 198. [Google Scholar] [CrossRef]
- Sève, P.; Ferry, T.; Charhon, A.; Calvet, A.; Broussolle, C. Systemic manifestations of Parvovirus B19 infections. Rev. Med. Interne 2004, 25, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Oiwa, H.; Shimada, T.; Hashimoto, M.; Kawaguchi, A.; Ueda, T.; Sugiyama, E.; Kamiya, T. Clinical findings in parvovirus B19 infection in 30 adult patients in Kyoto. Mod. Rheumatol. 2011, 21, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L. Parvovirus-associated arthritis. Curr. Opin. Rheumatol. 2000, 12, 289–294. [Google Scholar] [CrossRef]
- Serjeant, G.; Serjeant, B.; Thomas, P.; Anderson, M.; Patou, G.; Pattison, J. Human parvovirus infection in homozygous sickle cell disease. Lancet 1993, 341, 1237–1240. [Google Scholar] [CrossRef]
- Saarinen, U.M.; Chorba, T.L.; Tattersall, P. Human Parvovirus B19-Induced Epidemic Acute Red Cell Aplasia in Patients With Hereditary Hemolytic Anemia. Blood 1986, 67, 1411–1417. [Google Scholar] [CrossRef]
- Serjeant, B.E.; Hambleton, I.R.; Kerr, S.; Kilty, C.G.; Serjeant, G.R. Haematological response to parvovirus B19 infection in homozygous sickle-cell disease. Lancet 2001, 358, 1779–1780. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Sifer, C.; Schneider, V.; Allaume, X.; Servant, A.; Bernaudin, F.; Auguste, V.; Garbarg-Chenon, A. Novel human erythrovirus associated with transient aplastic anemia. J. Clin. Microbiol. 1999, 37, 2483–2487. [Google Scholar] [CrossRef] [PubMed]
- Douvoyiannis, M.; Litman, N.; Goldman, D.L. Neurologic manifestations associated with parvovirus B19 infection. Clin. Infect. Dis. 2009, 48, 1713–1723. [Google Scholar] [CrossRef]
- Barah, F.; Whiteside, S.; Batista, S.; Morris, J. Neurological aspects of human parvovirus B19 infection: A systematic review. Rev. Med. Virol. 2014, 24, 154–168. [Google Scholar] [CrossRef]
- Porter, H.J.; Khong, T.Y.; Evans, M.F.; Chan VT, W.; Fleming, K.A. Parvovirus as a cause of hydrops fetalis: Detection by in situ DNA hybridisation. J. Clin. Pathol. 1988, 41, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Rodis, J.F.; Quinn, D.L.; Gary, G.W.; Anderson, L.J.; Rosengren, S.; Cartter, M.L.; Campbell, W.A.; Vintzileos, A.M. Management and outcomes of pregnancies complicated by human B19 parvovirus infection: A prospective study. Am. J. Obstet. Gynecol. 1990, 163, 1168–1171. [Google Scholar] [CrossRef]
- Miller, E.; Fairley, C.K.; Cohen, B.J.; Seng, C. Immediate and long term outcome of human parvovirus B19 infection in pregnancy. Br. J. Obstet. Gynaecol. 1998, 105, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Enders, M.; Weidner, A.; Zoellner, I.; Searle, K.; Enders, G. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: Prospective evaluation of 1018 cases. Prenat. Diagn 2004, 24, 513–518. [Google Scholar] [CrossRef]
- Skjöldebrand-Sparre, L.; Tolfvenstam, T.; Papadogiannakis, N.; Wahren, B.; Broliden, K.; Nyman, M. Parvovirus B19 infection: Association with third-trimester intrauterine fetal death. BJOG Int. J. Obstet. Gynaecol. 2000, 107, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Salbetti, M.B.; Pedranti, M.S.; Barbero, P.; Molisani, P.; Lazzari, M.; Olivera, N.; Isa, M.B.; Bertoldi, A.; Moreno, L.; Adamo, M.P. Molecular screening of the human parvoviruses B19 and bocavirus 1 in the study of congenital diseases as applied to symptomatic pregnant women and children. Access Microbiol. 2019, 1, e000037. [Google Scholar] [CrossRef]
- Jain, A.; Kant, R. Genotypes of erythrovirus B19, their geographical distribution & circulation in cases with various clinical manifestations. Indian J. Med. Res 2018, 147, 239–247. [Google Scholar]
- Mor, O.; Ofir, I.; Pavel, R.; Bassal, R.; Kra-Oz, Z.; Cohen, D.; Shohat, T.; Mendelson, E. Parvovirus B19V infection in Israel: Prevalence and occurrence of acute infection between 2008 and 2013. Epidemiol. Infect. 2016, 144, 207–214. [Google Scholar] [CrossRef]
- Gigi, C.E.; Anumba, D.O.C. Parvovirus b19 infection in pregnancy—A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Israel Central Bureau of Statistics. Characterization and Classification of Geographical Units by the Socio-Economic Level of the Population 2015; Israel Central Bureau of Statistics: Jerusalem, Israel, 2019.
- Ben-Tov, A.; Lebwohl, B.; Banon, T.; Chodick, G.; Kariv, R.; Assa, A.; Gazit, S.; Patalon, T. BNT162b2 mRNA COVID-19 Vaccine Effectiveness in Patients with Coeliac Disease Autoimmunity: Real-World Data from Mass Vaccination Campaign. Viruses 2023, 15, 1968. [Google Scholar] [CrossRef] [PubMed]
- Hunter, L.A.; Ayala, N.K. Parvovirus B19 in Pregnancy: A Case Review. J. Midwifery Women’s Health 2021, 66, 385–390. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Chapman & Hall/CRC: London, UK, 1989. [Google Scholar]
- Lipsitch, M.; Tchetgen Tchetgen, E.; Cohen, T. Negative Controls: A tool for detecting confounding and bias in observational studies. Epidemiology 2010, 21, 383–388. [Google Scholar] [CrossRef]
- Paran, Y.; Shalev, V.; Steinvil, A.; Justo, D.; Zimmerman, O.; Finn, T.; Zeltser, D.; Weitzman, D.; Raz, R.; Chodick, G.; et al. Thrombosis following acute cytomegalovirus infection: A community prospective study. Ann Hematol 2013, 92, 969–974. [Google Scholar] [CrossRef]
- Messacar, K.; Baker, R.E.; Park, S.W.; Nguyen-Tran, H.; Cataldi, J.R.; Grenfell, B. Preparing for uncertainty: Endemic paediatric viral illnesses after COVID-19 pandemic disruption. Lancet 2022, 400, 1663–1665. [Google Scholar] [CrossRef]
- Molenaar-de Backer, M.W.; Hogema, B.M.; Koppelman, M.H.; van de Laar, T.J.; Slot, E.; Zaaijer, H.L. Lower Incidence of Parvovirus-B19 Infections in Dutch Blood Donors during SARS-CoV-2 Pandemic. Microbiol. Spectr. 2021, 9, e00253-21. [Google Scholar] [CrossRef]
- Sauleda, S.; Piron, M.; Bes, M.; Martinez-Llonch, N.; Puig, L. Changes in Parvovirus B19 positivity rates in plasma units for fractionation: An unexpected effect of non-pharmaceutical interventions against COVID-19? Vox Sang. 2022, 117, 626–627. [Google Scholar] [CrossRef]
- Williams, S.; Ratcliff, J.; Nguyen, D.; Simmonds, P.; Harvala, H. Detection frequencies and viral load distribution of parvovirus B19 DNA in blood and plasma donations in England. Transfus. Med. 2022, 32, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Fourgeaud, J.; Allali, S.; Toubiana, J.; Pinhas, Y.; Frange, P.; Leruez-Ville, M.; Cohen, J.F. Post-COVID-19 pandemic outbreak of severe Parvovirus B19 primary infections in Paris, France: 10-year interrupted time-series analysis (2012–2023). J. Clin. Virol. 2023, 167, 105576. [Google Scholar] [CrossRef]
- Nicolay, N.; Cotter, S. Clinical and epidemiological aspects of parvovirus B19 infections in Ireland, January 1996–June 2008. Eurosurveillance 2009, 14, 19249. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, E.; De Oronzo, M.A.; Iozza, I.; Di Natale, A.; Cianci, S.; Garofalo, G.; Giacobbe, A.M.; Politi, S. Parvovirus B19 during pregnancy: A review. J. Prenat. Med. 2010, 4, 63–66. [Google Scholar] [PubMed]


| No | Yes | Overall | |
|---|---|---|---|
| (N = 2,747,624) | (N = 3977) | (N = 2,751,601) | |
| Sex | |||
| Female | 1,401,306 (51.0%) | 2331 (58.6%) | 1,403,637 (51.0%) |
| Male | 1,346,318 (49.0%) | 1646 (41.4%) | 1,347,964 (49.0%) |
| Age | |||
| Mean (SD 1) | 34.9 (23.0) | 12.2 (13.1) | 34.8 (23.0) |
| Median [Min, Max] | 32.4 [0.0, 102] | 7.33 [0.0, 79.6] | 32.4 [0.0, 102] |
| SES 2 | |||
| High (8–10) | 927,084 (33.7%) | 2090 (52.6%) | 929,174 (33.8%) |
| Middle (5–7) | 1,491,338 (54.3%) | 1703 (42.8%) | 1,493,041 (54.3%) |
| Low (1–4) | 322,550 (11.7%) | 180 (4.5%) | 322,730 (11.7%) |
| Other | 6652 (0.2%) | 4 (0.1%) | 6656 (0.2%) |
| Social Sector | |||
| Jewish | 2,291,679 (83.4%) | 3622 (91.1%) | 2,295,301 (83.4%) |
| Arab | 175,545 (6.4%) | 27 (0.7%) | 175,572 (6.4%) |
| Orthodox Jewish | 280,400 (10.2%) | 328 (8.2%) | 280,728 (10.2%) |
| Immunocompromised | |||
| No | 2,738,155 (99.7%) | 3971 (99.8%) | 2,742,126 (99.7%) |
| Yes | 9469 (0.3%) | 6 (0.2%) | 9475 (0.3%) |
| Population | IRR 1 of B19V 2 Incidence in 2023 Compared to 2015–2022 | Adjusted IRR 1 of B19V 2 Incidence in 2023 Compared to 2015–2022 |
|---|---|---|
| Total population | 7.07 (6.78, 7.37) | 6.6 (6.33, 6.89) |
| All Children | 7.16 (6.84, 7.49) | 6.39 (6.1, 6.69) |
| Children aged [0, 6) | 5.92 (5.53, 6.34) | 5.24 (4.9, 5.62) |
| Children aged [6, 12) | 8.73 (8.18, 9.31) | 7.72 (7.22, 8.24) |
| Children aged [12, 18) | 7.21 (5.9, 8.81) | 6.2 (5.07, 7.58) |
| Pregnant women | 12.15 (10.06, 14.69) | 11.47 (9.44, 13.97) |
| Variable | Category | Adjusted IRR 1 |
|---|---|---|
| Social sector | ||
| Jewish | Ref | |
| Arab | 0.29 (0.24, 0.35) | |
| Ultra-orthodox | 0.98 (0.90, 1.06) |
| Year | Total Serology Tests | Positive IgM Tests | Proportion (%) of Positive IgM Tests out of Total Performed Tests |
|---|---|---|---|
| 2015 | 3180 | 112 | 3.5% |
| 2016 | 4682 | 268 | 5.7% |
| 2017 | 5560 | 248 | 4.5% |
| 2018 | 5738 | 68 | 1.2% |
| 2019 | 11,314 | 80 | 0.7% |
| 2020 | 32,650 | 207 | 0.6% |
| 2021 | 39,275 | 52 | 0.1% |
| 2022 | 41,129 | 64 | 0.2% |
| 2023 | 34,838 | 1053 | 3.0% |
| Serology Confirmed | Diagnosis Confirmed | |||
| Population | IRR 1 of B19V 2 Incidence in 2023 Compared to 2015–2022 | Adjusted IRR 1 of B19V 2 Incidence in 2023 Compared to 2015–2022 | IRR 1 of B19V 2 Incidence in 2023 Compared to 2015–2022 | Adjusted IRR 1 of B19V 2 Incidence in 2023 Compared to 2015–2022 |
| Total population | 9.43 (8.60, 10.34) | 8.51 (7.73, 9.36) | 6.89 (6.60, 7.20) | 6.48 (6.19, 6.78) |
| All Children | 7.18 (6.02, 8.55) | 6.21 (5.19, 7.44) | 7.23 (6.91, 7.57) | 6.45 (6.16, 6.77) |
| Children aged [0,6) | 6.65 (4.66, 9.41) | 5.77 (4.04, 8.18) | 5.96 (5.56, 6.38) | 5.28 (4.92, 5.66) |
| Children aged [6,12) | 7.44 (5.97, 9.26) | 6.45 (5.16, 8.06) | 8.88 (8.31, 9.49) | 7.86 (7.35, 8.40) |
| Children aged [12,18) | 7.00 (4.10, 11.80) | 5.93 (3.46, 10.01) | 7.27 (5.90, 8.94) | 6.25 (5.08, 7.69) |
| Pregnant women | 13.03 (10.65, 15.97) | 12.73 (10.33, 15.74) | 10.85 (8.34, 14.12) | 10.13 (7.74, 13.31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patalon, T.; Saciuk, Y.; Trotzky, D.; Pachys, G.; Ben-Tov, A.; Segal, Y.; Gazit, S. An Outbreak of Parvovirus B19 in Israel. Viruses 2023, 15, 2261. https://doi.org/10.3390/v15112261
Patalon T, Saciuk Y, Trotzky D, Pachys G, Ben-Tov A, Segal Y, Gazit S. An Outbreak of Parvovirus B19 in Israel. Viruses. 2023; 15(11):2261. https://doi.org/10.3390/v15112261
Chicago/Turabian StylePatalon, Tal, Yaki Saciuk, Daniel Trotzky, Gal Pachys, Amir Ben-Tov, Yaakov Segal, and Sivan Gazit. 2023. "An Outbreak of Parvovirus B19 in Israel" Viruses 15, no. 11: 2261. https://doi.org/10.3390/v15112261
APA StylePatalon, T., Saciuk, Y., Trotzky, D., Pachys, G., Ben-Tov, A., Segal, Y., & Gazit, S. (2023). An Outbreak of Parvovirus B19 in Israel. Viruses, 15(11), 2261. https://doi.org/10.3390/v15112261






