Tumor Tropism of DNA Viruses for Oncolytic Virotherapy
Abstract
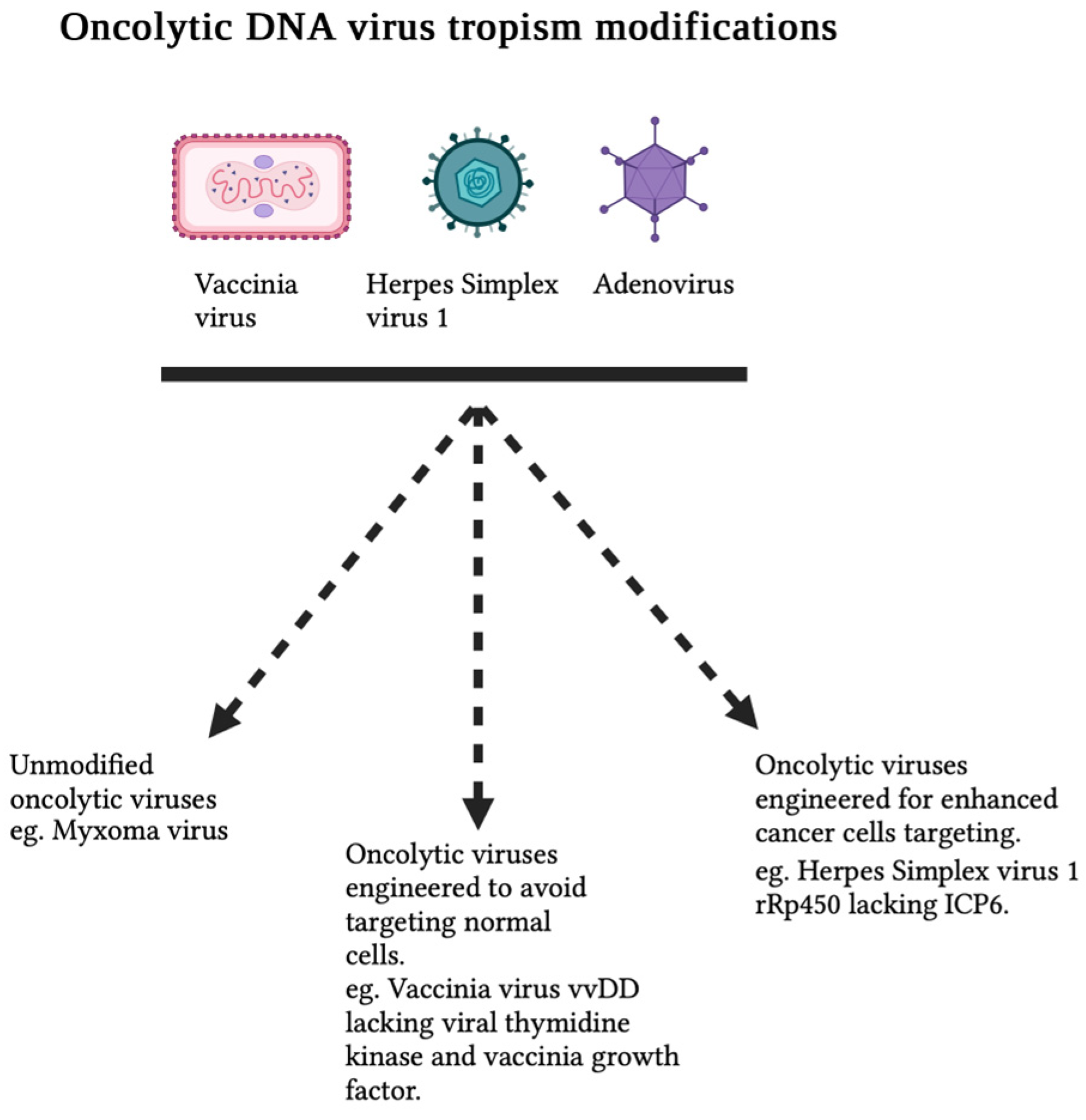
1. Introduction
2. Oncolytic Poxviruses
2.1. Natural Tropism of Poxviruses for Cancer Cells
2.2. Vaccinia Virus and Other Orthopoxviruses
2.2.1. Enhancing Tumor-Selective Replication of VACV
2.2.2. Deletion of Immune-Evasion Genes in VACV to Enhance Tumor Selectivity and Activation of Antitumor Immune Response
2.3. Other Poxviruses with Natural Tropism for Cancer Cells
2.4. Novel Chimeric Poxviruses with Enhanced Tropism for Cancer Cells
| Family | Virus | Natural Host | Mutant Names | Targeting Properties | Engineered Tropism | Introduced Transgene | Target Cancer Type | Ref. |
|---|---|---|---|---|---|---|---|---|
| Poxviridae | Vaccinia virus | Unknown | VACV-EEV | Enhanced virus spread in tumor beds. Viral inhibition of complement. Lack of IFN inhibition. | K151E mutation of viral protein A34 (A34 K151E) and B18R deletion. | N/A | PC3 human prostate cancer | [15] |
| Vaccinia virus WR strain | Unknown | vvDD | Attenuate infection of healthy cells but retain replicative ability in dividing cancer cells. | Viral thymidine kinase and vaccinia growth factor (VGF) gene deletion. | N/A | Human and murine adenocarcinoma cell lines | [20,21] | |
| Vaccinia virus Lister strain | Unknown | GLV-1H68 | Attenuate infection of healthy cells but retain tumor-selective replication. | Deletions of viral thymidine kinase (J2R), secretory signal peptide (F14.5L), and hemagglutinin (A56R) genes. | N/A | GI-101A human breast tumors xenograft mouse model | [23] | |
| Vaccinia virus LC16mO strain | Unknown | MDRVV | Tumor-selective virus for MAPK–ERK pathway. | Deletions of VGF and O1 genes. | N/A | Human pancreatic ductal adenocarcinoma xenograft mouse model | [25,26,27] | |
| Vaccinia virus WR strain | Unknown | ∆F4L∆J2R VACV | Selective targeting of cancer cells because of the high level of ribonucleotide reductase enzyme expression that the virus lacks. | Deletions of viral thymidine kinase (J2R) and F4L genes. | N/A | Xenograft human RT112-luc orthotopic bladder cancer model | [52] | |
| Vaccinia virus WR strain | Unknown | WR-Δ4 | Targeting of viral genes that act on metabolic, proliferation, and signaling pathways to confine viral tropism to cancer cells. | Deletions of viral thymidine kinase (J2R), soluble interferon receptor (B18R), vaccinia growth factor (C11R), and thymidine kinase (A48R) genes. | N/A | Mouse B16F10 syngeneic melanoma model | [29] | |
| Vaccinia virus WR/TK strain | Unknown | WR/TK−/3Δ | Enhanced tumor selectivity both in vitro and in vivo and enhanced IRF3 phosphorylation within the cancer cells. | Deletion of immune-evasion genes (C10L, N2L, C6L) that antagonize the TLR3-IRF3 pathway at different levels. | N/A | Mouse syngeneic Renca (kidney derived) tumor model | [30,31] | |
| Vaccinia virus WR strain F13L+ | Unknown | ΔN1L VV, ΔK1L VV, ΔK3L VV, ΔA46R VV, and ΔA52R VV | Effectively target and kill tumor cells by diminishing the viral antiviral properties. | Deleting the N1L, K1L, K3L, A46R, and A52R genes increased antitumor immune response and heightened production of virus-induced cytokines. | N/A | Human colorectal adenocarcinoma cell line DLD-1, human ovarian cancer cell line A2780 | [32] | |
| Vaccinia virus WR strain | Unknown | VVΔTKΔN1L-IL12 | Tumor-specific targeting by the virus, more outstanding viral-induced cytokine production, and elevated circulating NK-cell levels. | N1L deletion correlated with greater levels of virus-induced cytokines and elevated NK-cell levels. | IL12 | CT26 mouse metastatic colon adenocarcinoma and metastatic lung squamous cell carcinoma LLC. Surgical models of head and neck cancer in Syrian hamsters | [33] | |
| Tana poxvirus | Human | TPVΔ15L | Cancer-specific targeting. | TK (66L), neuregulin (NRG), and EGF-like growth factor (15L) have been deleted. | N/A | Human melanoma xenograft model in nude mice | [41] | |
| Chimeric Parapoxvirus | N/A | CF189 | Robust tumor cell targeting by virus. | Chimeric Parapoxviruses were constructed by co-infecting MDBK cells with Orf-virus strain NZ2 and pseudocowpox virus strain TJS. | N/A | Mouse models of human triple-negative breast cancer cell lines MDA-MB-468 | [46] | |
| Chimeric orthopoxvirus | N/A | CF33 | Superior ability to kill human pancreatic cancer cells. | The chimeric orthopox virus was created by co-infecting nine strains of the orthopox virus. (see section on novel chimeric poxviruses for the different viruses used.) | N/A | Mouse xenograft models of human pancreatic ductal adenocarcinoma | [47] | |
| Chimeric vaccinia virus | N/A | deVV5 | Improved cancer-killing capacity and tumor selectivity in vitro. | Chimera was created by co-infecting VACV strains Copenhagen, Western Reserve, Wyeth, and the attenuated VACV Ankara. The TK gene was deleted to attenuate virus replication in normal primary cells. | N/A | In vitro screen of human cancer cell lines | [51] | |
| Herpesviridae | Herpes simplex virus 1 | Humans | N/A | Reduced tropism for neuronal cells and selective targeting of cancer cells. | Deletion in viral protein ICP34.5 that is involved in inhibiting PKR. | N/A | The modified oHSV-1 is safe in Phase-I clinical trials in glioma and melanoma patients | [53] |
| Herpes simplex virus 1 | Humans | N/A | Tumor-selective replication due to high levels of ribonuclease reductase expression. | The viral gene (UL39) for the ICP6 protein is deleted. | N/A | Deleted ICP6 oHSV is currently in Phase I clinical trial liver metastasis and primary liver cancer | [54] | |
| Herpes simplex virus 1 | Humans | rRp450 | Enhanced tumor-selective targeting and minimal effect on normal cells. | Deletion of ICP6 gene. | Introduction of rat cytochrome P450 2B1 that serves as a prodrug enzyme for cyclophosphamide. | rRp450 showed therapeutic benefit in mouse models of diffuse colon cancer liver metastasis | [55] | |
| Herpes simplex virus 1 | Humans | G47∆ | Enhanced MHC-I antigen presentation, enhanced cytopathic effect in-vitro and tumor-selective targeting. | Deletion of ICP6 gene, ICP47 gene, and both copies of the ICP34.5 gene. | N/A | G47∆ has shown therapeutic efficacy against mouse models of Neuro2a neuroblastoma tumors and human U87MG glioma tumors | [56] | |
| Herpes simplex virus 1 | Humans | R-LM13 | Engineered to target HER-2 expressing tumor cells selectively. | Insertion of scFV targeting HER-2 into glycoprotein gD. | N/A | R-LM13 has demonstrated therapeutic efficacy against mice models of human ovarian cancer (SK-OV-3) and breast cancer (MDA-MB-453 and BT-474) | [57,58,59] | |
| Herpes simplex virus 1 | Humans | rQNestin34.5 | The virus is engineered to replicate selectively under the Nestin promoter’s influence. | Insertion of a Nestin-specific promoter within the ICP34.5 gene. | N/A | rQNestin34.5 treatment increased the survival rate of nude mice by >90% of 77.8% mice bearing intracerebral human U87EGFR glioma | [60] | |
| Adenoviridae | Adenovirus serotype 5 | Human | Ad5-3Δ-A20T | Ad5-3Δ-A20T is engineered to target αvβ6 integrin-expressing cells selectively. | The Ad5 mutant expresses the αvβ6-binding peptide that can infect and kill αvβ6-expressing cells. | αvβ6-binding peptide | Ad5-3Δ-A20T efficiently inhibited the growth of human pancreatic cancer xenograft murine models | [61] |
| Adenovirus serotype 5 | Human | AdV5 hTERT | AdV5 hTERT can selectively replicate in cells expressing high levels of telomerase activity (cancer cells). | AdV5 expressing the E1A and E1B genes under the influence of an hTERT promoter. | Human telomerase reverse transcriptase promoter. | AdV5 hTERT intratumoral injection resulted in the growth inhibition of human lung tumor models | [62] | |
| Adenovirus serotype 5 | Human | ONYX-15 | ONYX-15 can selectively replicate in tumors lacking a functional p53. | This virus lacks the 55kDa E1B gene region. | N/A | ONYX-15 resulted in antitumor effects in mouse–human tumor xenografts of cervical (C33A) and laryngeal (HLaC) carcinoma lacking functional p53. ONYX-15 is being used in clinical trials for head and neck cancer | [63] | |
| Adenovirus serotype 5 | Human | Gendicine® | Gendicine can selectively kill tumors by forcing them to undergo apoptosis. | Gendicine lacks the E1 gene region. | Introduction of the p53 protein driven by a Rous sarcoma virus promoter | Gendicine® is approved to treat head and neck squamous cell carcinoma in China. | [64,65] |
3. Oncolytic Herpes Simplex Virus (oHSV)
3.1. Enhancing Safety and Tumor-Selective Replication of oHSV
3.2. Engineered oHSVs for Tumor-Selective Tropism
4. Oncolytic Adenovirus
4.1. Molecular Basis for Adenovirus Cancer Tropism
4.2. Genetic Modifications for Cancer Tropism
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chan, W.M.; McFadden, G. Oncolytic Poxviruses. Annu. Rev. Virol. 2014, 1, 119. [Google Scholar] [CrossRef]
- Chan, W.M.; Rahman, M.M.; McFadden, G. Oncolytic Myxoma Virus: The Path to Clinic. Vaccine 2013, 31, 4252. [Google Scholar] [CrossRef]
- Rahman, M.M.; McFadden, G. Oncolytic Virotherapy with Myxoma Virus. J. Clin. Med. 2020, 9, 171. [Google Scholar] [CrossRef]
- Chahroudi, A.; Chavan, R.; Koyzr, N.; Waller, E.K.; Silvestri, G.; Feinberg, M.B. Vaccinia Virus Tropism for Primary Hematolymphoid Cells Is Determined by Restricted Expression of a Unique Virus Receptor. J. Virol. 2005, 79, 10397–10407. [Google Scholar] [CrossRef]
- Sánchez-Puig, J.M.; Sánchez, L.; Roy, G.; Blasco, R. Susceptibility of Different Leukocyte Cell Types to Vaccinia Virus Infection. Virol. J. 2004, 1, 10. [Google Scholar] [CrossRef]
- Shepherd, N.; Lan, J.; Li, W.; Rane, S.; Yu, Q. Primary Human B Cells at Different Differentiation and Maturation Stages Exhibit Distinct Susceptibilities to Vaccinia Virus Binding and Infection. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef]
- Villa, N.Y.; Wasserfall, C.H.; Meacham, A.M.; Wise, E.; Chan, W.; Wingard, J.R.; McFadden, G.; Cogle, C.R. Myxoma Virus Suppresses Proliferation of Activated T Lymphocytes yet Permits Oncolytic Virus Transfer to Cancer Cells. Blood 2015, 125, 3778. [Google Scholar] [CrossRef]
- Byrd, D.; Amet, T.; Hu, N.; Lan, J.; Hu, S.; Yu, Q. Primary Human Leukocyte Subsets Differentially Express Vaccinia Virus Receptors Enriched in Lipid Rafts. J. Virol. 2013, 87, 9301. [Google Scholar] [CrossRef]
- Kim, M.; Madlambayan, G.J.; Rahman, M.M.; Smallwood, S.E.; Meacham, A.M.; Hosaka, K.; Scott, E.W.; Cogle, C.R.; McFadden, G. Myxoma Virus Targets Primary Human Leukemic Stem and Progenitor Cells While Sparing Normal Hematopoietic Stem and Progenitor Cells. Leukemia 2009, 23, 2313–2317. [Google Scholar] [CrossRef]
- Moss, B. Membrane Fusion during Poxvirus Entry. Semin. Cell Dev. Biol. 2016, 60, 89. [Google Scholar] [CrossRef]
- Moss, B. Poxvirus Cell Entry: How Many Proteins Does It Take? Viruses 2012, 4, 688. [Google Scholar] [CrossRef]
- Guo, Z.S.; Lu, B.; Guo, Z.; Giehl, E.; Feist, M.; Dai, E.; Liu, W.; Storkus, W.J.; He, Y.; Liu, Z.; et al. Vaccinia Virus-Mediated Cancer Immunotherapy: Cancer Vaccines and Oncolytics. J. Immunother. Cancer 2019, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dong, L.; Zhao, C.; Zheng, P.; Zhang, X.; Xu, J. Vaccinia Virus-Based Vector against Infectious Diseases and Tumors. Hum. Vaccin. Immunother. 2021, 17, 1578. [Google Scholar] [CrossRef]
- Fields Virology: Fundamentals. Available online: https://shop.lww.com/Fields-Virology--Fundamentals/p/9781975112516 (accessed on 19 September 2023).
- Kirn, D.H.; Wang, Y.; Liang, W.; Contag, C.H.; Thorne, S.H. Enhancing Poxvirus Oncolytic Effects through Increased Spread and Immune Evasion. Cancer Res. 2008, 68, 2071–2075. [Google Scholar] [CrossRef] [PubMed]
- Vanderplasschen, A.; Mathew, E.; Hollinshead, M.; Sim, R.B.; Smith, G.L. Extracellular Enveloped Vaccinia Virus Is Resistant to Complement Because of Incorporation of Host Complement Control Proteins into Its Envelope. Proc. Natl. Acad. Sci. USA 1998, 95, 7544. [Google Scholar] [CrossRef] [PubMed]
- Kirn, D.H.; Thorne, S.H. Targeted and Armed Oncolytic Poxviruses: A Novel Multi-Mechanistic Therapeutic Class for Cancer. Nat. Rev. Cancer 2009, 9, 64–71. [Google Scholar] [CrossRef]
- Nakatake, M.; Kurosaki, H.; Kuwano, N.; Horita, K.; Ito, M.; Kono, H.; Okamura, T.; Hasegawa, K.; Yasutomi, Y.; Nakamura, T. Partial Deletion of Glycoprotein B5R Enhances Vaccinia Virus Neutralization Escape While Preserving Oncolytic Function. Mol. Ther. Oncolytics 2019, 14, 159. [Google Scholar] [CrossRef]
- Kinase, V.M.L.T. Systemic Cancer Therapy with a Tumor-Selective Vaccinia Virus Mutant Lacking Thymidine Kinase and Vaccinia Growth Factor Genes. Cancer Res. 2001, 61, 8751–8757. [Google Scholar]
- Puhlmann, M.; Brown, C.K.; Gnant, M.; Huang, J.; Libutti, S.K.; Alexander, H.R.; Bartlett, D.L. Vaccinia as a Vector for Tumor-Directed Gene Therapy: Biodistribution of a Thymidine Kinase-Deleted Mutant. Cancer Gene Ther. 2000, 7, 66–73. [Google Scholar] [CrossRef]
- Buller, R.M.L.; Chakrabarti, S.; Moss, B.; Fredricksont, T. Cell Proliferative Response to Vaccinia Virus Is Mediated by VGF. Virology 1988, 164, 182–192. [Google Scholar] [CrossRef]
- Ricordel, M.; Foloppe, J.; Pichon, C.; Findeli, A.; Tosch, C.; Cordier, P.; Cochin, S.; Quémeneur, E.; Camus-Bouclainville, C.; Bertagnoli, S.; et al. Oncolytic Properties of Non-Vaccinia Poxviruses. Oncotarget 2018, 9, 35891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, Y.A.; Wang, E.; Chen, N.; Danner, R.L.; Munson, P.J.; Marincola, F.M.; Szalay, A.A. Eradication of Solid Human Breast Tumors in Nude Mice with an Intravenously Injected Light-Emitting Oncolytic Vaccinia Virus. Cancer Res. 2007, 67, 10038–10046. [Google Scholar] [CrossRef]
- Ricordel, M.; Foloppe, J.; Pichon, C.; Sfrontato, N.; Antoine, D.; Tosch, C.; Cochin, S.; Cordier, P.; Quemeneur, E.; Camus-Bouclainville, C.; et al. Cowpox Virus: A New and Armed Oncolytic Poxvirus. Mol. Ther. Oncolytics 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, H.; Nakatake, M.; Sakamoto, T.; Kuwano, N.; Yamane, M.; Ishii, K.; Fujiwara, Y.; Nakamura, T. Anti-Tumor Effects of Mapk-Dependent Tumor-Selective Oncolytic Vaccinia Virus Armed with Cd/Uprt against Pancreatic Ductal Adenocarcinoma in Mice. Cells 2021, 10, 985. [Google Scholar] [CrossRef]
- Andrade, A.A.; Silva, P.N.G.; Pereira, A.C.T.C.; De Sousa, L.P.; Ferreira, P.C.P.; Gazzinelli, R.T.; Kroon, E.G.; Ropert, C.; Bonjardim, C.A. The Vaccinia Virus-Stimulated Mitogen-Activated Protein Kinase (MAPK) Pathway Is Required for Virus Multiplication. Biochem. J. 2004, 381, 437. [Google Scholar] [CrossRef] [PubMed]
- Schweneker, M.; Lukassen, S.; Späth, M.; Wolferstätter, M.; Babel, E.; Brinkmann, K.; Wielert, U.; Chaplin, P.; Suter, M.; Hausmann, J. The Vaccinia Virus O1 Protein Is Required for Sustained Activation of Extracellular Signal-Regulated Kinase 1/2 and Promotes Viral Virulence. J. Virol. 2012, 86, 2323. [Google Scholar] [CrossRef] [PubMed]
- Arulanandam, R.; Batenchuk, C.; Angarita, F.A.; Ottolino-Perry, K.; Cousineau, S.; Mottashed, A.; Burgess, E.; Falls, T.J.; De Silva, N.; Tsang, J.; et al. VEGF-Mediated Induction of PRD1-BF1/Blimp1 Expression Sensitizes Tumor Vasculature to Oncolytic Virus Infection. Cancer Cell 2015, 28, 210–224. [Google Scholar] [CrossRef]
- Mejías-Pérez, E.; Carreño-Fuentes, L.; Esteban, M. Development of a Safe and Effective Vaccinia Virus Oncolytic Vector WR-Δ4 with a Set of Gene Deletions on Several Viral Pathways. Mol. Ther. Oncolytics 2018, 8, 27. [Google Scholar] [CrossRef]
- Riederer, S.; Fux, R.; Lehmann, M.H.; Volz, A.; Sutter, G.; Rojas, J.J. Activation of Interferon Regulatory Factor 3 by Replication-Competent Vaccinia Viruses Improves Antitumor Efficacy Mediated by T Cell Responses. Mol. Ther. Oncolytics 2021, 22, 399. [Google Scholar] [CrossRef]
- Smith, G.L.; Benfield, C.T.O.; Maluquer de Motes, C.; Mazzon, M.; Ember, S.W.J.; Ferguson, B.J.; Sumner, R.P. Vaccinia Virus Immune Evasion: Mechanisms, Virulence and Immunogenicity. J. Gen. Virol. 2013, 94, 2367–2392. [Google Scholar] [CrossRef]
- Ho, T.Y.; Mealiea, D.; Okamoto, L.; Stojdl, D.F.; McCart, J.A. Deletion of Immunomodulatory Genes as a Novel Approach to Oncolytic Vaccinia Virus Development. Mol. Ther. Oncolytics 2021, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Chard, L.S.; Yuan, M.; Wang, J.; Howells, A.; Li, Y.; Li, H.; Zhang, Z.; Lu, S.; Gao, D.; et al. A New Oncolytic Vacciniavirus Augments Antitumor Immune Responses to Prevent Tumor Recurrence and Metastasis after Surgery. J. Immunother. Cancer 2020, 8, e000415. [Google Scholar] [CrossRef] [PubMed]
- Gratz, M.S.; Suezer, Y.; Kremer, M.; Volz, A.; Majzoub, M.; Hanschmann, K.-M.; Kalinke, U.; Schwantes, A.; Sutter, G. N1L Is an Ectromelia Virus Virulence Factor and Essential for In Vivo Spread upon Respiratory Infection. J. Virol. 2011, 85, 3557. [Google Scholar] [CrossRef]
- Bartlett, N.; Symons, J.A.; Tscharke, D.C.; Smith, G.L. The Vaccinia Virus N1L Protein Is an Intracellular Homodimer That Promotes Virulence. J. Gen. Virol. 2002, 83, 1965–1976. [Google Scholar] [CrossRef]
- Ren, H.; Ferguson, B.J.; de Motes, C.M.; Sumner, R.P.; Harman, L.E.R.; Smith, G.L. Enhancement of CD8+ T-Cell Memory by Removal of a Vaccinia Virus Nuclear Factor-ΚB Inhibitor. Immunology 2015, 145, 34. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, N.; Bartlett, N.W.; Clark, R.H.; Smith, G.L. Vaccinia Virus Lacking the Bcl-2-like Protein N1 Induces a Stronger Natural Killer Cell Response to Infection. J. Gen. Virol. 2008, 89, 2877. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Johnston, S. Monkeypox and other Poxviruses. In Feigin and Cherry’s Textbook of Pediatric Infectious Diseases, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 2101–2108. [Google Scholar] [CrossRef]
- Nazarian, S.H.; Barrett, J.W.; Stanford, M.M.; Johnston, J.B.; Essani, K.; McFadden, G. Tropism of Tanapox Virus Infection in Primary Human Cells. Virology 2007, 368, 32–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suryawanshi, Y.; Zhang, T.; Razi, F.; Essani, K. Tanapoxvirus: From Discovery towards Oncolytic Immunovirotherapy. J. Cancer Res. Ther. 2020, 16, 708–712. [Google Scholar] [CrossRef]
- Zhang, T.; Suryawanshi, Y.R.; Kordish, D.H.; Woyczesczyk, H.M.; Jeng, D.; Essani, K. Tanapoxvirus Lacking a Neuregulin-like Gene Regresses Human Melanoma Tumors in Nude Mice. Virus Genes. 2017, 53, 52. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lee, J.; McCart, J.A.; Xu, H.; Moss, B.; Alexander, H.R.; Bartlett, D.L. Yaba-Like Disease Virus: An Alternative Replicating Poxvirus Vector for Cancer Gene Therapy. J. Virol. 2001, 75, 10300. [Google Scholar] [CrossRef]
- Rintoul, J.L.; Lemay, C.G.; Tai, L.H.; Stanford, M.M.; Falls, T.J.; De Souza, C.T.; Bridle, B.W.; Daneshmand, M.; Ohashi, P.S.; Wan, Y.; et al. ORFV: A Novel Oncolytic and Immune Stimulating Parapoxvirus Therapeutic. Mol. Ther. 2012, 20, 1148. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Y.; Liu, F.; Luo, S. Orf Virus: A Promising New Therapeutic Agent. Rev. Med. Virol. 2019, 29, e2013. [Google Scholar] [CrossRef]
- Fiebig, H.H.; Siegling, A.; Volk, H.D.; Friebe, A.; Knolle, P.; Limmer, A.; Weber, O. Inactivated Orf Virus (Parapoxvirus ovis) Induces Antitumoral Activity in Transplantable Tumor Models. Anticancer Res. 2021, 31, 4185–4190. [Google Scholar]
- Choi, A.H.; O’Leary, M.P.; Chaurasiya, S.; Lu, J.; Kim, S.I.; Fong, Y.; Chen, N.G. Novel Chimeric Parapoxvirus CF189 as an Oncolytic Immunotherapy in Triple-Negative Breast Cancer. Surgery 2018, 163, 336–342. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.P.; Choi, A.H.; Kim, S.I.; Chaurasiya, S.; Lu, J.; Park, A.K.; Woo, Y.; Warner, S.G.; Fong, Y.; Chen, N.G. Novel Oncolytic Chimeric Orthopoxvirus Causes Regression of Pancreatic Cancer Xenografts and Exhibits Abscopal Effect at a Single Low Dose. J. Transl. Med. 2018, 16, 110. [Google Scholar] [CrossRef]
- Choi, A.H.; O’Leary, M.P.; Lu, J.; Kim, S.I.; Fong, Y.; Chen, N.G. Endogenous Akt Activity Promotes Virus Entry and Predicts Efficacy of Novel Chimeric Orthopoxvirus in Triple-Negative Breast Cancer. Mol. Ther. Oncolytics 2018, 9, 22–29. [Google Scholar] [CrossRef]
- Hammad, M.; Cornejo, Y.; Flores, L.; Hyde, C.; Ngai, H.W.; Li, M.; Dellinger, T.H.; Lu, J.; Chen, N.G.; Mooney, R.; et al. Novel Chimeric Poxvirus CF17 Improves Survival in a Murine Model of Intraperitoneal Ovarian Cancer Metastasis. Mol. Ther. Oncolytics 2020, 19, 278. [Google Scholar] [CrossRef]
- Chaurasiya, S.; Chen, N.G.; Lu, J.; Martin, N.; Shen, Y.; Kim, S.I.; Warner, S.G.; Woo, Y.; Fong, Y. A Chimeric Poxvirus with J2R (Thymidine Kinase) Deletion Shows Safety and Anti-Tumor Activity in Lung Cancer Models. Cancer Gene Ther. 2020, 27, 125. [Google Scholar] [CrossRef]
- Ricordel, M.; Foloppe, J.; Antoine, D.; Findeli, A.; Kempf, J.; Cordier, P.; Gerbaud, A.; Grellier, B.; Lusky, M.; Quemeneur, E.; et al. Vaccinia Virus Shuffling: DeVV5, a Novel Chimeric Poxvirus with Improved Oncolytic Potency. Cancers 2018, 10, 231. [Google Scholar] [CrossRef]
- Potts, K.G.; Irwin, C.R.; Favis, N.A.; Pink, D.B.; Vincent, K.M.; Lewis, J.D.; Moore, R.B.; Hitt, M.M.; Evans, D.H. Deletion of F4L (Ribonucleotide Reductase) in Vaccinia Virus Produces a Selective Oncolytic Virus and Promotes Anti-tumor Immunity with Superior Safety in Bladder Cancer Models. EMBO Mol. Med. 2017, 9, 638. [Google Scholar] [CrossRef]
- Liu, B.L.; Robinson, M.; Han, Z.Q.; Branston, R.H.; English, C.; Reay, P.; McGrath, Y.; Thomas, S.K.; Thornton, M.; Bullock, P.; et al. ICP34.5 Deleted Herpes Simplex Virus with Enhanced Oncolytic, Immune Stimulating, and Anti-Tumour Properties. Gene Ther. 2003, 10, 292–303. [Google Scholar] [CrossRef]
- Peters, C.; Rabkin, S.D. Designing Herpes Viruses as Oncolytics. Mol. Ther. Oncolytics 2015, 2, 15010. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Nakamura, H.; Mullen, J.T.; Kasuya, H.; Yoon, S.S.; Chandrasekhar, S.; Chiocca, E.A.; Tanabe, K.K. Prodrug Bioactivation and Oncolysis of Diffuse Liver Metastases by a Herpes Simplex Virus 1 Mutant That Expresses the CYP2B1 Transgene. Cancer 2002, 95, 1171–1181. [Google Scholar] [CrossRef]
- Todo, T.; Martuza, R.L.; Rabkin, S.D.; Johnson, P.A. Oncolytic Herpes Simplex Virus Vector with Enhanced MHC Class I Presentation and Tumor Cell Killing. Proc. Natl. Acad. Sci. USA 2001, 98, 6396–6401. [Google Scholar] [CrossRef] [PubMed]
- Nanni, P.; Gatta, V.; Menotti, L.; de Giovanni, C.; Ianzano, M.; Palladini, A.; Grosso, V.; Dall’Ora, M.; Croci, S.; Nicoletti, G.; et al. Preclinical Therapy of Disseminated HER-2+ Ovarian and Breast Carcinomas with a HER-2-Retargeted Oncolytic Herpesvirus. PLoS Pathog. 2013, 9, e1003155. [Google Scholar] [CrossRef]
- Menotti, L.; Nicoletti, G.; Gatta, V.; Croci, S.; Landuzzi, L.; De Giovanni, C.; Nanni, P.; Lollini, P.L.; Campadelli-Fiume, G. Inhibition of Human Tumor Growth in Mice by an Oncolytic Herpes Simplex Virus Designed to Target Solely HER-2-Positive Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 9039–9044. [Google Scholar] [CrossRef]
- Menotti, L.; Cerretani, A.; Hengel, H.; Campadelli-Fiume, G. Construction of a Fully Retargeted Herpes Simplex Virus 1 Recombinant Capable of Entering Cells Solely via Human Epidermal Growth Factor Receptor 2. J. Virol. 2008, 82, 10153–10161. [Google Scholar] [CrossRef] [PubMed]
- Kambara, H.; Okano, H.; Chiocca, E.A.; Saeki, Y. An Oncolytic HSV-1 Mutant Expressing ICP34.5 under Control of a Nestin Promoter Increases Survival of Animals Even When Symptomatic from a Brain Tumor. Cancer Res. 2005, 65, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.K.S.; Davies, J.A.; Coughlan, L.; Pantelidou, C.; Blázquez-Moreno, A.; Marshall, J.F.; Parker, A.L.; Halldén, G. The Novel Oncolytic Adenoviral Mutant Ad5-3Δ-A20T Retargeted to Avβ6 Integrins Efficiently Eliminates Pancreatic Cancer Cells. Mol. Cancer Ther. 2018, 17, 575–587. [Google Scholar] [CrossRef]
- Kawashima, T.; Kagawa, S.; Kobayashi, N.; Shirakiya, Y.; Umeoka, T.; Teraishi, F.; Taki, M.; Kyo, S.; Tanaka, N.; Fujiwara, T. Telomerase-Specific Replication-Selective Virotherapy for Human Cancer. Clin. Cancer Res. 2004, 10, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Khuri, F.R.; Nemunaitis, J.; Ganly, I.; Arseneau, J.; Tannock, I.F.; Romel, L.; Gore, M.; Ironside, J.; MacDougall, R.H.; Heise, C.; et al. A Controlled Trial of Intratumoral ONYX-015, a Selectively-Replicating Adenovirus, in Combination with Cisplatin and 5-Fluorouracil in Patients with Recurrent Head and Neck Cancer. Nat. Med. 2000, 6, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Lu, W.; Chen, M.; Gao, W.; Zhang, C.; Guo, B.; Yang, J. Combined Effect of Recombinant Human Adenovirus P53 and Curcumin in the Treatment of Liver Cancer. Exp. Ther. Med. 2020, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.X.; Zheng, L.H.; Liu, S.Y.; He, X.H. RAd-P53 Enhances the Sensitivity of Human Gastric Cancer Cells to Chemotherapy. World J. Gastroenterol. 2011, 17, 4289–4297. [Google Scholar] [CrossRef] [PubMed]
- Aldrak, N.; Alsaab, S.; Algethami, A.; Bhere, D.; Wakimoto, H.; Shah, K.; Alomary, M.N.; Zaidan, N. Oncolytic Herpes Simplex Virus-Based Therapies for Cancer. Cells 2021, 10, 1541. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic Viruses: A New Class of Immunotherapy Drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Kelly, E.; Russell, S.J. History of Oncolytic Viruses: Genesis to Genetic Engineering. Mol. Ther. 2007, 15, 651–659. [Google Scholar] [CrossRef]
- Karasneh, G.A.; Shukla, D. Herpes Simplex Virus Infects Most Cell Types in Vitro: Clues to Its Success. Virol. J. 2011, 8, 481. [Google Scholar] [CrossRef]
- Kesari, S.; Lee, V.M.Y.; Brown, S.M.; Trojanowski, J.Q.; Fraser, N.W. Selective Vulnerability of Mouse CNS Neurons to Latent Infection with a Neuroattenuated Herpes Simplex Virus-1. J. Neurosci. 1996, 16, 5644–5653. [Google Scholar] [CrossRef]
- Cesaro, T.; Michiels, T. Inhibition of PKR by Viruses. Front. Microbiol. 2021, 12, 757238. [Google Scholar] [CrossRef]
- Pan, S.; Liu, X.; Ma, Y.; Cao, Y.; He, B. Herpes Simplex Virus 1 Γ134.5 Protein Inhibits STING Activation That Restricts Viral Replication. J. Virol. 2018, 92, e01015-18. [Google Scholar] [CrossRef]
- Aghi, M.; Visted, T.; DePinho, R.A.; Chiocca, E.A. Oncolytic Herpes Virus with Defective ICP6 Specifically Replicates in Quiescent Cells with Homozygous Genetic Mutations in P16. Oncogene 2008, 27, 4249–4254. [Google Scholar] [CrossRef]
- Chase, M.; Chung, R.Y.; Antonio Chiocca, E. An Oncolytic Viral Mutant That Delivers the CYP2B1 Transgene and Augments Cyclophosphamide Chemotherapy. Nat. Biotechnol. 1998, 16, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Jugovic, P.; York, L.; Russ, G.; Bennink, J.; Yewdell, J.; Ploegh, H.; Johnson, D. Herpes Simplex Virus Turns off the TAP to Evade Host Immunity. Nature 1995, 375, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.T.; Edelmann, K.H.; Vieira, J.; Corey, L.; Raulet, D.H.; Wilson, C.B. Inhibition of MHC Class I Is a Virulence Factor in Herpes Simplex Virus Infection of Mice. PLoS Pathog. 2005, 1, 0062–0071. [Google Scholar] [CrossRef] [PubMed]
- Backovic, M.; DuBois, R.M.; Cockburn, J.J.; Sharff, A.J.; Vaney, M.C.; Granzow, H.; Klupp, B.G.; Bricogne, G.; Mettenleiter, T.C.; Rey, F.A. Structure of a Core Fragment of Glycoprotein H from Pseudorabies Virus in Complex with Antibody. Proc. Natl. Acad. Sci. USA 2010, 107, 22635–22640. [Google Scholar] [CrossRef] [PubMed]
- Campadelli-Fiume, G.; Amasio, M.; Avitabile, E.; Cerretani, A.; Forghieri, C.; Gianni, T.; Menotti, L. The Multipartite System That Mediates Entry of Herpes Simplex Virus into the Cell. Rev. Med. Virol. 2007, 17, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Chowdary, T.K.; Cairns, T.M.; Atanasiu, D.; Cohen, G.H.; Eisenberg, R.J.; Heldwein, E.E. Crystal Structure of the Conserved Herpesvirus Fusion Regulator Complex GH-GL. Nat. Struct. Mol. Biol. 2010, 17, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Browell, D.; Gautrey, H.; Tyson-Capper, A. Clinical Significance of HER-2 Splice Variants in Breast Cancer Progression and Drug Resistance. Int. J. Cell Biol. 2013, 2013, 973584. [Google Scholar] [CrossRef] [PubMed]
- Menotti, L.; Cerretani, A.; Campadelli-Fiume, G. A Herpes Simplex Virus Recombinant That Exhibits a Single-Chain Antibody to HER2/Neu Enters Cells through the Mammary Tumor Receptor, Independently of the GD Receptors. J. Virol. 2006, 80, 5531–5539. [Google Scholar] [CrossRef]
- Menotti, L.; Avitabile, E.; Gatta, V.; Malatesta, P.; Petrovic, B.; Campadelli-Fiume, G. HSV as A Platform for the Generation of Retargeted, Armed, and Reporter-Expressing Oncolytic Viruses. Viruses 2018, 10, 352. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Nakashima, H.; Kasai, K.; Fernandez, S.A.; Oglesbee, M. Preclinical Toxicology of RQNestin34.5v.2: An Oncolytic Herpes Virus with Transcriptional Regulation of the ICP34.5 Neurovirulence Gene. Mol. Ther. Methods Clin. Dev. 2020, 17, 871–893. [Google Scholar] [CrossRef] [PubMed]
- Ghebremedhin, B.; Ghebremedhin, B. Human Adenovirus: Viral Pathogen with Increasing Importance. Eur. J. Microbiol. Immunol. 2014, 4, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Lin, Z.; Zhang, Y.; Lv, F.; Li, H.; Zhang, X.; Lin, L.; Zhu, H.H.; Xu, Z.; Li, C.; et al. The Epidemiology, Molecular, and Clinical of Human Adenoviruses in Children Hospitalized With Acute Respiratory Infections. Front. Microbiol. 2021, 12, 629971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bergelson, J.M. Adenovirus Receptors. J. Virol. 2005, 79, 12125–12131. [Google Scholar] [CrossRef] [PubMed]
- Nemerow, G.R.; Stewart, P.L. Role of Alpha(v) Integrins in Adenovirus Cell Entry and Gene Delivery. Microbiol. Mol. Biol. Rev. 1999, 63, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Davydova, J.; Wang, M.; Siegal, G.P.; Krasnykh, V.; Vickers, S.M.; Curiel, D.T. Infectivity Enhanced, Cyclooxygenase-2 Promoter-Based Conditionally Replicative Adenovirus for Pancreatic Cancer. Gastroenterology 2003, 125, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Davydova, J.; Ono, H.A.; Akiyama, H.; Hirai, S.I.; Ohno, S.; Takeshita, E.; Aoki, K.; Ochiya, T.; Yamamoto, M.; et al. Imaging and Antitumoral Effect of a Cyclo-Oxygenase 2-Specific Replicative Adenovirus for Small Metastatic Gastric Cancer Lesions. Anticancer Res. 2015, 35, 5201–5210. [Google Scholar]
- Tazawa, H.; Hasei, J.; Yano, S.; Kagawa, S.; Ozaki, T.; Fujiwara, T. Bone and Soft-Tissue Sarcoma: A New Target for Telomerase-Specific Oncolytic Virotherapy. Cancers 2020, 12, 478. [Google Scholar] [CrossRef]
- Heise, C.; Sampson-Johannes, A.; Williams, A.; McCormick, F.; Von Hoff, D.D.; Kirn, D.H. ONYX-015, an E1B Gene-Attenuated Adenovirus, Causes Tumor-Specific Cytolysis and Antitumoral Efficacy That Can Be Augmented by Standard Chemotherapeutic Agents. Nat. Med. 1997, 3, 639–645. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enow, J.A.; Sheikh, H.I.; Rahman, M.M. Tumor Tropism of DNA Viruses for Oncolytic Virotherapy. Viruses 2023, 15, 2262. https://doi.org/10.3390/v15112262
Enow JA, Sheikh HI, Rahman MM. Tumor Tropism of DNA Viruses for Oncolytic Virotherapy. Viruses. 2023; 15(11):2262. https://doi.org/10.3390/v15112262
Chicago/Turabian StyleEnow, Junior A., Hummad I. Sheikh, and Masmudur M. Rahman. 2023. "Tumor Tropism of DNA Viruses for Oncolytic Virotherapy" Viruses 15, no. 11: 2262. https://doi.org/10.3390/v15112262
APA StyleEnow, J. A., Sheikh, H. I., & Rahman, M. M. (2023). Tumor Tropism of DNA Viruses for Oncolytic Virotherapy. Viruses, 15(11), 2262. https://doi.org/10.3390/v15112262





