Transcriptomic Profiling of Influenza A Virus-Infected Mouse Lung at Recovery Stage Using RNA Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Mouse Infection and Lung Sample Collection
2.3. RNA Extraction and Purification
2.4. Library Preparation and RNA-Seq
2.5. Sequence Alignment and Gene Annotation
2.6. Statistical Analysis of DEGs
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Javanian, M.; Barary, M.; Ghebrehewet, S.; Koppolu, V.; Vasigala, V.; Ebrahimpour, S. A brief review of influenza virus infection. J. Med. Virol. 2021, 93, 4638–4646. [Google Scholar] [CrossRef] [PubMed]
- Uyeki, T.M.; Hui, D.S.; Zambon, M.; Wentworth, D.E.; Monto, A.S. Influenza. Lancet 2022, 400, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.A., 2nd; Gong, M.; Bhagwanjee, S.; Sevransky, J. Global burden of influenza as a cause of cardiopulmonary morbidity and mortality. Glob. Heart 2014, 9, 325–336. [Google Scholar] [CrossRef]
- Al Hajjar, S.; McIntosh, K. The first influenza pandemic of the 21st century. Ann. Saudi Med. 2010, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, S.; Goraya, M.U.; Maarouf, M.; Huang, S.; Chen, J.L. Host Immune Response to Influenza A Virus Infection. Front. Immunol. 2018, 9, 320. [Google Scholar] [CrossRef]
- Girard, M.P.; Tam, J.S.; Assossou, O.M.; Kieny, M.P. The 2009 A (H1N1) influenza virus pandemic: A review. Vaccine 2010, 28, 4895–4902. [Google Scholar] [CrossRef]
- Thompson, M.G.; Dittmar, M.; Mallory, M.J.; Bhat, P.; Ferretti, M.B.; Fontoura, B.M.; Cherry, S.; Lynch, K.W. Viral-induced alternative splicing of host genes promotes influenza replication. eLife 2020, 9, e55500. [Google Scholar] [CrossRef]
- Pociask, D.A.; Robinson, K.M.; Chen, K.; McHugh, K.J.; Clay, M.E.; Huang, G.T.; Benos, P.V.; Janssen-Heininger, Y.M.W.; Kolls, J.K.; Anathy, V.; et al. Epigenetic and Transcriptomic Regulation of Lung Repair during Recovery from Influenza Infection. Am. J. Pathol. 2017, 187, 851–863. [Google Scholar] [CrossRef]
- Bauer, D.L.V.; Tellier, M.; Martínez-Alonso, M.; Nojima, T.; Proudfoot, N.J.; Murphy, S.; Fodor, E. Influenza Virus Mounts a Two-Pronged Attack on Host RNA Polymerase II Transcription. Cell Rep. 2018, 23, 2119–2129.e2113. [Google Scholar] [CrossRef]
- Heinz, S.; Texari, L.; Hayes, M.G.B.; Urbanowski, M.; Chang, M.W.; Givarkes, N.; Rialdi, A.; White, K.M.; Albrecht, R.A.; Pache, L.; et al. Transcription Elongation Can Affect Genome 3D Structure. Cell 2018, 174, 1522–1536.e1522. [Google Scholar] [CrossRef]
- Zhao, N.; Sebastiano, V.; Moshkina, N.; Mena, N.; Hultquist, J.; Jimenez-Morales, D.; Ma, Y.; Rialdi, A.; Albrecht, R.; Fenouil, R.; et al. Influenza virus infection causes global RNAPII termination defects. Nat. Struct. Mol. Biol. 2018, 25, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Bercovich-Kinori, A.; Tai, J.; Gelbart, I.A.; Shitrit, A.; Ben-Moshe, S.; Drori, Y.; Itzkovitz, S.; Mandelboim, M.; Stern-Ginossar, N. A systematic view on influenza induced host shutoff. eLife 2016, 5, e18311. [Google Scholar] [CrossRef] [PubMed]
- Gaucherand, L.; Porter, B.K.; Levene, R.E.; Price, E.L.; Schmaling, S.K.; Rycroft, C.H.; Kevorkian, Y.; McCormick, C.; Khaperskyy, D.A.; Gaglia, M.M. The Influenza A Virus Endoribonuclease PA-X Usurps Host mRNA Processing Machinery to Limit Host Gene Expression. Cell Rep. 2019, 27, 776–792.e777. [Google Scholar] [CrossRef] [PubMed]
- McCall, M.N.; Murakami, P.N.; Lukk, M.; Huber, W.; Irizarry, R.A. Assessing affymetrix GeneChip microarray quality. BMC Bioinform. 2011, 12, 137. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, J.; Zhang, Z.; Wang, X.; Hu, S.; Yu, J. Ribogenomics: The science and knowledge of RNA. Genom. Proteom. Bioinform. 2014, 12, 57–63. [Google Scholar] [CrossRef]
- Söllner, J.F.; Leparc, G.; Hildebrandt, T.; Klein, H.; Thomas, L.; Stupka, E.; Simon, E. An RNA-Seq atlas of gene expression in mouse and rat normal tissues. Sci. Data 2017, 4, 170185. [Google Scholar] [CrossRef]
- Picelli, S.; Björklund, Å.K.; Faridani, O.R.; Sagasser, S.; Winberg, G.; Sandberg, R. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 2013, 10, 1096–1098. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Jurgens, H.A.; Amancherla, K.; Johnson, R.W. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J. Neurosci. 2012, 32, 3958–3968. [Google Scholar] [CrossRef]
- Strickland, D.H.; Fear, V.; Shenton, S.; Wikstrom, M.E.; Zosky, G.; Larcombe, A.N.; Holt, P.G.; Berry, C.; von Garnier, C.; Stumbles, P.A. Persistent and compartmentalised disruption of dendritic cell subpopulations in the lung following influenza A virus infection. PLoS ONE 2014, 9, e111520. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Society. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Huda Al-Shalan. Molecular and Histopathological Characterisation of Peripheral Nerve and Immune Cell Distributions in Normal and Virus Infected Mouse Tissues. 2022. Available online: https://researchportal.murdoch.edu.au/esploro/outputs/doctoral/Molecular-and-histopathological-characterisation-of-peripheral/991005541220007891 (accessed on 7 August 2023).
- Chen, J.; Wu, J.; Hao, S.; Yang, M.; Lu, X.; Chen, X.; Li, L. Long term outcomes in survivors of epidemic Influenza A (H7N9) virus infection. Sci. Rep. 2017, 7, 17275. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Wilk, E.; Michaelsen-Preusse, K.; Gerhauser, I.; Baumgärtner, W.; Geffers, R.; Schughart, K.; Korte, M. Long-Term Neuroinflammation Induced by Influenza A Virus Infection and the Impact on Hippocampal Neuron Morphology and Function. J. Neurosci. 2018, 38, 3060–3080. [Google Scholar] [CrossRef]
- Natarajan, A.; Shetty, A.; Delanerolle, G.; Zeng, Y.; Zhang, Y.; Raymont, V.; Rathod, S.; Halabi, S.; Elliot, K.; Shi, J.Q.; et al. A systematic review and meta-analysis of long COVID symptoms. Syst. Rev. 2023, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Perumal, R.; Shunmugam, L.; Naidoo, K.; Abdool Karim, S.S.; Wilkins, D.; Garzino-Demo, A.; Brechot, C.; Parthasarathy, S.; Vahlne, A.; Nikolich, J. Long COVID: A review and proposed visualization of the complexity of long COVID. Front. Immunol. 2023, 14, 1117464. [Google Scholar] [CrossRef] [PubMed]
- Altmann, D.M.; Whettlock, E.M.; Liu, S.; Arachchillage, D.J.; Boyton, R.J. The immunology of long COVID. Nat. Rev. Immunol. 2023, 23, 618–634. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Pommerenke, C.; Wilk, E.; Srivastava, B.; Schulze, A.; Novoselova, N.; Geffers, R.; Schughart, K. Global transcriptome analysis in influenza-infected mouse lungs reveals the kinetics of innate and adaptive host immune responses. PLoS ONE 2012, 7, e41169. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Fan, W.; Zhang, S.; Li, Y.; Gu, J.; Zhou, J.; Liu, W. Transcriptome Profiling Reveals Differential Effect of Interleukin-17A Upon Influenza Virus Infection in Human Cells. Front. Microbiol. 2019, 10, 2344. [Google Scholar] [CrossRef]
- Dissanayake, T.K.; Schäuble, S.; Mirhakkak, M.H.; Wu, W.L.; Ng, A.C.; Yip, C.C.Y.; López, A.G.; Wolf, T.; Yeung, M.L.; Chan, K.H.; et al. Comparative Transcriptomic Analysis of Rhinovirus and Influenza Virus Infection. Front. Microbiol. 2020, 11, 1580. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Barr, J.; Jaquish, A.; Xu, J.; Verheyden, J.M.; Sun, X. Identification of lung innervating sensory neurons and their target specificity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L50–L63. [Google Scholar] [CrossRef] [PubMed]
- Frankl, S.; Coffin, S.E.; Harrison, J.B.; Swami, S.K.; McGuire, J.L. Influenza-Associated Neurologic Complications in Hospitalized Children. J. Pediatr. 2021, 239, 24–31.e21. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.; Zurynski, Y.; Buttery, J.; Marshall, H.; Richmond, P.C.; Dale, R.C.; Royle, J.; Gold, M.; Snelling, T.; Whitehead, B.; et al. Neurologic complications of influenza A(H1N1)pdm09: Surveillance in 6 pediatric hospitals. Neurology 2012, 79, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Jantarabenjakul, W.; Paprad, T.; Paprad, T.; Anugulruengkitt, S.; Pancharoen, C.; Puthanakit, T.; Chomtho, K. Neurological complications associated with influenza in hospitalized children. Influenza Other Respir. Viruses 2023, 17, e13075. [Google Scholar] [CrossRef]
- Jang, H.; Boltz, D.; Sturm-Ramirez, K.; Shepherd, K.R.; Jiao, Y.; Webster, R.; Smeyne, R.J. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc. Natl. Acad. Sci. USA 2009, 106, 14063–14068. [Google Scholar] [CrossRef]
- Hosseini, S.; Michaelsen-Preusse, K.; Schughart, K.; Korte, M. Long-Term Consequence of Non-neurotropic H3N2 Influenza A Virus Infection for the Progression of Alzheimer’s Disease Symptoms. Front. Cell. Neurosci. 2021, 15, 643650. [Google Scholar] [CrossRef]
- Hara, H.; Chida, J.; Uchiyama, K.; Pasiana, A.D.; Takahashi, E.; Kido, H.; Sakaguchi, S. Neurotropic influenza A virus infection causes prion protein misfolding into infectious prions in neuroblastoma cells. Sci. Rep. 2021, 11, 10109. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.; Santos, L.E.; Rangel, B. The Impact of Non-Neurotropic Influenza Strains on the Brain: A Role for Microglial Priming? J. Neurosci. 2018, 38, 7758–7760. [Google Scholar] [CrossRef]
- De Virgiliis, F.; Di Giovanni, S. Lung innervation in the eye of a cytokine storm: Neuroimmune interactions and COVID-19. Nat. Rev. Neurol. 2020, 16, 645–652. [Google Scholar] [CrossRef]
- Belvisi, M.G. Overview of the innervation of the lung. Curr. Opin. Pharmacol. 2002, 2, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yang, L.; Han, X.; Ding, X.; Li, J.; Yang, J. Local sympathetic innervations modulate the lung innate immune responses. Sci. Adv. 2020, 6, eaay1497. [Google Scholar] [CrossRef] [PubMed]
- Acanfora, D.; Nolano, M.; Acanfora, C.; Colella, C.; Provitera, V.; Caporaso, G.; Rodolico, G.R.; Bortone, A.S.; Galasso, G.; Casucci, G. Impaired Vagal Activity in Long-COVID-19 Patients. Viruses 2022, 14, 1035. [Google Scholar] [CrossRef]
- Gu, L.; Zhou, Y.; Wang, G.; Deng, H.; Song, X.; He, X.; Wang, T.; Chen, X.; Dai, J.; Li, R. Spatial learning and memory impaired after infection of non-neurotropic influenza virus in BALB/c male mice. Biochem. Biophys. Res. Commun. 2021, 540, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bohmwald, K.; Andrade, C.A.; Mora, V.P.; Muñoz, J.T.; Ramírez, R.; Rojas, M.F.; Kalergis, A.M. Neurotrophin Signaling Impairment by Viral Infections in the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5817. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, A.; Pulitanò, S.; Conti, G.; Barone, G.; Buonsenso, D.; Manni, L.; Capozzi, D.; Ria, F.; Riccardi, R. Interleukin and neurotrophin up-regulation correlates with severity of H1N1 infection in children: A case-control study. Int. J. Infect. Dis. 2013, 17, e1186–e1193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Porter, G.A.; O’Connor, J.C. Brain-derived neurotrophic factor and inflammation in depression: Pathogenic partners in crime? World J. Psychiatry 2022, 12, 77–97. [Google Scholar] [CrossRef]
- Bernstein, B.W.; Maloney, M.T.; Bamburg, J.R. Actin and Diseases of the Nervous System. Adv. Neurobiol. 2011, 5, 201–234. [Google Scholar] [CrossRef]
- Linfield, D.T.; Gao, N.; Raduka, A.; Harford, T.J.; Piedimonte, G.; Rezaee, F. RSV attenuates epithelial cell restitution by inhibiting actin cytoskeleton-dependent cell migration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L189–L203. [Google Scholar] [CrossRef]
- Kee, Z.; Kodji, X.; Brain, S.D. The Role of Calcitonin Gene Related Peptide (CGRP) in Neurogenic Vasodilation and Its Cardioprotective Effects. Front. Physiol. 2018, 9, 1249. [Google Scholar] [CrossRef] [PubMed]
- Garelja, M.L.; Hay, D.L. A narrative review of the calcitonin peptide family and associated receptors as migraine targets: Calcitonin gene-related peptide and beyond. Headache 2022, 62, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Veres, T.Z.; Rochlitzer, S.; Shevchenko, M.; Fuchs, B.; Prenzler, F.; Nassenstein, C.; Fischer, A.; Welker, L.; Holz, O.; Müller, M.; et al. Spatial interactions between dendritic cells and sensory nerves in allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 2007, 37, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Assas, B.M.; Pennock, J.I.; Miyan, J.A. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front. Neurosci. 2014, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Gárate, G.; Pascual, M.; Rivero, M.; Toriello, M.; Pérez-Pereda, S.; González-Quintanilla, V.; Madera, J.; Gutiérrez-Cuadra, M.; Fariñas, M.D.C.; Hernández, J.L.; et al. Serum Calcitonin Gene-Related Peptide α and β Levels are Increased in COVID-19 Inpatients. Arch. Med. Res. 2023, 54, 56–63. [Google Scholar] [CrossRef]
- Boahen, A.; Hu, D.; Adams, M.J.; Nicholls, P.K.; Greene, W.K.; Ma, B. Bidirectional crosstalk between the peripheral nervous system and lymphoid tissues/organs. Front. Immunol. 2023, 14, 1254054. [Google Scholar] [CrossRef]
- Vigil, F.A.; Carver, C.M.; Shapiro, M.S. Pharmacological Manipulation of K (v) 7 Channels as a New Therapeutic Tool for Multiple Brain Disorders. Front. Physiol. 2020, 11, 688. [Google Scholar] [CrossRef]
- Charlton, F.W.; Pearson, H.M.; Hover, S.; Lippiat, J.D.; Fontana, J.; Barr, J.N.; Mankouri, J. Ion Channels as Therapeutic Targets for Viral Infections: Further Discoveries and Future Perspectives. Viruses 2020, 12, 844. [Google Scholar] [CrossRef]
- Devasani, K.; Yao, Y. Expression and functions of adenylyl cyclases in the CNS. Fluids Barriers CNS 2022, 19, 23. [Google Scholar] [CrossRef]
- Wang, H.; Pineda, V.V.; Chan, G.C.; Wong, S.T.; Muglia, L.J.; Storm, D.R. Type 8 adenylyl cyclase is targeted to excitatory synapses and required for mossy fiber long-term potentiation. J. Neurosci. 2003, 23, 9710–9718. [Google Scholar] [CrossRef]
- Ago, Y.; Asano, S.; Hashimoto, H.; Waschek, J.A. Probing the VIPR2 Microduplication Linkage to Schizophrenia in Animal and Cellular Models. Front. Neurosci. 2021, 15, 717490. [Google Scholar] [CrossRef]
- Miotto, D.; Boschetto, P.; Bononi, I.; Zeni, E.; Cavallesco, G.; Fabbri, L.M.; Mapp, C.E. Vasoactive intestinal peptide receptors in the airways of smokers with chronic bronchitis. Eur. Respir. J. 2004, 24, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Delgado, M. Role of vasoactive intestinal peptide in inflammation and autoimmunity. Curr. Opin. Investig. Drugs 2005, 6, 1116–1123. [Google Scholar] [PubMed]
- Yousefi, H.; Fong, J.; Alahari, S.K. NR4A Family Genes: A Review of Comprehensive Prognostic and Gene Expression Profile Analysis in Breast Cancer. Front. Oncol. 2022, 12, 777824. [Google Scholar] [CrossRef] [PubMed]
- Rothe, T.; Ipseiz, N.; Faas, M.; Lang, S.; Perez-Branguli, F.; Metzger, D.; Ichinose, H.; Winner, B.; Schett, G.; Krönke, G. The Nuclear Receptor Nr4a1 Acts as a Microglia Rheostat and Serves as a Therapeutic Target in Autoimmune-Driven Central Nervous System Inflammation. J. Immunol. 2017, 198, 3878–3885. [Google Scholar] [CrossRef]
- Howard, F.H.N.; Kwan, A.; Winder, N.; Mughal, A.; Collado-Rojas, C.; Muthana, M. Understanding Immune Responses to Viruses-Do Underlying Th1/Th2 Cell Biases Predict Outcome? Viruses 2022, 14, 1493. [Google Scholar] [CrossRef]
- Paiva, I.A.; Badolato-Corrêa, J.; Familiar-Macedo, D.; de-Oliveira-Pinto, L.M. Th17 Cells in Viral Infections-Friend or Foe? Cells 2021, 10, 1159. [Google Scholar] [CrossRef]
- Aghbash, P.S.; Hemmat, N.; Nahand, J.S.; Shamekh, A.; Memar, M.Y.; Babaei, A.; Baghi, H.B. The role of Th17 cells in viral infections. Int. Immunopharmacol. 2021, 91, 107331. [Google Scholar] [CrossRef]
- Martonik, D.; Parfieniuk-Kowerda, A.; Rogalska, M.; Flisiak, R. The Role of Th17 Response in COVID-19. Cells 2021, 10, 1550. [Google Scholar] [CrossRef]
- Pauwels, A.M.; Trost, M.; Beyaert, R.; Hoffmann, E. Patterns, Receptors, and Signals: Regulation of Phagosome Maturation. Trends Immunol. 2017, 38, 407–422. [Google Scholar] [CrossRef]
- Sciacchitano, S.; Sacconi, A.; De Vitis, C.; Blandino, G.; Piaggio, G.; Salvati, V.; Napoli, C.; Marchetti, P.; Taurelli, B.S.; Coluzzi, F.; et al. H-Ras gene takes part to the host immune response to COVID-19. Cell Death Discov. 2021, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, Y.; Tsuda, M.; Nanbo, A.; Hattori, T.; Sasaki, J.; Sasaki, T.; Miyazaki, T.; Ohba, Y. A Ca2+-dependent signalling circuit regulates influenza A virus internalization and infection. Nat. Commun. 2013, 4, 2763. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Lin, K.; Cai, W.; Lin, Y.; Liu, X.; Guo, L.; Zhang, J.; Xu, W.; Lin, Z.; Wong, C.W.; et al. Tumors driven by RAS signaling harbor a natural vulnerability to oncolytic virus M1. Mol. Oncol. 2020, 14, 3153–3168. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, L.; von Wasielewski, R.; Lindenmaier, W.; Dittmar, K.E.J. Serial sectioning and three-dimensional reconstruction of mouse Peyer’s patch. Micron 2008, 39, 967–975. [Google Scholar] [CrossRef]
- Ma, B.; Yin, C.; Hu, D.; Newman, M.; Nicholls, P.K.; Wu, Z.; Greene, W.K.; Shi, Z. Distribution of non-myelinating Schwann cells and their associations with leukocytes in mouse spleen revealed by immunofluorescence staining. Eur. J. Histochem. 2018, 62, 2890. [Google Scholar] [CrossRef]
- Ma, B.; Li, M.; Fuchs, S.; Bischoff, I.; Hofmann, A.; Unger, R.E.; Kirkpatrick, C.J. Short-term hypoxia promotes vascularization in co-culture system consisting of primary human osteoblasts and outgrowth endothelial cells. J. Biomed. Mater. Res. A 2020, 108, 7–18. [Google Scholar] [CrossRef]

| B Cell Infiltration/Plasma Cells | T Cell Infiltration | Airway Repair |
|---|---|---|
| Igh; Igk; Cd79a; Cd22; Mzb1; Rgs13; Tnfrsf17; Tnfrsf13c; Spib; Pou2af1; Fcamr; Aicda; Jchain; Cd52 | Gzmk; Cxcr3; Tigit; Thy1; Cd4; Cd3; Izumo1r; Pbk; Cd52 | Krt5; Krt14; Krt15; Krt17; Mef2b; Slpi; Tff2 |
| Leukocyte Chemotaxis | Inflammation and Edema | Immune Modulation |
| Ccl20; Oit1; Ccl8; Cxcl9; Cxcl10; Cxcl13 | Itln1; Ubd; Aqp3; Agr2; Clca1; Il9r | Pla2g2d; Tigit; Calcb; Sectm1b; Il21r; Akr1c18 |
| Cell Proliferation | Innate Immunity | Platelets/Platelet Activation |
| Cdca5; Ccna1; Ccna2; Ccnb1; Plk1; Ska1; Cdk1; Cdkn3; Cdca3; Cdca8; Cdc20; Cenpm; Cep55; Mxd3; Melk; Top2a | Ltf; Dmbt1; Reg3g; Pglyrp1; Padi1; Bpifa1; Gp2 | Alox12 |
| Granulocyte/Monocyte Infiltration | Hypoxia | Smooth Muscle Activation |
| Prg2; Ms4a7; Cd177 | Dmbt1 | Stra6; Adh7; Dnase1l3; Birc5 |
| Mesothelium | MHC | Cell Signaling and Apoptosis |
| Msln | H2-EB2; H2-M2 | Stra6; Adh7; Dnase1l3; Birc5 |
| Complement | Neuronal | |
| C1qa; C1qb | Calcb; Kcna6 |
| Innate Immune Response | Specific Immune Response | Inflammatory Response |
|---|---|---|
| Spon2 | Slc39a10; S1pr1 | Abi3bp; Adcy8; Mylk; Tspan18 |
| Anti-inflammation | Neuroinflammation | Brain Development |
| Tek | Adcy8 | Pcdh18; Dnm1l; Tmod2; Adcy8 |
| Gene Expression/Transcription Regulation | Cell Division | Neurotrophin Signaling Pathway |
| Rnf187; Nox4; Zeb1; Aff1; Ier2; Tfcp2l1; Zeb2; Tfdp2; Klf11; Sox7 | Pard6g; Usp39; Specc1l | Gucy1a1; Tek; Slc39a10; Calml3; Map3k1 |
| Cell Death (i.e., Apoptosis) | cAMP Synthesis | Actin Organization |
| Peg3; Mylk; Dnm1l; Tnfrsf19 | Adcy8 | Abi3bp; Vcl; Cdc42ep1; Slc9a3r2; Clasp2; Ablim3; Fermt2 |
| Cell Adhesion | Neuronal | Growth Factor Activity |
| Spon2; Vcl; Ptprm | Ier2; Sema6d; Zdhhc3; Map6; Zeb1; Gm38399; Scn3a; Peg3; Atp7a; Tmod2; Abcd2; Map3k11; Vcl; Adcy8; Luzp1; Amph; Sema6d; mylk | Ier2; Reps2; Cpm; Rnf187; Ogn; Flt4; Tek |
| Gene | Full Name | Molecular Functions | Log2 FC | Fold Up | p-Value |
|---|---|---|---|---|---|
| Ighv14-2 | Immunoglobulin heavy variable 14-2 | Humoral immune response | 5.41 | 42.56 | 4.70 × 10−9 |
| AICDA | Activation-induced cytidine deaminase | Somatic hypermutation and antibody class switching | 5.40 | 42.33 | 3.89 × 10−7 |
| GZMK | Granzyme K | Cytolytic granules of cytotoxic T lymphocytes and natural killer cells; Inhibitor of influenza virus replication | 3.92 | 15.08 | 0.000107 |
| TIGIT | T cell immunoreceptor with Ig and ITIM domains | Induces IL-10; Suppresses T cell activation by generating immunoregulatory dendritic cells (DCs) | 3.20 | 9.19 | 4.57 × 10−5 |
| PRG2 | Proteoglycan 2 (bone marrow) | Induces non-cytolytic histamine release; Eosinophil major basic protein | 4.70 | 26.03 | 1.80 × 10−6 |
| KRT14 | Keratin 14 | Airway repair via epithelial cell differentiation | 6.47 | 88.94 | 1.24 × 10−5 |
| ALOX12E | Arachidonate lipoxygenase, epidermal | Prothrombotic/inflammatory response during influenza virus infection; Regulates gene expression | 7.35 | 162.72 | 1.29 × 10−6 |
| ITLN1 | Intelectin 1 | Airway inflammation in mucsa | 5.35 | 40.87 | 1.95 × 10−8 |
| LTF | Lactotransferrin | Negatively regulates viral processes and inhibits viral genome replication | 4.77 | 27.26 | 4.35 × 10−6 |
| DMBT1 | Deleted in malignant brain tumors 1 | Mucosal immune defense, epithelial differentiation, and tumor suppression | 4.43 | 21.55 | 2.83 × 10−5 |
| CCL20 | Chemokine (C-C motif) ligand 20 | Induces strong chemotactic for lymphocytes | 4.49 | 22.52 | 8.50 × 10−8 |
| BPIFA1 | BPI fold-containing family A member 1 | Neutrophil recruitment and interferon signaling; Inhibits viral proliferation; Immune response in upper airway | 3.92 | 15.17 | 0.00039 |
| Igkv5-48 | Immunoglobulin kappa variable 5-48 | Humoral immune response | 6.02 | 64.83 | 1.11 × 10−9 |
| MEF2B | Myocyte enhancer factor 2B | Smooth muscle-specific and/or growth-factor-related transcription | 4.48 | 22.36 | 1.37 × 10−6 |
| CD177 | CD177 antigen | Mediates activation of TNF-α primed neutrophils | 1.87 | 3.66 | 0.000317 |
| CALCB | Calcitonin-related polypeptide, beta | Neuroimmune communicator to regulate lymphocytes; Suppresses appetite | 3.41 | 10.67 | 9.85 × 10−5 |
| KCNA6 | Potassium voltage-gated channel, shaker-related, subfamily A, member 6 | Provide instructions for making voltage -gated potassium channels; Regulates neurotransmitter release, heart rate, insulin secretion, neuronal excitability, epithelial electrolyte transport, smooth muscle contraction, and cell volume | 1.69 | 3.22 | 0.00022 |
| MSLN | Mesothelin | Cellular adhesion | 3.41 | 10.66 | 1.92 × 10−6 |
| PLA2G2D | Phospholipase A2, group IID | Anti-inflammatory and immunosuppressive functions; Generates lipid mediators for pathogen clearance | 3.11 | 8.63 | 1.38 × 10−5 |
| H2-EB2 | Histocompatibility 2, class II antigen E beta2 | Adaptive immune response; Antigen processing; Presentation of peptide via MHCII | 2.92 | 7.55 | 1.36 × 10−5 |
| Gene | Full Name | Molecular Functions | Log2 FC | Fold Up | p-Value |
|---|---|---|---|---|---|
| Abi3bp | ABI family member 3 binding protein | Actin filament and collagen binding; Inflammatory response | −1.19 | 0.44 | 7.10 × 10−7 |
| Vcl | Vinculin | Cell-matrix and cell–cell adhesion | −1.49 | 0.36 | 7.62 × 10−7 |
| Adcy8 | Adenylate cyclase 8 | Neuroinflammatory response and brain functions such as memory; cAMP signaling activation | −1.35 | 0.39 | 2.62 × 10−6 |
| Vipr2 | Vasoactive intestinal peptide receptor 2 | Receptor for VIP; Water and ion flux in lungs and intestinal epithelia | −1.45 | 0.37 | 9.96 × 10−6 |
| Pcdhac2 | Protocadherin alpha subfamily C, 2 | Nervous system development; calcium-dependent cell-adhesion protein | −2.57 | 0.17 | 1.03 × 10−5 |
| Spon2 | Spondin 2 | Cell adhesion and innate immune response | −2.74 | 0.15 | 2.34 × 10−13 |
| Elmo2 | Engulfment and cell motility 2 | Cytoskeletal rearrangements for phagocytosis of apoptotic cells | −0.90 | 0.54 | 1.58 × 10−5 |
| Eng | Endoglin | Regulates angiogenesis and CNS vasculogenesis | −1.30 | 0.40 | 2.78 × 10−5 |
| Cers2 | Ceramide synthase 2 | Negative regulation of Schwann cell migration and proliferation in axon regeneration | −0.82 | 0.56 | 0.000188 |
| Atp1b2 | ATPase, Na + /K + transporting, beta 2 polypeptide | Mediates cell adhesion of neurons and astrocytes; Catalyzes the hydrolysis of ATP | −0.86 | 0.55 | 0.000168 |
| Hsph1 | Heat shock protein 1 105 kDa | Positive regulation of natural killer T cell activation | −1.12 | 0.46 | 0.00017 |
| Nr4a1 | Nuclear receptor subfamily 4 group A member 1 | Neurotransmitter secretion; Cell cycle mediation, inflammation, and apoptosis | −1.01 | 0.50 | 0.000215 |
| Nr4a3 | Nuclear receptor subfamily 4 group A member 3 | Inflammatory response; Mediates survival of neurons and smooth muscle cells | −1.17 | 0.44 | 0.000206 |
| Tmod2 | Tropomodulin 2 | Neuron–neuron synaptic transmission; Learning or memory | −0.81 | 0.57 | 0.000123 |
| Ednra | Endothelin receptor type A | Development of enteric nervous system and neural crest cells | −0.78 | 0.58 | 4.06 × 10−5 |
| S1pr1 | Sphingosine 1- phosphate receptor 1 | Immune response; Transport of mature T cells from the thymus into the blood and peripheral lymphoid organs | −1.01 | 0.50 | 6.91 × 10−5 |
| Mylk | Myosin light chain kinase | Inflammatory response; Regulating the actin–myosin interaction of smooth muscle | −0.83 | 0.56 | 8.89 × 10−5 |
| Crtc3 | CREB-regulated transcription coactivator 3 | cAMP response element building | −1.07 | 0.48 | 9.65 × 10−5 |
| Slc39a10 | Solute carrier family 39, member 10 | B cell proliferation and B cell receptor signaling pathway | −0.82 | 0.57 | 0.000119 |
| Usp39 | Ubiquitin-specific peptidase 39 | Cell cycle and cell division; Pre-mRNA splicing; NF-κB-mediated inflammatory responses | −1.12 | 0.46 | 0.000153 |
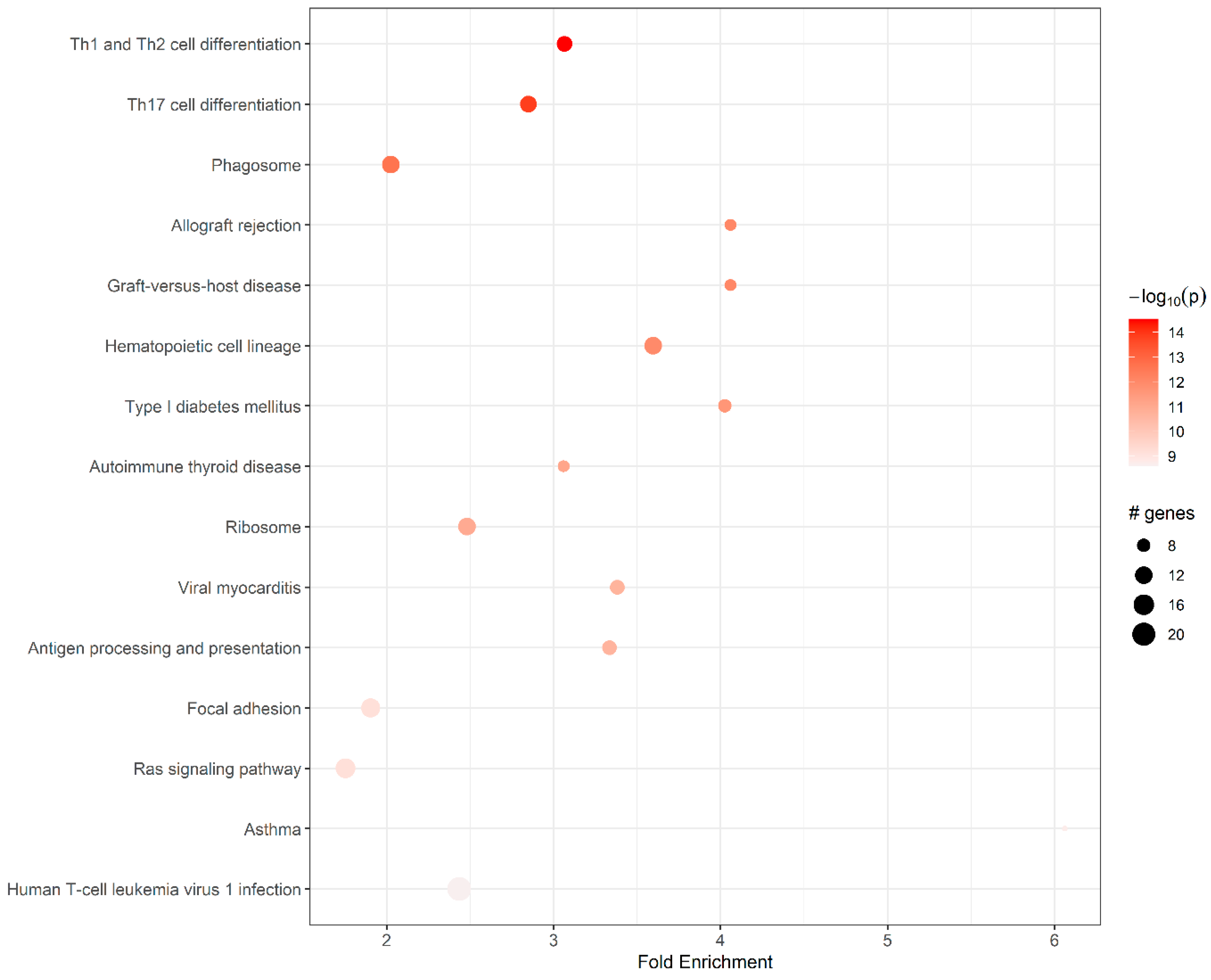
| ID | Pathway | Fold En. | Up-Regulated Genes | Down-Regulated Genes |
|---|---|---|---|---|
| mmu04658 | Th1 and Th2 cell differentiation | 3.07 | H2-Eb1, H2-DMa, H2-Oa, H2-Ob, H2-Eb2, Cd4, Cd3g, Cd3d, Lat | Fos |
| mmu04659 | Th17 cell differentiation | 2.85 | Il21r, H2-Eb1, H2-DMa, H2-Oa, H2-Ob, H2-Eb2, Cd4, Cd3g, Cd3d, Lat | Fos |
| mmu04145 | Phagosome | 2.03 | H2-M2, H2-Q6, H2-Eb1, H2-DMa, H2-Oa, H2-Ob, H2-Eb2, Tap2, Cd14 | Sec61a1, Thbs1, Cd209b |
| mmu05330 | Allograft rejection | 4.06 | H2-M2, H2-Q6, H2-Eb1, H2-DMa, H2-Oa, H2-Ob, H2-Eb2 | |
| mmu05332 | Graft-versus-host disease | 4.06 | H2-Eb1, H2-DMa, H2-Oa, H2-Ob, H2-Eb2, H2-M2, H2-Q6 | |
| mmu04640 | Hematopoietic cell lineage | 3.60 | H2-Eb1, H2-DMa, H2-Oa, H2-Ob, H2-Eb2, Cd4, Cd3d, Cd3g, Cd22, Cd14, | Kit |
| mmu04940 | Type I diabetes mellitus | 4.03 | H2-Eb1, H2-DMa, H2-Oa, H2-Ob, H2-Eb2, H2-M2, H2-Q6 | Ica1 |
| mmu05320 | Autoimmune thyroid disease | 3.06 | H2-Eb1, H2-DMa, H2-Oa, H2-Ob, H2-Eb2, H2-M2, H2-Q6 | |
| mmu03010 | Ribosome | 2.48 | Rps7, Rps8, Rps10, Rps20, Rps23, Rps27a, Rpsa, Rpl11, Rpl22, Rpl22l1, Rpl30, Rpl35a | |
| mmu05416 | Viral myocarditis | 3.38 | H2-Eb1, H2-DMa, H2-Oa, H2-Ob, H2-Eb2, H2-M2, H2-Q6 | Cav1, Icam1 |
| mmu04612 | Antigen processing and presentation | 3.33 | H2-M2, H2-Q6, Tap2, H2- Eb1, H2-DMa, H2-Oa, H2- Ob, H2-Eb2, Cd4 | |
| mmu04510 | Focal adhesion | 1.90 | Prkcb | |
| mmu04014 | Ras signaling pathway | 1.75 | Efna5, Lat, Calml3, Pla2g5, Pla2g2d, Prkcb | |
| mmu05310 | Asthma | 6.06 | H2-Eb1, H2-DMa, H2-Oa, H2-Ob, H2-Eb2 | |
| mmu05166 | Human T cell leukemia virus 1 infection | 2.44 | Cd4, H2-M2, H2-Q6, Mad2l1, Cdc20, Ccnb2, Ccna2, Ccna1, Map3k1, Cd3d, Cd3g, Tnfrsf13c, H2-Eb1, H2-DMa, H2-Oa, H2-Ob, H2-Eb2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shalan, H.A.M.; Hu, D.; Wang, P.; Uddin, J.; Chopra, A.; Greene, W.K.; Ma, B. Transcriptomic Profiling of Influenza A Virus-Infected Mouse Lung at Recovery Stage Using RNA Sequencing. Viruses 2023, 15, 2198. https://doi.org/10.3390/v15112198
Al-Shalan HAM, Hu D, Wang P, Uddin J, Chopra A, Greene WK, Ma B. Transcriptomic Profiling of Influenza A Virus-Infected Mouse Lung at Recovery Stage Using RNA Sequencing. Viruses. 2023; 15(11):2198. https://doi.org/10.3390/v15112198
Chicago/Turabian StyleAl-Shalan, Huda A. M., Dailun Hu, Penghao Wang, Jasim Uddin, Abha Chopra, Wayne K. Greene, and Bin Ma. 2023. "Transcriptomic Profiling of Influenza A Virus-Infected Mouse Lung at Recovery Stage Using RNA Sequencing" Viruses 15, no. 11: 2198. https://doi.org/10.3390/v15112198
APA StyleAl-Shalan, H. A. M., Hu, D., Wang, P., Uddin, J., Chopra, A., Greene, W. K., & Ma, B. (2023). Transcriptomic Profiling of Influenza A Virus-Infected Mouse Lung at Recovery Stage Using RNA Sequencing. Viruses, 15(11), 2198. https://doi.org/10.3390/v15112198







