The Cyclophilin Inhibitor Rencofilstat Decreases HCV-Induced Hepatocellular Carcinoma Independently of Its Antiviral Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Antibodies
2.2. Animal Care
2.3. HCV Chimeric Mouse Study
2.4. Quantification of HCV RNA by Real-Time Reverse Transcription PCR
2.5. HCC Analyses
3. Results
3.1. HCV Infection Induces the Development of HCC in Humanized Liver Mice
3.2. HCV Infection Induces a Progressive HCC Development in Humanized Liver Mice
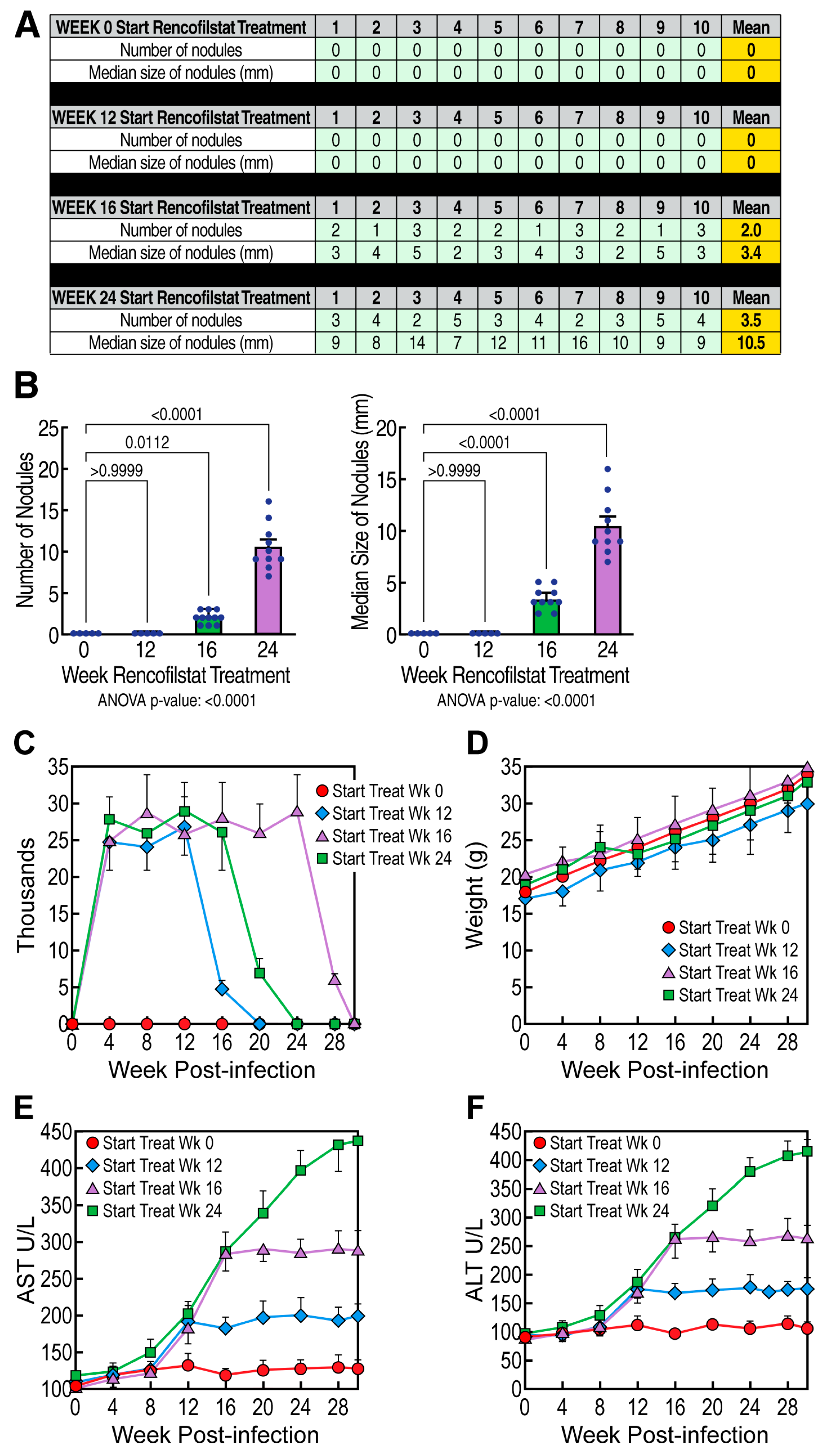
3.3. Rencofilstat Treatment Reduces HCV-Induced HCC
3.4. Rencofilstat’s Anti-HCC Activity Is Partly Independent of Its Anti-HCV Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Handschumacher, R.E.; Harding, M.W.; Rice, J.; Drugge, R.J.; Speicher, D.W. Cyclophilin: A specific cytosolic binding protein for cyclosporin A. Science 1984, 226, 544–547. [Google Scholar] [CrossRef]
- Fischer, G.; Wittmann-Liebold, B.; Lang, K.; Kiefhaber, T.; Schmid, F.X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 1989, 337, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Schmidpeter, P.A.M.; Koch, J.R.; Schmid, F.X. Control of protein function by prolyl isomerization. Biochim. Biophys. Acta 2015, 1850, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.P.; Finn, G.; Lee, T.H.; Nicholson, L.K. Prolyl cis-trans isomerization as a molecular timer. Nat. Chem. Biol. 2007, 3, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Reichman, C.; Saleh, T.; Birge, R.B.; Kalodimos, C.G. Proline cis-trans isomerization controls autoinhibition of a signaling protein. Mol. Cell 2007, 25, 413–426. [Google Scholar] [CrossRef]
- Lummis, S.C.R.; Beene, D.L.; Lee, L.W.; Lester, H.A.; Broadhurst, R.W.; Dougherty, D.A. Cis–trans isomerization at a proline opens the pore of a neurotransmitter-gated ion channel. Nature 2005, 438, 248–252. [Google Scholar] [CrossRef]
- Sarkar, P.; Saleh, T.; Tzeng, S.-R.; Birge, R.B.; Kalodimos, C.G. Structural basis for regulation of the Crk signaling protein by a proline switch. Nat. Chem. Biol. 2011, 7, 51–57. [Google Scholar] [CrossRef]
- Aumüller, T.; Jahreis, G.; Fischer, G.; Schiene-Fischer, C. Role of prolyl cis/trans isomers in cyclophilin-assisted Pseudomonas syringae Avrrpt2 protease activation. Biochemistry 2010, 49, 1042–1052. [Google Scholar] [CrossRef]
- Watts, R.; Clunie, G.; Hall, F.; Marshall, T. Rheumatology; Oxford University Press: Oxford, UK, 2009; p. 558. ISBN 978-0-19-922999-4. [Google Scholar]
- Davis, T.L.; Walker, J.R.; Campagna-Slater, V.; Finerty, P.J., Jr.; Paramanathan, R.; Bernstein, G.; MacKenzie, F.; Tempel, W.; Ouyang, H.; Lee, W.H.; et al. Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases. PLoS Biol. 2010, 8, e1000439. [Google Scholar] [CrossRef]
- Sigal, N.H.; Dumont, F.; Durette, P.; Siekierka, J.J.; Peterson, L.; Rich, D.H.; Dunlap, B.E.; Staruch, M.J.; Melino, M.R.; Koprak, S.L. Is cyclophilin involved in the immunosuppressive and nephrotoxic mechanism of action of cyclosporin A? J. Exp. Med. 1991, 173, 619–628. [Google Scholar] [CrossRef]
- Quesniaux, V.F.J.; Schreier, M.H.; Wenger, R.M.; Hiestand, P.C.; Harding, M.W.; Van Regenmortel, M.H. Cyclophilin binds to the region of cyclosporine involved in its immunosup-pressive activity. Eur. J. Immunol. 1987, 17, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, Z.K.; Fu, J.; Wiedmann, B. From chemical tools to clinical medicines: Nonimmunosuppressive cyclophilin inhibitors de-rived from the cyclosporin and sanglifehrin scaffolds. J. Med. Chem. 2014, 57, 7145–7159. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.; Bobardt, M.; Chatterji, U.; Mayo, P.R.; Trepanier, D.J.; Foster, R.T.; Gallay, P.; Ure, D.R. A Pan-Cyclophilin Inhibitor, CRV431, Decreases Fibrosis and Tumor Development in Chronic Liver Disease Models. J. Pharmacol. Exp. Ther. 2019, 371, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Mayo, P.R.; Hobbs, T.M.; Canizares, C.; Foster, E.P.; Zhao, C.; Ure, D.R.; Trepanier, D.J.; Greytok, J.A.; Foster, R.T. Rencofilstat, a cyclophilin inhibitor: A phase 2a, multicenter, single-blind, placebo-controlled study in F2/F3 NASH. Hepatol. Commun. 2022, 6, 3379–3392. [Google Scholar] [CrossRef]
- Gallay, P.A. Cyclophilin inhibitors: A novel class of promising host-targeting anti-HCV agents. Immunol. Res. 2012, 52, 200–210. [Google Scholar] [CrossRef]
- Naoumov, N.V. Cyclophilin inhibition as potential therapy for liver diseases. J. Hepatol. 2014, 61, 1166–1174. [Google Scholar] [CrossRef]
- Hopkins, S.; Gallay, P.A. The role of immunophilins in viral infection. Biochim. Biophys. Acta 2015, 1850, 2103–2110. [Google Scholar] [CrossRef]
- Lin, K.; Gallay, P. Curing a viral infection by targeting the host: The example of cyclophilin inhibitors. Antivir. Res. 2013, 99, 68–77. [Google Scholar] [CrossRef]
- Hopkins, S.; Gallay, P. Cyclophilin inhibitors: An emerging class of therapeutics for the treatment of chronic hepatitis C infection. Viruses 2012, 4, 2558–2577. [Google Scholar] [CrossRef]
- Gallay, P.A.; Chatterji, U.; Bobardt, M.D.; Long, Z.; Zhang, S.; Su, Z. Characterization of the Anti-HCV Activities of the New Cyclophilin Inhibitor STG-175. PLoS ONE 2016, 11, e0152036. [Google Scholar] [CrossRef]
- Gregory, M.A.; Bobardt, M.; Obeid, S.; Chatterji, U.; Coates, N.J.; Foster, T.; Gallay, P.; Leyssen, P.; Moss, S.J.; Neyts, J.; et al. Preclinical characterization of naturally occurring polyketide cyclophilin inhibitors from the sanglifehrin family. Antimicrob. Agents Chemother. 2011, 55, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Hansson, M.J.; Moss, S.J.; Bobardt, M.; Chatterji, U.; Coates, N.; Garcia-Rivera, J.A.; Elmér, E.; Kendrew, S.; Leyssen, P.; Neyts, J.; et al. Bioengineering and semisynthesis of an optimized cyclophilin inhibitor for treatment of chronic viral infection. Chem. Biol. 2015, 22, 285–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coelmont, L.; Hanoulle, X.; Chatterji, U.; Berger, C.; Snoeck, J.; Bobardt, M.; Lim, P.; Vliegen, I.; Paeshuyse, J.; Vuagniaux, G.; et al. DEB025 (Alisporivir) inhibits hepatitis C virus replication by preventing a cyclophilin A induced cis-trans isomerisation in domain II of NS5A. PLoS ONE 2010, 5, e13687. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.; Bobardt, M.; Chatterji, U.; Garcia-Rivera, J.A.; Lim, P.; Gallay, P.A. The cyclophilin inhibitor SCY-635 disrupts hepatitis C virus NS5A-cyclophilin A complexes. Antimicrob. Agents Chemother. 2012, 56, 3888–3897. [Google Scholar] [CrossRef]
- Chatterji, U.; Bobardt, M.; Selvarajah, S.; Yang, F.; Tang, H.; Sakamoto, N.; Vuagniaux, G.; Parkinson, T.; Gallay, P. The isomerase active site of cyclophilin a is critical for hepatitis C virus replication. J. Biol. Chem. 2009, 284, 16998–17005. [Google Scholar] [CrossRef]
- Chatterji, U.; Lim, P.; Bobardt, M.D.; Wieland, S.; Cordek, D.G.; Vuagniaux, G.; Chisari, F.; Cameron, C.E.; Targett-Adams, P.; Parkinson, T.; et al. HCV resistance to cyclosporin A does not correlate with a resistance of the NS5A–cyclophilin A interaction to cyclophilin inhibitors. J. Hepatol. 2010, 53, 50–56. [Google Scholar] [CrossRef]
- Chatterji, U.; Bobardt, M.; Tai, A.; Wood, M.; Gallay, P.A. Cyclophilin and NS5A Inhibitors, but not other anti-hepatitis C virus (HCV) agents, preclude HCV-mediated formation of double-membrane-vesicle viral factories. Antimicrob. Agents Chemother. 2015, 59, 2496–2507. [Google Scholar] [CrossRef]
- Chatterji, U.; Bobardt, M.; Schaffer, L.; Wood, M.; Gallay, P.A. Cyclophilin Inhibitors Remodel the Endoplasmic Reticulum of HCV-Infected Cells in a Unique Pattern Rendering Cells Impervious to a Reinfection. PLoS ONE 2016, 11, e0159511. [Google Scholar] [CrossRef]
- Hopkins, S.; DiMassimo, B.; Rusnak, P.; Heuman, D.; Lalezari, J.; Sluder, A.; Scorneaux, B.; Mosier, S.; Kowalczyk, P.; Ribeill, Y.; et al. The cyclophilin inhibitor SCY-635 suppresses viral replication and induces endogenous interferons in patients with chronic HCV genotype 1 infection. J. Hepatol. 2012, 57, 47–54. [Google Scholar] [CrossRef]
- Garcia-Rivera, J.A.; Lin KHopkins, S.; Gregory, M.A.; Wilkinson, B.; Gallay, P.A. Development of a flow cytometry live cell assay for the screening of inhibitors of hepatitis C virus (HCV) replication. Open Virol. J. 2012, 6, 97–102. [Google Scholar] [CrossRef]
- Garcia-Rivera, J.A.; Bobardt, M.; Chatterji, U.; Hopkins, S.; Gregory, M.A.; Wilkinson, B.; Lin, K.; Gallay, P.A. Multiple mutations in HCV NS5A domain II are required to confer a significant level of resistance to alisporivir. Antimicrob. Agents Chemother. 2012, 56, 5113–5121. [Google Scholar] [CrossRef] [PubMed]
- Bobardt, M.; Hopkins, S.; Baugh, J.; Chatterji, U.; Hernandez, F.; Hiscott, J.; Sluder, A.; Lin, K.; Gallay, P.A. HCV NS5A and IRF9 compete for CypA binding. J. Hepatol. 2013, 58, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, S.; Flisiak, R.; Vierling, J.M.; Mazur, W.; Mazzella, G.; Thongsawat, S.; Abdurakhmanov, D.; Van Kính, N.; Calistru, P.; Heo, J.; et al. Randomised clinical trial: Alisporivir combined with peginterferon and ribavirin in treatment-naïve patients with chronic HCV genotype 1 infection (ESSENTIAL II). Aliment. Pharmacol. Ther. 2015, 42, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.; Flisiak, R.; Sarin, S.K.; Rasenack, J.; Piratvisuth, T.; Chuang, W.; Peng, C.; Foster, G.R.; Shah, S.; Wedemeyer, H.; et al. Alisporivir plus ribavirin, interferon free or in combination with pegylated interferon, for hepatitis C virus genotype 2 or 3 infection. Hepatology 2015, 62, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Jaroszewicz, J.; Flisiak, I.; Łapiński, T. Update on alisporivir in treatment of viral hepatitis C. Expert Opin. Investig. Drugs 2012, 21, 375–382. [Google Scholar] [CrossRef]
- Flisiak, R.; Feinman, S.V.; Jablkowski, M.; Horban, A.; Kryczka, W.; Pawlowska, M.; Heathcote, J.E.; Mazzella, G.; Vandelli, C.; Nicolas-Métral, V.; et al. The cyclophilin inhibitor Debio 025 combined with PEG IFNalpha2a significantly reduces viral load in treatment-naïve hepatitis C patients. Hepatology 2009, 49, 1460–1468. [Google Scholar] [CrossRef]
- Dorner, M.; Horwitz, J.A.; Donovan, B.M.; Labitt, R.N.; Budell, W.C.; Friling, T.; Vogt, A.; Catanese, M.T.; Satoh, T.; Kawai, T.; et al. Completion of the entire hepatitis C virus life cycle in genetically humanized mice. Nature 2013, 501, 237–241. [Google Scholar] [CrossRef]
- Bobardt, M.; Hansson, M.J.; Mayo, P.; Ure, D.; Foster, R.; Gallay, P. Structurally distinct cyclosporin and sanglifehrin analogs CRV431 and NV556 suppress established HCV infection in humanized-liver mice. PLoS ONE 2020, 15, e0237236. [Google Scholar] [CrossRef]
- Pawlotsky, J.-M. Interferon-Free Hepatitis C Virus Therapy. Cold Spring Harb. Perspect. Med. 2020, 10, a036855. [Google Scholar] [CrossRef]
- Flores, A.; Marrero, J.A. Emerging Trends in Hepatocellular Carcinoma: Focus on Diagnosis and Therapeutics. Clin. Med. Insights Oncol. 2014, 8, 71–76. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bruix, J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008, 48, 1312–1327. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M.; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Pravisani, R.; Baccarani, U.; Isola, M.; Adani, G.; Lorenzin, D.; Terrosu, G.; Risaliti, A. Impact of surgical complications on the risk of hepatocellular carcinoma recurrence after hepatic resection. Updat. Surg. 2017, 70, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Buonfiglioli, F.; Scuteri, A.; Crespi, C.; Bolondi, L.; Caraceni, P.; Foschi, F.G.; Lenzi, M.; Mazzella, G.; Verucchi, G.; et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol. 2016, 65, 727–733. [Google Scholar] [CrossRef]
- Boix, L.; Mariño, Z.; Torres, F.; Forns, X.; Bruix, J.; Reig, M. Liver Cancer Emergence Associated with Antiviral Treatment: An Immune Surveillance Failure? Semin. Liver Dis. 2017, 37, 109–118. [Google Scholar] [CrossRef]
- The Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef]
- The European Union HCV Collaborators. Hepatitis C virus prevalence and level of intervention required to achieve the WHO targets for elimination in the European Union by 2030: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 325–336. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; Clinical Practice Guidelines Panel: Chair; EASL Governing Board Representative. EASL recommendations on treatment of hepatitis C: Final update of the series☆. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, N.; Tesfaye, A.; Feinstone, S.; Kumar, A. HCV infection-associated hepatocellular carcinoma in humanized mice. Infect. Agents Cancer 2015, 10, 24. [Google Scholar] [CrossRef]
- Takeuchi, T.; Katsume, A.; Tanaka, T.; Abe, A.; Inoue, K.; Tsukiyama-Kohara, K.; Kawaguchi, R.; Tanaka, S.; Kohara, M. Real-time detection system for quantification of hepatitis C virus genome. Gastroenterology 1999, 116, 636–642. [Google Scholar] [CrossRef]
- Kuo, J.; Serrano, S.S.; Grönberg, A.; Massoumi, R.; Hansson, M.J.; Gallay, P. Cyclophilin Inhibitor NV556 Reduces Fibrosis and Hepatocellular Carcinoma Development in Mice With Non-Alcoholic Steatohepatitis. Front. Pharmacol. 2019, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Balogh, J.; Victor, D., 3rd; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Tunissiolli, N.M.; Castanhole-Nunes, M.M.U.; Biselli-Chicote, P.M.; Pavarino, E.C.; da Silva, R.F.; da Silva, R.C.; Goloni-Bertollo, E.M. Hepatocellular Carcinoma: A Comprehensive Review of Biomarkers, Clinical Aspects, and Therapy. Asian Pac. J. Cancer Prev. 2017, 18, 863–872. [Google Scholar] [PubMed]
- Waller, L.P.; Deshpande, V.; Pyrsopoulos, N. Hepatocellular carcinoma: A comprehensive review. World J. Hepatol. 2015, 7, 2648–2663. [Google Scholar] [CrossRef]
- Calvaruso, V.; Cabibbo, G.; Cacciola, I.; Petta, S.; Madonia, S.; Bellia, A.; Tinè, F.; Distefano, M.; Giannitrapani, L.; Prestileo, T.; et al. Early occurrence of hepatocellular carcinoma in patients with HCV cirrhosis treated with direct-acting antivirals. Dig. Liver Dis. 2017, 49, e60. [Google Scholar] [CrossRef]
- Issachar, A.; Sneh-Arbib, O.; Braun, M.; Shlomai, A.; Oxtrud, E.; Harif, Y.; Karavani, C.; Kaspa, R.T.; Cohen-Naftaly, M. Occurrence and Recurrence of Malignancies Post DAA Treatment in 5.1% of Patients- Single Center Experience. J. Hepatol. 2017, 66, S97. [Google Scholar] [CrossRef]
- Grandhe, S.; Frenette, C.T. Occurrence and Recurrence of Hepatocellular Carcinoma After Successful Direct-Acting Antiviral Therapy for Patients With Chronic Hepatitis C Virus Infection. Gastroenterol. Hepatol. 2017, 13, 421–425. [Google Scholar]
- Bielen, R.; Moreno, C.; Van Vlierberghe, H.; Bourgeois, S.; Mulkay, J.; Vanwolleghem, T.; Verlinden, W.; Brixco, C.; Decaestecker, J.; de Galocsy, C.; et al. The risk of early occurrence and recurrence of hepatocellular carcinoma in hepatitis C-infected patients treated with direct-acting antivirals with and without pegylated interferon: A Belgian experience. J. Viral Hepat. 2017, 24, 976–981. [Google Scholar] [CrossRef]
- Cabibbo, G.; Petta, S.; Calvaruso, V.; Cacciola, I.; Cannavò, M.R.; Madonia, S.; Distefano, M.; Larocca, L.; Prestileo, T.; Tinè, F.; et al. Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? A prospective multicentre study. Aliment. Pharmacol. Ther. 2017, 46, 688–695. [Google Scholar] [CrossRef]
- Reig, M.; Mariño, Z.; Perelló, C.; Iñarrairaegui, M.; Ribeiro, A.; Lens, S.; Díaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef]
- Romano, A.; Capra, F.; Carolo, G.; Scroccaro, G.; Piovesan, S.; Chemello, L.; Cavalletto, L.; Anastassopulos, G.; Vincenzi, V.; Scotton, P.; et al. Incidence and pattern of de novo hepa-tocellular carcinoma in HCV patients treated with oral DAAs. Hepatology 2016, 64, 10A. [Google Scholar]
- Cardoso, H.; Vale, A.M.; Rodrigues, S.; Gonçalves, R.; Albuquerque, A.; Pereira, P.; Lopes, S.; Silva, M.; Andrade, P.; Morais, R.; et al. High incidence of hepatocellular carcinoma following successful interferon-free antiviral therapy for hepatitis C associated cirrhosis. J. Hepatol. 2016, 65, 1070–1071. [Google Scholar] [CrossRef] [PubMed]
- Kozbial, K.; Moser, S.; Schwarzer, R.; Laferl, H.; Al-Zoairy, R.; Stauber, R.; Stättermayer, A.F.; Beinhardt, S.; Graziadei, I.; Freissmuth, C.; et al. Unexpected high incidence of hepatocellular carci-noma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J. Hepatol. 2016, 65, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Waidmann, O.P.; Vermehren, J.; Moreno, C.; Vögeli, I.; Berg, T.; Semela, D.; Zeuzem, S.; Dufour, J.F. Hepatocellular carcinoma recurrence after direct antiviral agent treatment: A European multicentre study. J. Hepatol. 2017, 67, 876–888. [Google Scholar]
- Howell, J.; Papaluca, T.; Glasgow, S.; New, K.; Hong, T.; Snell, J.; Iser, D.; Ryan, M.; Bell, S.; Desmond, P.; et al. In hepatitis C patients with cirrhosis who achieve SVR with treatment, reduction in transient elastography measures does not translate to reduced risk of hepatocellular carcinoma: A prospective cohort study. J. Hepatol. 2018, 68, S535–S536. [Google Scholar] [CrossRef]
- Flisiak, R.; Łucejko, M.; Zarebska-Michaluk, D.; Tomasiewicz, K.; Rostkowska, K.; Tudrujek, M.; Grzegorz, M.; Krzysztof, S.; Biatkowska, J.; Jablkowski, M. Risk of de novo hepatocellular carcinoma after DAA treatment within two years following treatment with Ombitasvir/Paritaprevir/ritonavir ± Dasabuvir ± Ribavirin in the AMBER—Real world experience study. J. Hepatol. 2018, 68, S536. [Google Scholar] [CrossRef]
- Idilman, R.; Demir, M.; Aladag, M.; Kaymakoglu, S.; Erol, C.; Cavus, B.; Iliaz, R.; Akarca, U.S.; Koklu, S.; Cakaloglu, Y.; et al. Recurrence and occurrence of hepatocellular car-cinoma following ledipasvir and sofosbuvir treatment for chronic hepatitis C in patients with advanced liver disease: Turkish multicenter early access program. J. Hepatol. 2018, 68, S301–S302. [Google Scholar] [CrossRef]
- Ruiz, P.; Deiss, L.; Buendía, L.; Erdozain, I.; Álvarez, C.; Gutiérrez, P.; Pascual, J.J.; Baranda, A.; Blanco, S.; Menéndez, F.; et al. De novo hepatocellular carcinoma in patients with cirrhosis due hepatitis C virus infection after treatment with direct antiviral agents. J. Hepatol. 2018, 68, S531–S532. [Google Scholar] [CrossRef]
- Ozeki, I.; Suii, H.; Tatsumi, R.; Yamaguchi, M.; Kimura, M.; Arakawa, T.; Kuwata, Y.; Ohmura, T.; Hige, S.; Karino, Y.; et al. Recurrence of hepatocellular carcinoma in patients with a history of HCC after SVR to DAA for chronic hepatitis C. J. Hepatol. 2018, 68, S535. [Google Scholar] [CrossRef]
- Bandiera, S.; Hamdane, N.; Davidson, I.; Thumann, C.; Zeisel, M.; Hoshida, Y.; Bardeesy, N.; Baumert, T. Deciphering Epigenetic Reprogramming in Persistent Hepatitis C Virus Infection Reveals Candidate Drivers of Hepatocarcinogenesis. J. Hepatol. 2016, 64, S420–S421. [Google Scholar] [CrossRef]
- Prenner, S.B.; VanWagner, L.B.; Flamm, S.L.; Salem, R.; Lewandowski, R.J.; Kulik, L. Hepatocellular carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals. J. Hepatol. 2017, 66, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Minuk, G.Y.; Bautista, W.; Klein, J. Evidence of Hepatitis B Virus Infection in Cancer and Noncancer Stem Cells Associated with Human Hepatocellular Carcinoma. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 8931591. [Google Scholar] [CrossRef] [PubMed]
- Faria, L.C.; Gigou, M.; Roque–Afonso, A.M.; Sebagh, M.; Roche, B.; Fallot, G.; Ferrari, T.C.; Guettier, C.; Dussaix, E.; Castaing, D.; et al. Hepatocellular carcinoma is associated with an increased risk of hepatitis B virus recurrence after liver transplantation. Gastroenterology 2008, 134, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Debes, J.D.; van Tilborg, M.; Groothuismink, Z.M.A.; Hansen, B.E.; Schulze Zur Wiesch, J.; von Felden, J.; de Knegt, R.J.; Boonstra, A. Levels of Cytokines in Serum Associate with Development of Hepatocellular Carcinoma in Patients with HCV Infection Treated With Direct-Acting Antivirals. Gastroenterology 2018, 154, 515–517.e3. [Google Scholar] [CrossRef] [PubMed]
- Faillaci, F.; Marzi, L.; Critelli, R.; Milosa, F.; Schepis, F.; Turola, E.; Andreani, S.; Vandelli, G.; Bernabucci, V.; Lei, B.; et al. Liver Angiopoietin-2 is a key predictor of de novo or recurrent hepatocellular cancer after HCV direct-acting antivirals. Hepatology 2018, 68, 1010–1024. [Google Scholar] [CrossRef]
- Villani, R.; Facciorusso, A.; Bellanti, F.; Tamborra, R.; Piscazzi, A.; Landriscina, M.; Vendemiale, G.; Serviddio, G. DAAs Rapidly Reduce Inflammation but Increase Serum VEGF Level: A Rationale for Tumor Risk during Anti-HCV Treatment. PLoS ONE 2016, 11, e0167934. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zhou, R.; He, B.; Wang, W.; Zhang, B. Cyclophilin D: Guardian or Executioner for Tumor Cells? Front. Oncol. 2022, 12, 939588. [Google Scholar] [CrossRef]
- Machida, K.; Ohta, Y.; Osada, H. Suppression of apoptosis by cyclophilin D via stabilization of hexokinase II mitochondrial binding in cancer cells. J. Biol. Chem. 2006, 281, 14314–14320. [Google Scholar] [CrossRef]
- Nederlof, R.v.d.; Elshout, M.A.M.; Koeman, A.; Uthman, L.; Koning, I.; Eerbeek, O.; Weber, N.C.; Hollmann, M.W.; Zuurbier, C.J. Cyclophilin D ablation is associated with increased end-ischemic mitochondrial hexokinase activity. Sci. Rep. 2017, 7, 12749. [Google Scholar] [CrossRef]
- Klawitter, J.; Klawitter, J.; Pennington, A.T.; Kirkpatrick, B.; Roda, G.; Kotecha, N.C.; Thurman, J.M.; Christians, U. Cyclophilin D knockout protects the mouse kidney against cyclosporin A-induced oxidative stress. Am. J. Physiol Renal. Physiol. 2019, 317, F683–F694. [Google Scholar] [CrossRef]
- Elrod, J.W.; Molkentin, J.D. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ. J. 2013, 77, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Schinzel, A.C.; Takeuchi, O.; Huang, Z.; Fisher, J.K.; Zhou, Z.; Rubens, J.; Hetz, C.; Danial, N.N.; Moskowitz, M.A.; Korsmeyer, S.J. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. USA 2005, 102, 12005–12010. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Zhang, L.; Liu, B.; Zhu, Z.; He, Y.; Chen, J.; Zhu, J.; Yu, H. CypD-mPTP axis regulates mitochondrial functions contributing to osteogenic dysfunction of MC3T3-E1 cells in inflammation. J. Physiol. Biochem. 2018, 74, 395–402. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stauffer, W.; Bobardt, M.; Ure, D.; Foster, R.; Gallay, P. The Cyclophilin Inhibitor Rencofilstat Decreases HCV-Induced Hepatocellular Carcinoma Independently of Its Antiviral Activity. Viruses 2023, 15, 2099. https://doi.org/10.3390/v15102099
Stauffer W, Bobardt M, Ure D, Foster R, Gallay P. The Cyclophilin Inhibitor Rencofilstat Decreases HCV-Induced Hepatocellular Carcinoma Independently of Its Antiviral Activity. Viruses. 2023; 15(10):2099. https://doi.org/10.3390/v15102099
Chicago/Turabian StyleStauffer, Winston, Michael Bobardt, Daren Ure, Robert Foster, and Philippe Gallay. 2023. "The Cyclophilin Inhibitor Rencofilstat Decreases HCV-Induced Hepatocellular Carcinoma Independently of Its Antiviral Activity" Viruses 15, no. 10: 2099. https://doi.org/10.3390/v15102099
APA StyleStauffer, W., Bobardt, M., Ure, D., Foster, R., & Gallay, P. (2023). The Cyclophilin Inhibitor Rencofilstat Decreases HCV-Induced Hepatocellular Carcinoma Independently of Its Antiviral Activity. Viruses, 15(10), 2099. https://doi.org/10.3390/v15102099






