HIV Drug Resistance Patterns and Characteristics Associated with Clinically Significant Drug Resistance among Children with Virologic Failure on Antiretroviral Treatment in Kenya: Findings from the Opt4Kids Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Procedures
2.2. Study Setting
2.3. Study Population
2.4. Data Collection and Management
2.5. Primary Analytic Outcome
2.6. Exposures and Covariates
2.7. Statistical Analysis
3. Results
3.1. Drug Resistance among Children on ART with Virologic Failure
3.2. Characteristics Associated with Major Drug Resistance
3.3. Clinical Management and Outcomes of Children with DRT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penazzato, M.; Irvine, C.; Vicari, M.; Essajee, S.M.; Sharma, A.; Puthanakit, T.; Abrams, E.J.; Doherty, M. A Global Research Agenda for Pediatric HIV. J. Acquir. Immune Defic. Syndr. 2018, 78 (Suppl. S1), S10–S15. [Google Scholar] [CrossRef]
- Abrams, E.J.; Strasser, S. 90-90-90—Charting a steady course to end the paediatric HIV epidemic. J. Int. AIDS Soc. 2015, 18, 20296. [Google Scholar] [CrossRef]
- Boerma, R.S.; Boender, T.S.; Bussink, A.P.; Calis, J.C.J.; Bertagnolio, S.; de Wit, T.F.R.; van Hensbroek, M.B.; Sigaloff, K.C. Suboptimal Viral Suppression Rates Among HIV-Infected Children in Low- and Middle-Income Countries: A Meta-analysis. Clin. Infect. Dis. 2016, 63, 1645–1654. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Early Warning Indicators of HIV Drug Resistance; World Health Organization: Geneva, Switzerland, 2016.
- World Health Organization (WHO). Global Action Plan on HIV Drug Resistance 2017–2021; WHO: Geneva, Switzerland, 2021.
- World Health Organization. HIV Drug Resistance Report 2021; WHO: Geneva, Switzerland, 2021.
- Boerma, R.S.; Sigaloff, K.C.; Akanmu, A.S.; Inzaule, S.; Boele van Hensbroek, M.; Rinke de Wit, T.F.; Calis, J.C. Alarming increase in pretreatment HIV drug resistance in children living in sub-Saharan Africa: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2017, 72, 365–371. [Google Scholar] [CrossRef]
- Kityo, C.; Boerma, R.S.; Sigaloff, K.C.E.; Kaudha, E.; Calis, J.C.J.; Musiime, V.; Balinda, S.; Nakanjako, R.; Boender, T.S.; Mugyenyi, P.N.; et al. Pretreatment HIV drug resistance results in virological failure and accumulation of additional resistance mutations in Ugandan children. J. Antimicrob. Chemother. 2017, 72, 2587–2595. [Google Scholar] [CrossRef]
- Wamalwa, D.C.; Lehman, D.A.; Benki-Nugent, S.; Gasper, M.A.; Gichohi, R.; Maleche-Obimbo, E.; Farquhar, C.; John-Stewart, G.C.; Overbaugh, J. Long-term virologic response and genotypic resistance mutations in HIV-1 infected Kenyan children on combination antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2013, 62, 267–274. [Google Scholar] [CrossRef]
- Hackett, S.; Teasdale, C.A.; Pals, S.; Muttiti, A.; Mogashoa, M.; Chang, J.; Zeh, C.; Ramos, A.; Rivadeneira, E.D.; DeVos, J.; et al. Drug Resistance Mutations Among South African Children Living With HIV on WHO-recommended ART Regimens. Clin. Infect. Dis. 2021, 73, e2217–e2225. [Google Scholar] [CrossRef]
- Crowell, C.S.; Maiga, A.I.; Sylla, M.; Taiwo, B.; Kone, N.; Oron, A.P.; Murphy, R.L.; Marcelin, A.G.; Traore, B.; Fofana, D.B.; et al. High Rates of Baseline Drug Resistance and Virologic Failure Among ART Naive HIV-Infected Children in Mali. Pediatr. Infect. Dis. J. 2017, 36, e258–e263. [Google Scholar] [CrossRef]
- Soeria-Atmadja, S.; Amuge, P.; Nanzigu, S.; Bbuye, D.; Rubin, J.; Eriksen, J.; Kekitiinwa, A.; Obua, C.; Gustafsson, L.L.; Navér, L. Pretreatment HIV drug resistance predicts accumulation of new mutations in ART-naïve Ugandan children. Acta Paediatr. 2020, 109, 2706–2716. [Google Scholar] [CrossRef]
- World Health Organization. Priorities for Antiretroviral Drug Optimization in Adults and Children: Report of a CADO, PADO and HIVResNet Joint Meeting. Available online: https://www.who.int/publications/i/item/9789240053038 (accessed on 20 June 2022).
- Turkova, A.; White, E.; Mujuru, H.A.; Kekitiinwa, A.R.; Kityo, C.M.; Violari, A.; Lugemwa, A.; Cressey, T.R.; Musoke, P.; Variava, E.; et al. Dolutegravir as First- or Second-Line Treatment for HIV-1 Infection in Children. N. Engl. J. Med. 2021, 385, 2531–2543. [Google Scholar] [CrossRef]
- Turkova, A.; Namusoke-Magongo, E. Debate: DTG Transition: A Switch to DTG Must be Accompanied by Change in NRTI Backbone in Children. In Proceedings of the International Workshop on HIV and Pediatrics, Montréal, QC, Canada, 27–28 July 2022. [Google Scholar]
- Joint United Nations Programme on HIV/AIDS. UNAIDS Kenya-HIV and AIDS Estimates 2022. Available online: https://www.unaids.org/en/regionscountries/countries/kenya (accessed on 20 June 2022).
- Kenya Ministry of Health. Report of the 2013 Cross-Sectional Survey of Acquired HIV Drug Resistance among Adults and Children on Antiretroviral Therapy at Sentinel Sites in Kenya; National AIDS and STI Control Program, Kenya Ministry of Health: Nairobi, Kenya, 2016.
- Patel, R.C. Impact of point-of-care HIV viral load and targeted drug resistance mutation testing on viral suppression among Kenyan children on antiretroviral therapy: Results from an open-label, randomized controlled trial (Opt4Kids). Lancet Child Adolesc. Health 2022, 6, 681–691. [Google Scholar] [CrossRef]
- Patel, R.C.; Oyaro, P.; Odeny, B.; Mukui, I.; Thomas, K.K.; Sharma, M.; Wagude, J.; Kinywa, E.; Oluoch, F.; Odhiambo, F.; et al. Optimizing viral load suppression in Kenyan children on antiretroviral therapy (Opt4Kids). Contemp. Clin. Trials Commun. 2020, 20, 100673. [Google Scholar] [CrossRef]
- Bwana, P.; Ageng’o, J.; Mwau, M. Performance and usability of Cepheid GeneXpert HIV-1 qualitative and quantitative assay in Kenya. PLoS ONE 2019, 14, e0213865. [Google Scholar] [CrossRef]
- World Health Organization. List of WHO-Designated Laboratories for HIV Drug Resistance Surveillance, November 2022; WHO: Geneva, Switzerland, 2022.
- National Public Health Laboratory. National HIV Research Lab 2022. Available online: https://nphl.go.ke/national-hiv-reference-laboratory-nhrl (accessed on 8 August 2023).
- Stanford University. HIV Drug Resistance Database 2021. Available online: https://hivdb.stanford.edu (accessed on 20 June 2022).
- Kenya Ministry of Health. New Guidance on ART Transition for Children and Adolescents Less than 15 Years of Age Living with HIV (CALHIV) in Kenya; National AIDS & STI Control Program Kenya Ministry of Health: Nairobi, Kenya, 2018.
- Kenya Ministry of Health. Use of Dolutegravir Based Regimen in Adolescent Girls and Women Living with HIV Nairobi, Kenya; National AIDS & STI Control Program Kenya Ministry of Health: Nairobi, Kenya, 2019.
- Patel, R.C.; Oyaro, P.; Thomas, K.K.; Wagude, J.; Mukui, I.; Brown, E.; Hassan, S.A.; Kinywa, E.; Oluoch, F.; Odhiambo, F.; et al. Point-of-care HIV viral load and targeted drug resistance mutation testing versus standard care for Kenyan children on antiretroviral therapy (Opt4Kids): An open-label, randomised controlled trial. Lancet Child. Adolesc. Health 2022, 6, 681–691. [Google Scholar] [CrossRef]
- Muri, L.; Gamell, A.; Ntamatungiro, A.J.; Glass, T.R.; Luwanda, L.B.; Battegay, M.; Furrer, H.; Hatz, C.; Tanner, M.; Felger, I.; et al. Development of HIV drug resistance and therapeutic failure in children and adolescents in rural Tanzania: An emerging public health concern. AIDS 2017, 31, 61–70. [Google Scholar] [CrossRef]
- Nyandiko, W.; Holland, S.; Vreeman, R.; DeLong, A.K.; Manne, A.; Novitsky, V.; Sang, F.; Ashimosi, C.; Ngeresa, A.; Chory, A.; et al. HIV-1 Treatment Failure, Drug Resistance, and Clinical Outcomes in Perinatally Infected Children and Adolescents Failing First-Line Antiretroviral Therapy in Western Kenya. J. Acquir. Immune Defic. Syndr. 2022, 89, 231–239. [Google Scholar] [CrossRef]
- Boender, T.S.; Kityo, C.M.; Boerma, R.S.; Hamers, R.L.; Ondoa, P.; Wellington, M.; Siwale, M.; Nankya, I.; Kaudha, E.; Akanmu, A.S.; et al. Accumulation of HIV-1 drug resistance after continued virological failure on first-line ART in adults and children in sub-Saharan Africa. J. Antimicrob. Chemother. 2016, 71, 2918–2927. [Google Scholar] [CrossRef]
- Koay, W.L.A.; Kose-Otieno, J.; Rakhmanina, N. HIV Drug Resistance in Children and Adolescents: Always a Challenge? Curr. Epidemiol. Rep. 2021, 8, 97–107. [Google Scholar] [CrossRef]
- Cahn, P.; Pozniak, A.L.; Mingrone, H.; Shuldyakov, A.; Brites, C.; Andrade-Villanueva, J.F.; Richmond, G.; Buendia, C.B.; Fourie, J.; Ramgopal, M.; et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: Week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013, 382, 700–708. [Google Scholar] [CrossRef]
- Aboud, M.; Kaplan, R.; Lombaard, J.; Zhang, F.; Hidalgo, J.A.; Mamedova, E.; Losso, M.H.; Chetchotisakd, P.; Brites, C.; Sievers, J.; et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): An open-label, non-inferiority, phase 3b trial. Lancet Infect. Dis. 2019, 19, 253–264. [Google Scholar] [CrossRef]
- Steegen, K.; Chandiwana, N.; Sokhela, S.; Venter, W.D.F.; Hans, L. Impact of rilpivirine cross-resistance on long-acting cabotegravir-rilpivirine in low and middle-income countries. AIDS 2023, 37, 1009–1011. [Google Scholar] [CrossRef]
- Cervo, A.; Russo, A.; Di Carlo, D.; De Vito, A.; Fabeni, L.; D’Anna, S.; Duca, L.; Colpani, A.; Fois, M.; Zauli, B.; et al. Long-acting combination of cabotegravir plus rilpivirine: A picture of potential eligible and ineligible HIV-positive individuals from the Italian ARCA cohort. J. Glob. Antimicrob. Resist. 2023, 34, 141–144. [Google Scholar] [CrossRef]

| Total n = 704 | Children without Any Drug Resistance Testing n = 598 | Children with DRT | ||
|---|---|---|---|---|
| Children with Any Drug Resistance n = 106 | Children with Clinically Significant Drug Resistance Only n = 93 | |||
| Characteristics | N (%) 1 | N (%) | N (%) | N (%) |
| Age, median (IQR) | 9 (7, 12) | 9 (7, 12) | 9 (4, 12) | 9 (4, 12) |
| Age category (years) | ||||
| 1–4 | 55 (7.8) | 34 (5.7) | 21 (19.8) | 17 (18.3) |
| 5–10 | 325 (46.2) | 284 (47.5) | 41 (38.7) | 36 (38.7) |
| 11–14 | 324 (46.0) | 280 (46.8) | 44 (41.5) | 40 (43.0) |
| Sex | ||||
| Male | 360 (51.1) | 311 (52.0) | 49 (46.2) | 42 (45.2) |
| Female | 344 (48.9) | 287 (48.0) | 57 (53.8) | 51 (54.8) |
| Time on ART (years) median (IQR) | 5 (3, 8) | 6 (3, 8) | 4 (1, 8) | 4 (1, 8) |
| Time on ART categorical | ||||
| <2 years | 110 (15.6) | 76 (12.7) | 34 (32.1) | 29 (31.2) |
| 2–5 years | 186 (26.4) | 162 (27.1) | 24 (22.6) | 18 (19.4) |
| >5 years | 400 (56.8) | 354 (59.2) | 46 (43.4) | 44 (47.3) |
| Missing | 8 (1.1) | 6 (1.0) | 2 (1.9.0) | 2 (2.2) |
| ART regimen at enrollment | ||||
| NNRTI-based | 382 (54.3) | 336 (56.2) | 46 (43.4) | 38 (40.9) |
| PI-based | 294 (41.8) | 238 (39.8) | 56 (52.8) | 51 (54.8) |
| Integrase-based | 27 (3.8) | 23 (3.8) | 4 (3.8) | 4 (4.3) |
| Other | 1 (0.1) | 1 (0.2) | 0 | 0 |
| NRTI ART regimen at enrollment | ||||
| ABC+3TC | 498 (70.7) | 422 (70.6) | 76 (71.7) | 64 (68.8) |
| AZT+3TC | 113 (16.1) | 93 (15.6) | 20 (18.9) | 19 (20.4) |
| TDF+3TC | 93 (13.2) | 83 (13.8) | 10 (9.4) | 10 (10.8) |
| ART regimen at 1st DRT | ||||
| NNRTI-based | N/A | N/A | 46 (43.4) | 38 (40.9) |
| PI-based | 56 (52.8) | 51 (54.8) | ||
| INSTI-based | 4 (3.8) | 4 (4.3) | ||
| WHO stage | ||||
| 1 or 2 | 487 (69.2) | 410 (68.6) | 77 (72.6) | 67 (72.0) |
| 3 or 4 | 152 (21.6) | 134 (22.4) | 18 (17.0) | 17 (18.3) |
| Missing | 65 (9.2) | 54 (9.0) | 11 (10.4) | 9 (9.7) |
| History of virologic failure within 2 years prior to study | ||||
| Yes | 144 (20.5) | 87 (14.5) | 57 (53.8) | 55 (59.1) |
| No | 504 (71.6) | 470 (78.6) | 34 (32.0) | 27 (29.0) |
| Missing | 56 (8.0) | 41 (6.9) | 15 (14.2) | 11 (11.8) |
| Primary Caregiver | ||||
| Mother | 482 (68.4) | 409 (68.4) | 73 (68.9) | 63 (67.7) |
| Father | 59 (8.4) | 53 (8.9) | 6 (5.7) | 5 (5.4) |
| Other biological | 124 (17.6) | 107 (17.9) | 17 (16.0) | 17 (18.3) |
| Other non-biological | 39 (5.5) | 29 (4.8) | 10 (9.4) | 8 (8.6) |
| Primary caregiver HIV status | ||||
| Positive | 568 (80.7) | 485 (81.1 | 83 (78.3) | 74 (79.8) |
| Negative | 135 (19.2) | 112 (18.7) | 23 (21.7) | 19 (20.2) |
| Unknown | 1 (0.1) | 1 (0.2) | 0 | 0 |
| Household commodities ownership | ||||
| Electricity | 403 (57.2) | 346 (57.9) | 57 (53.8) | 50 (53.8) |
| Radio | 554 (78.7) | 472 (78.9) | 82 (77.4) | 73 (78.5) |
| Television | 375 (53.3) | 318 (53.2) | 57 (53.8) | 52 (56.0) |
| Phone | 679 (96.4) | 577 (96.4) | 101 (95.3) | 89 (95.7) |
| More than one room | 580 (82.4) | 494 (82.6) | 86 (81.1) | 74 (79.6) |
| Clinic volume | ||||
| Heavy | 421 (59.8) | 362 (60.5) | 59 (55.7) | 55 (59.0) |
| Medium | 158 (22.4) | 130 (21.7) | 28 (26.4) | 24 (26.0) |
| Light | 125 (17.8) | 106 (17.7) | 19 (18.0) | 14 (15.0) |
| Clinic location | ||||
| Urban | 421 (59.8) | 362 (60.5) | 59 (55.7) | 55 (59.1) |
| Seri-urban | 158 (22.4) | 130 (21.7) | 28 (26.4) | 24 (25.8) |
| Rural | 125 (17.8) | 106 (17.7) | 19 (18.0) | 14 (15.1) |
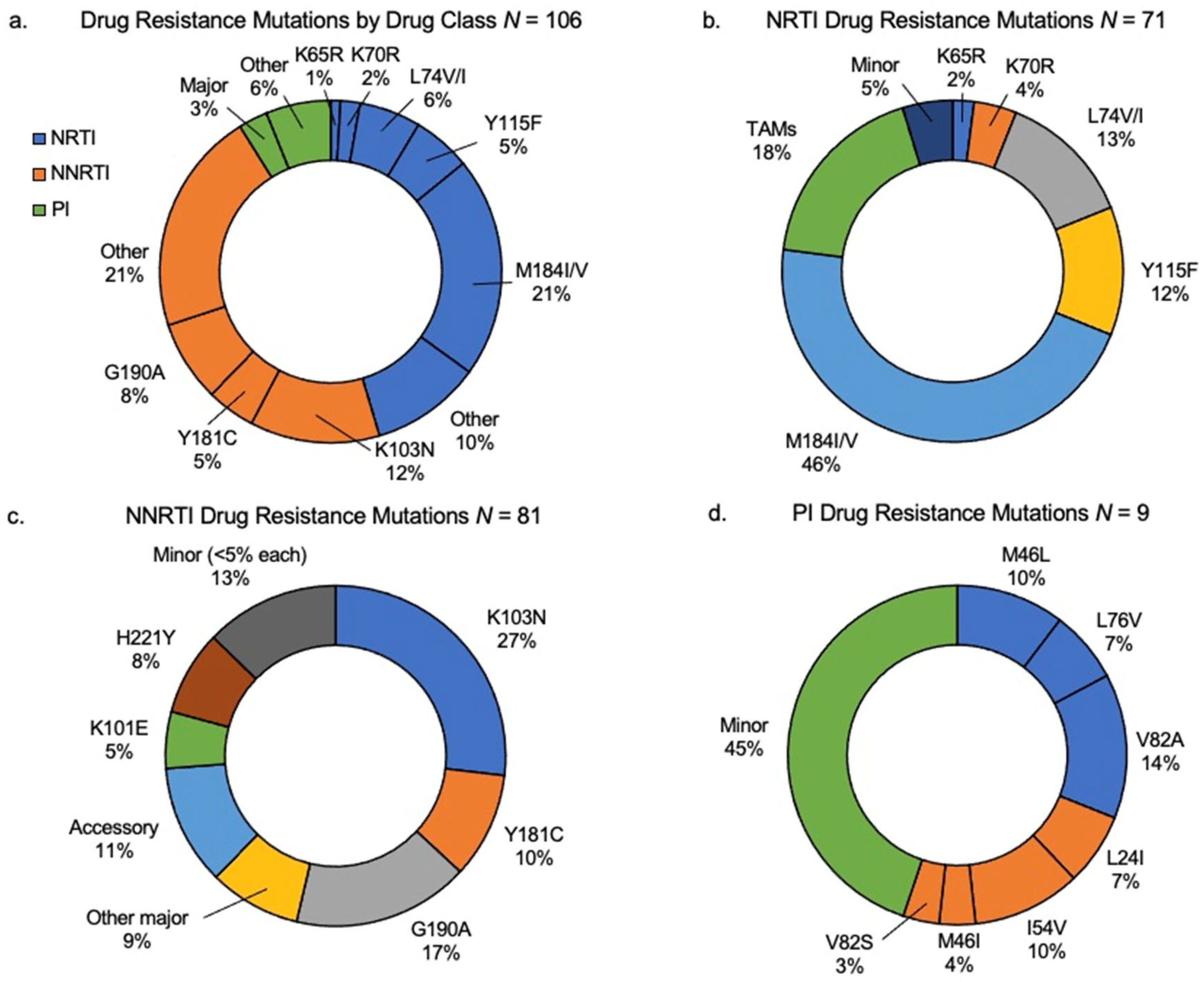
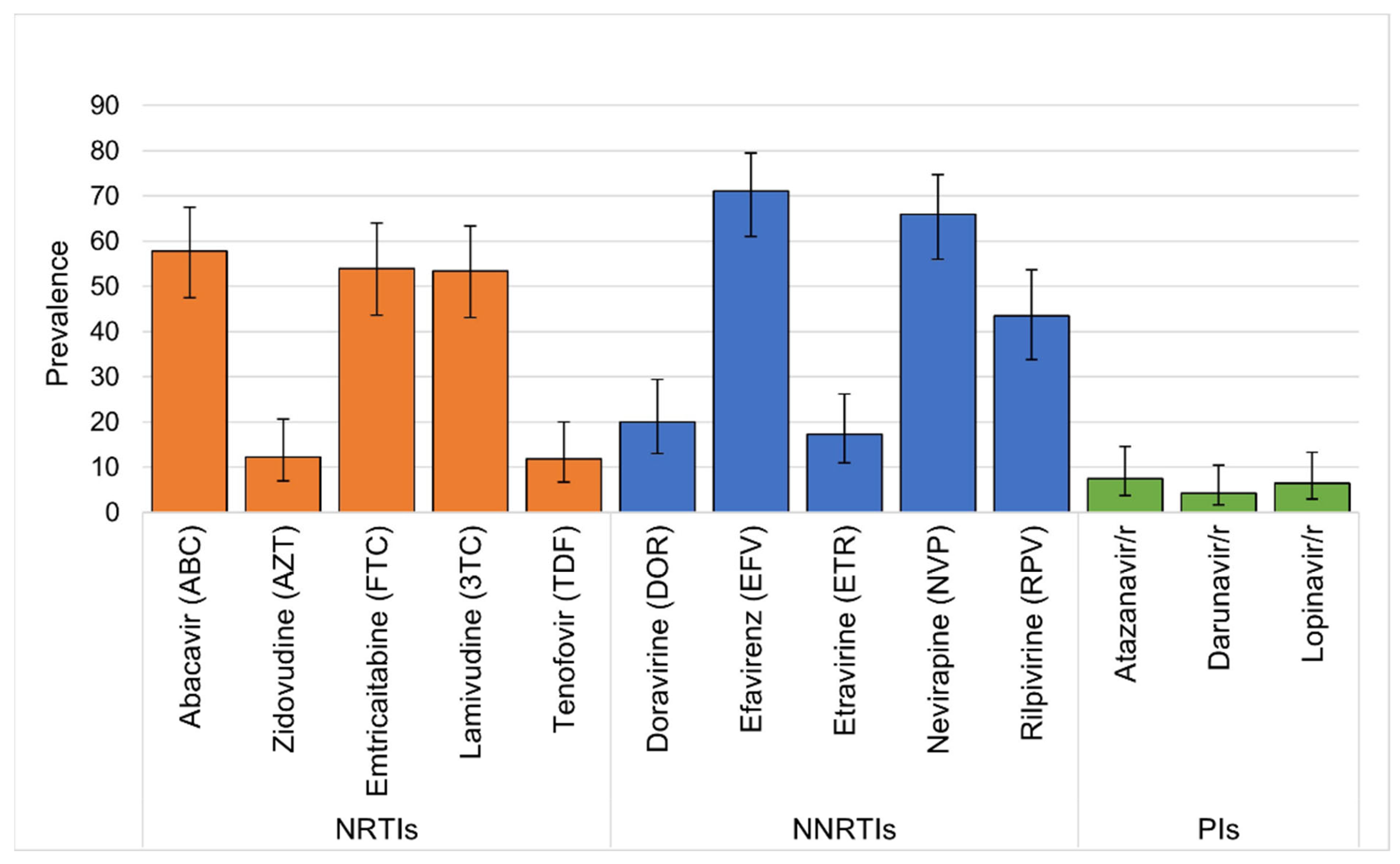
| Drug Class | Drug Resistance Mutation | CHIV with DRM n = 106 N | Prevalence (%) (95% Confidence Interval) |
|---|---|---|---|
| Nucleoside reverse transcriptase inhibitor (NRTIs) | |||
| K65R | 3 | 2.8 (0.01, 0.08) | |
| Y115F | 17 | 16.0 (0.10, 0.24) | |
| L74V/I | 18 | 17.0 (0.11, 0.25) | |
| M184V | 61 | 57.5 (0.48, 0.67) | |
| Total NRTI | 99 | 93.4 (0.87, 0.97) | |
| Non-nucleoside reverse transcriptase (NNRTIs) | |||
| Y181C | 14 | 13.2 (0.08, 0.21) | |
| G190A | 25 | 23.6 (0.17, 0.33) | |
| K103N | 38 | 35.8 (0.27, 0.45) | |
| Total NNRTI | 77 | 72.6 (0.63, 0.80) |
| Characteristics | Children with Major Drug Resistance (n = 93) | Children without Major Drug Resistance (n = 611) | Unadjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Age of child 1 | ||||||
| 1–4 | 17 (31.0) | 38 (69.0) | 3.18 (1.61, 6.10) | <0.001 | 2.01 (0.84, 4.76) | 0.113 |
| 5–10 | 36 (11.1) | 289 (89.0) | 0.88 (0.55, 1.43) | 0.615 | 0.84 (0.48, 1.46) | 0.534 |
| 11–14 (ref) | 40 (12.3) | 284 (87.7) | 1 | 1 | ||
| Gender | ||||||
| Male (ref) | 42 (11.7) | 318 (88.3) | 1 | |||
| Female | 51 (14.8) | 293 (85.2) | 1.32 (0.85, 2.05) | 0.217 | ||
| Time on ART (years) | ||||||
| <2 | 29 (26.4) | 81 (73.6) | 2.90 (1.70, 4.89) | <0.001 | 2.18 (1.08, 4.31) | 0.027 |
| 2–5 | 18 (9.8) | 168 (90.3) | 0.87 (0.48, 1.52) | 0.628 | 1.03 (0.54, 1.91) | 0.934 |
| >5 (ref) | 44 (11.0) | 356 (89.0) | 1 | 1 | ||
| ART regimen type at enrollment | ||||||
| NNRTI (ref) | 38 (10.0) | 344 (90.0) | 1 | |||
| PI | 51 (17.3) | 243 (82.7) | 1.90 (1.21, 3.00) | 0.005 | 1.07 (0.67, 1.87) | 0.809 2 |
| Integrase | 4 (15.0) | 23 (85.0) | 1.57 (0.44, 4.36) | 0.424 | 1.64 (0.41, 5.40) | 0.442 |
| Type NRTI ART at enrollment | ||||||
| ABC (ref) | 64 (13.0) | 434 (87.0) | 1 | |||
| AZT | 19 (17.0) | 94 (83.0) | 1.37 (0.77, 2.36) | 0.269 | ||
| TDF | 10 (11.0) | 83 (89.0) | 0.82 (0.38, 1.59) | 0.575 | ||
| ART regimen at 1st DRT | ||||||
| NNRTI-based (ref) | 38 (10.0) | 344 (90.0) | 1 | 1 | ||
| PI-based | 51 (17.3) | 244 (82.7) | 1.89 (1.21, 2.99) | 0.005 | 1.06 (0.61, 1.86) | 0.831 |
| Integrase-based | 4 (14.8) | 23 (85.2) | 1.57 (0.44, 4.36) | 0.424 | 1.65 (0.41, 5.42) | 0.437 |
| History of virologic failure | ||||||
| No (ref) | 27 (5.4) | 477 (94.6) | 1 | 1 | ||
| Yes | 55 (38.2) | 89 (61.8) | 10.92 (6.60, 18.47) | <0.001 | 9.57 (5.61, 16.70) | <0.001 |
| WHO stage | ||||||
| 1–2 | 67 (13.8) | 420 (86.2) | 1.27 (0.74, 2.30) | 0.413 | ||
| 3–4 (ref) | 17 (11.2) | 135 (88.8) | 1 | |||
| Clinic Volume | ||||||
| Heavy (ref) | 55 (13.0) | 366 (87.0) | 1 | |||
| Medium | 24 (15.0) | 134 (85.0) | 1.19 (0.70, 1.98) | 0.507 | ||
| Light | 14 (11.0) | 111 (89.0) | 0.84 (0.43, 1.53) | 0.582 | ||
| Clinic Location | ||||||
| Urban (ref) | 55 (13.0) | 366 (87.0) | 1 | |||
| Peri-urban | 24 (15.0) | 134 (85.0) | 1.19 (0.70, 1.98) | 0.507 | ||
| Rural | 14 (11.0) | 111 (89.0) | 0.84 (0.43, 1.53) | 0.582 |
| Total Children with DRT Results n = 77 (%) | Children Recommended to Change ART after DRT Review n = 36 (%) | Children Recommended Not to Change ART after DRT Review 1 n = 31 (%) | Children Changing ART Due to Transition to DTG n = 10 (%) | |
|---|---|---|---|---|
| 12-month viral load result | ||||
| Suppressed | 32 (42) | 21 (58) | 12 (39) | 9 (90) |
| Not suppressed | 21 (27) | 10 (28) | 10 (32) | 1 (10) |
| Lost to follow up | 9 (12) | 3 (8) | 6 (19) | 0 (0) |
| Died | 1 (1) | 0 (0) | 1 (3) | 0 (0) |
| Missing | 4 (5) | 2 (6) | 2 (6) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuogi, L.; Oyaro, P.; Wakjira, G.; Thomas, K.K.; Scallon, A.J.; Mukui, I.; Chohan, B.H.; Brown, E.; Karauki, E.; Yongo, N.; et al. HIV Drug Resistance Patterns and Characteristics Associated with Clinically Significant Drug Resistance among Children with Virologic Failure on Antiretroviral Treatment in Kenya: Findings from the Opt4Kids Randomized Controlled Trial. Viruses 2023, 15, 2083. https://doi.org/10.3390/v15102083
Abuogi L, Oyaro P, Wakjira G, Thomas KK, Scallon AJ, Mukui I, Chohan BH, Brown E, Karauki E, Yongo N, et al. HIV Drug Resistance Patterns and Characteristics Associated with Clinically Significant Drug Resistance among Children with Virologic Failure on Antiretroviral Treatment in Kenya: Findings from the Opt4Kids Randomized Controlled Trial. Viruses. 2023; 15(10):2083. https://doi.org/10.3390/v15102083
Chicago/Turabian StyleAbuogi, Lisa, Patrick Oyaro, Garoma Wakjira, Katherine K. Thomas, Andrea J. Scallon, Irene Mukui, Bhavna H. Chohan, Evelyn Brown, Enericah Karauki, Nashon Yongo, and et al. 2023. "HIV Drug Resistance Patterns and Characteristics Associated with Clinically Significant Drug Resistance among Children with Virologic Failure on Antiretroviral Treatment in Kenya: Findings from the Opt4Kids Randomized Controlled Trial" Viruses 15, no. 10: 2083. https://doi.org/10.3390/v15102083
APA StyleAbuogi, L., Oyaro, P., Wakjira, G., Thomas, K. K., Scallon, A. J., Mukui, I., Chohan, B. H., Brown, E., Karauki, E., Yongo, N., Ahmed, B., Hassan, S. A., Wagude, J., Kinywa, E., Otieno, L., Kingwara, L., Oyaro, B., Frenkel, L. M., John-Stewart, G., & Patel, R. C. (2023). HIV Drug Resistance Patterns and Characteristics Associated with Clinically Significant Drug Resistance among Children with Virologic Failure on Antiretroviral Treatment in Kenya: Findings from the Opt4Kids Randomized Controlled Trial. Viruses, 15(10), 2083. https://doi.org/10.3390/v15102083






