Novel Epitope Mapping of African Swine Fever Virus pI215L Protein Using Monoclonal Antibodies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Virus, Serum and Animals
2.2. I215L Recombinant Plasmid Construction
2.3. Expression and Purification of Recombinant pI215L (rpI215L) Protein
2.4. Anti-rpI215L mAbs Preparation
2.5. Indirect ELISA
2.6. SDS-PAGE and Western Blot Analysis
2.7. Indirect Immunofluorescence Assay (IFA)
2.8. Monoclonal Antibodies Secretion Stability and Isotype Determination
2.9. Epitope Mapping and Analysis
3. Results
3.1. Antigen Preparation
3.2. Generation of mAbs against ASFV rpI215L
3.3. Monoclonal Antibody Secretion Stability Analysis
3.4. Monoclonal Antibody Isotype Determination
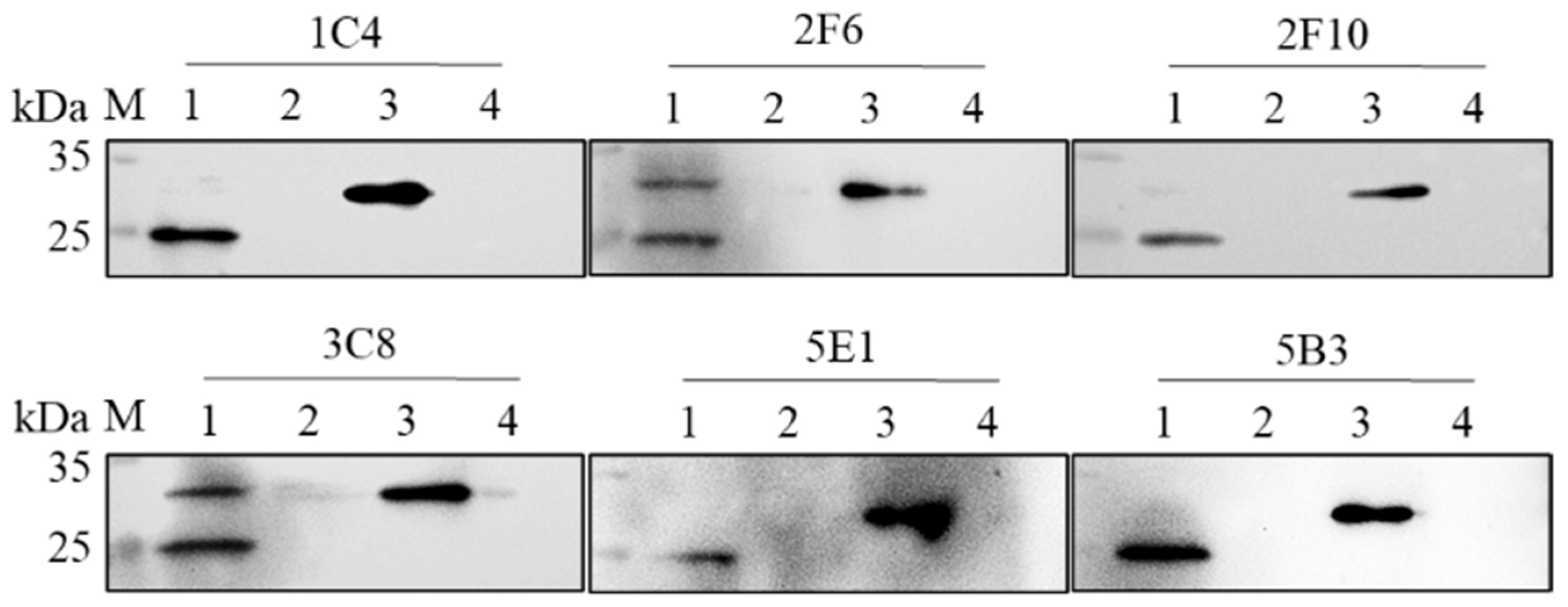
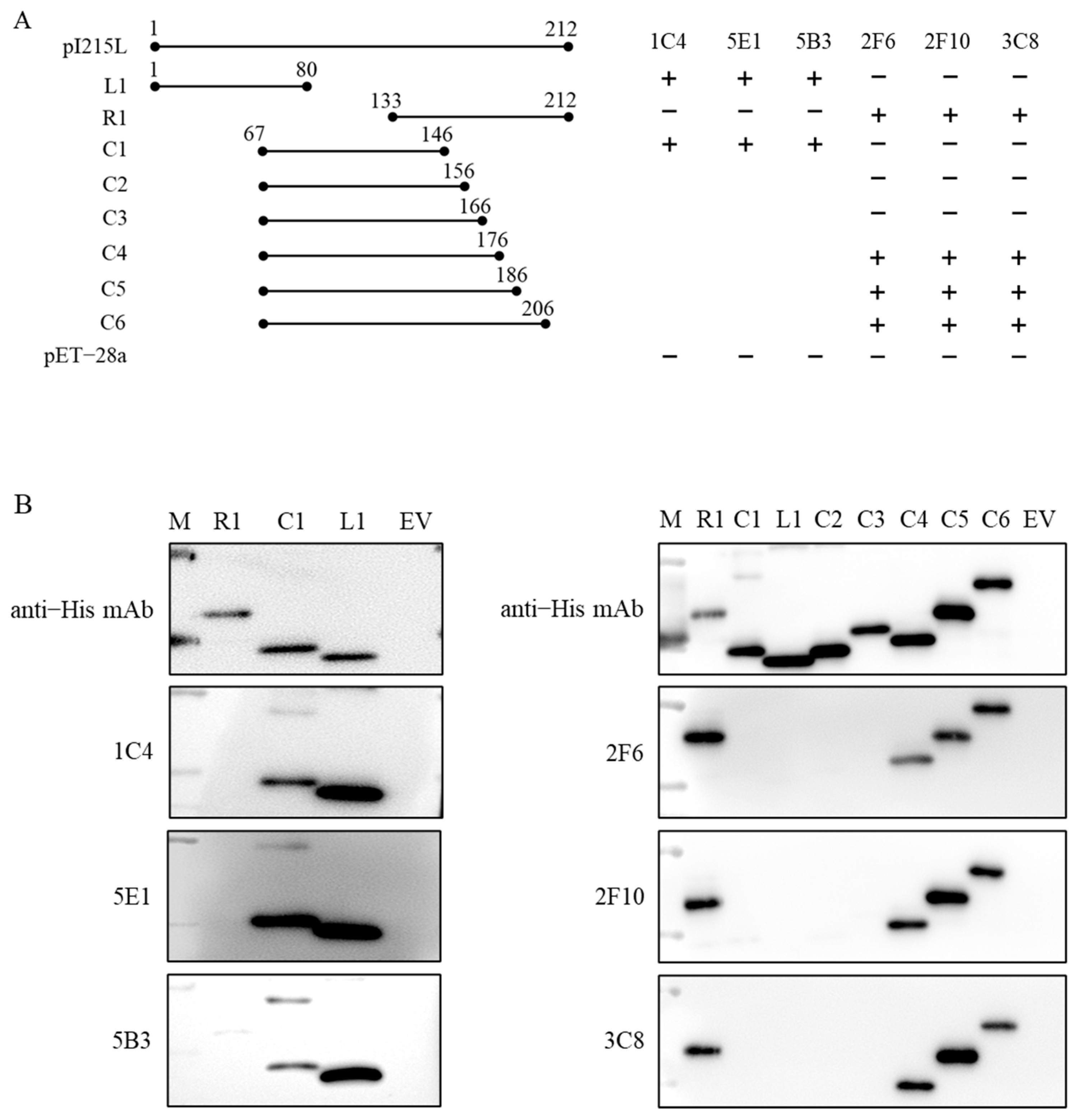
3.5. RpI215L Epitopes Identification
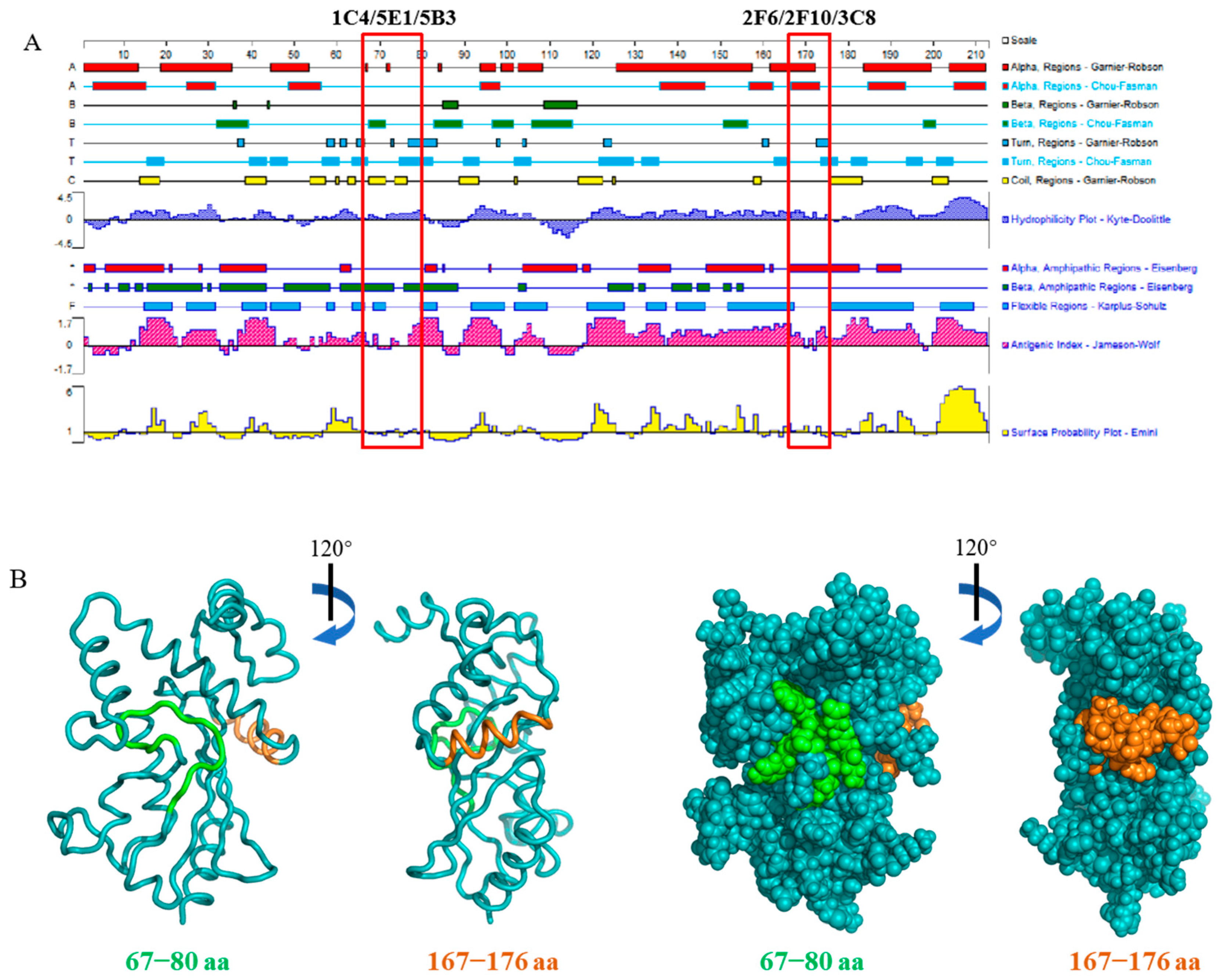
3.6. Epitope Characteristic Analysis
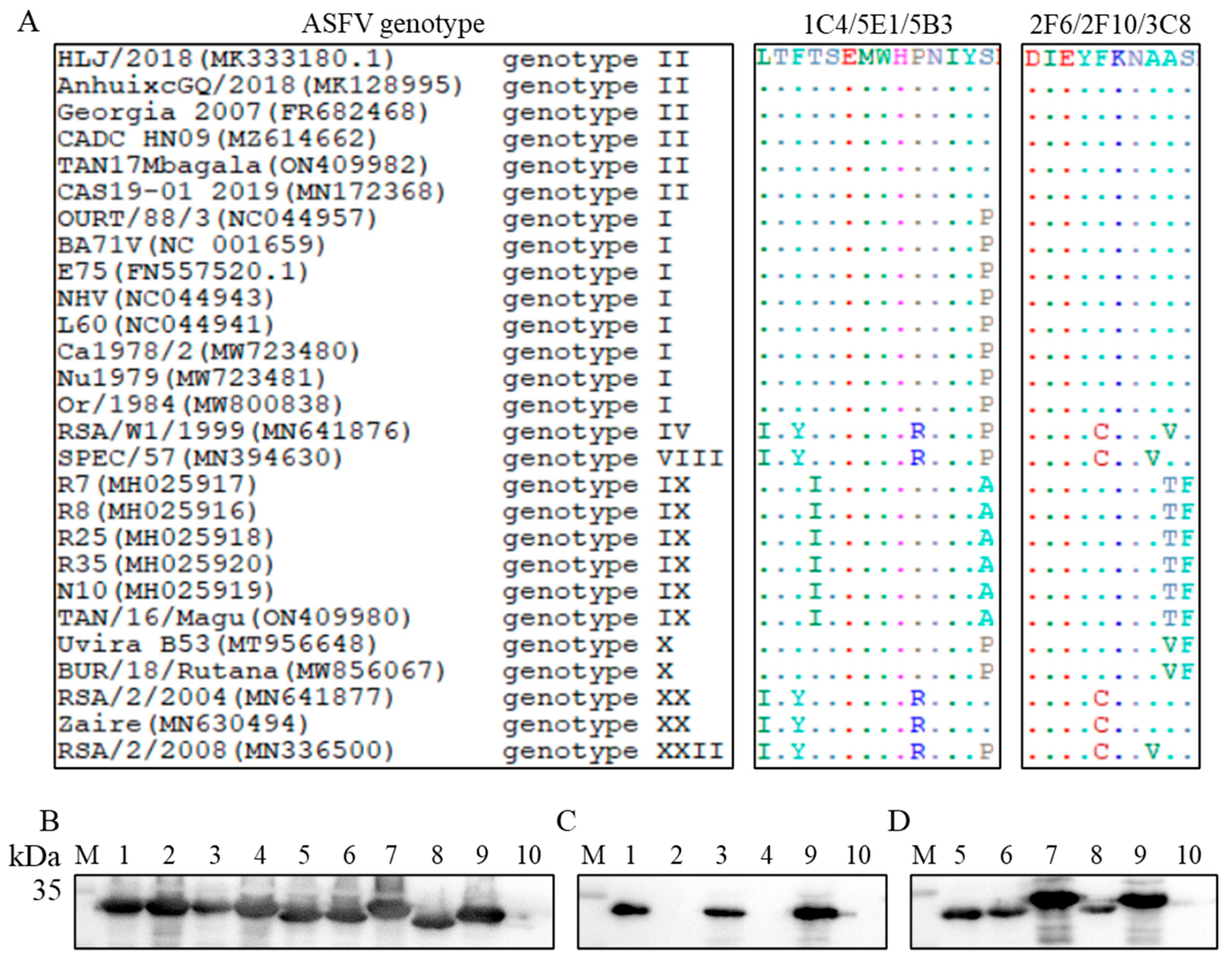
3.7. Conservative Analysis of the Epitopes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Parker, J.; Plowright, W.; Pierce, M.A. The epizootiology of African swine fever in Africa. Vet. Rec. 1969, 85, 668–674. [Google Scholar] [PubMed]
- Mulumba-Mfumu, L.K.; Saegerman, C.; Dixon, L.K.; Madimba, K.C.; Kazadi, E.; Mukalakata, N.T.; Oura, C.A.L.; Chenais, E.; Masembe, C.; Ståhl, K.; et al. African swine fever: Update on Eastern, Central and Southern Africa. Transbound. Emerg. Dis. 2019, 66, 1462–1480. [Google Scholar] [CrossRef] [PubMed]
- Karger, A.; Pérez-Núñez, D.; Urquiza, J.; Hinojar, P.; Alonso, C.; Freitas, F.B.; Revilla, Y.; Le Potier, M.-F.; Montoya, M. An Update on African Swine Fever Virology. Viruses 2019, 11, 864. [Google Scholar] [CrossRef]
- Njau, E.P.; Machuka, E.M.; Cleaveland, S.; Shirima, G.M.; Kusiluka, L.J.; Okoth, E.A.; Pelle, R. African Swine Fever Virus (ASFV): Biology, Genomics and Genotypes Circulating in Sub-Saharan Africa. Viruses 2021, 13, 2285. [Google Scholar] [CrossRef]
- Dixon, L.K.; Chapman, D.A.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Du, Y.; Li, R.; Sun, X.; Chen, Y.; Li, M.; Fan, L.; Liu, S.; Wang, S.; Ding, P.; et al. Development and characterization of monoclonal antibody against the critical loop structure of african swine fever virus P72 protein. Vet. Microbiol. 2023, 283, 109776. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhu, X.; Chen, X.; Li, D.; Xu, Q.; Yao, L.; Sun, Q.; Ghonaim, A.H.; Ku, X.; Fan, S.; et al. Establishment of a Blocking ELISA Detection Method for Against African Swine Fever Virus p30 Antibody. Front. Vet. Sci. 2021, 8, 781373. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiao, S.; Li, G.; Tong, W.; Dong, S.; Liu, J.; Guo, Z.; Zheng, H.; Zhao, R.; Tong, G.; et al. The Indirect ELISA and Monoclonal Antibody against African Swine Fever Virus p17 Revealed Efficient Detection and Application Prospects. Viruses 2022, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jiang, D.; Li, L.; Wang, J.; Wang, P.; Shi, X.; Zhao, Q.; Liu, B.; Ji, P.; Zhang, G. Identification of Two Novel Linear B Cell Epitopes on the CD2v Protein of African Swine Fever Virus Using Monoclonal Antibodies. Viruses 2022, 15, 131. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, T.; Bai, J.; Zhang, L.; Zheng, H.; Jiang, P. Preparation of Monoclonal Antibodies against the Viral p54 Protein and a Blocking ELISA for Detection of the Antibody against African Swine Fever Virus. Viruses 2022, 14, 2335. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiao, S.; Liu, J.; Zhou, Y.; Tong, W.; Dong, S.; Liu, C.; Jiang, Y.; Guo, Z.; Zheng, H.; et al. A highly efficient indirect ELISA aand monoclonal antibody established against African swine fever virus pK205R. Front. Immunol. 2023, 13, 1103166. [Google Scholar] [CrossRef]
- Hagoss, Y.T.; Shen, D.; Zhang, Z.; Li, F.; Bu, Z.; Zhao, D. Novel Epitopes Mapping of African Swine Fever Virus CP312R Protein Using Monoclonal Antibodies. Viruses 2023, 15, 557. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Zhang, L.; Xia, T.; Yang, X.; Zhang, K.; Gao, Y.; Jiang, P. A new B cell epitope of pC129R protein of African swine fever virus identified by monoclonal antibodies. Vet. Microbiol. 2023, 282, 109744. [Google Scholar] [CrossRef]
- Riera, E.; García-Belmonte, R.; Madrid, R.; Pérez-Núñez, D.; Revilla, Y. African swine fever virus ubiquitin-conjugating enzyme pI215L inhibits IFN-I signaling pathway through STAT2 degradation. Front. Microbiol. 2023, 13, 1081035. [Google Scholar] [CrossRef] [PubMed]
- Hingamp, P.M.; Leyland, M.L.; Webb, J.; Twigger, S.; Mayer, R.J.; Dixon, L.K. Characterization of a ubiquitinated protein which is externally located in African swine fever virions. J. Virol. 1995, 69, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.B.; Frouco, G.; Martins, C.; Ferreira, F. African swine fever virus encodes for an E2-ubiquitin conjugating enzyme that is mono- and di-ubiquitinated and required for viral replication cycle. Sci. Rep. 2018, 8, 3471. [Google Scholar] [CrossRef]
- Barrado-Gil, L.; Del Puerto, A.; Galindo, I.; Cuesta-Geijo, M.Á.; García-Dorival, I.; de Motes, C.M.; Alonso, C. African Swine Fever Virus Ubiquitin-Conjugating Enzyme Is an Immunomodulator Targeting NF-kappaB Activation. Viruses 2021, 13, 1160. [Google Scholar] [CrossRef]
- Li, L.; Fu, J.; Li, J.; Guo, S.; Chen, Q.; Zhang, Y.; Liu, Z.; Tan, C.; Chen, H.; Wang, X. African Swine Fever Virus pI215L Inhibits Type I Interferon Signaling by Targeting Interferon Regulatory Factor 9 for Autophagic Degradation. J. Virol. 2022, 96, e0094422. [Google Scholar] [CrossRef] [PubMed]
- Barrado-Gil, L.; Del Puerto, A.; Muñoz-Moreno, R.; Galindo, I.; Cuesta-Geijo, M.; Urquiza, J.; Nistal-Villán, E.; de Motes, C.M.; Alonso, C. African Swine Fever Virus Ubiquitin-Conjugating Enzyme Interacts with Host Translation Machinery to Regulate the Host Protein Synthesis. Front. Microbiol. 2020, 11, 622907. [Google Scholar] [CrossRef] [PubMed]
- De StGroth, S.F.; Scheidegger, D. Production of monoclonal antibodies: Strategy and tactics. J. Immunol. Methods 1980, 35, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.G.; Pérez-Núñez, D.; Revilla, Y. Development of vaccines against African swine fever virus. Virus Res. 2019, 265, 150–155. [Google Scholar] [CrossRef]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Gomez-Villamandos, J.C.; Carrasco, L. An Update on the Epidemiology and Pathology of African Swine Fever. J. Comp. Pathol. 2015, 152, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Cubillos, C.; Gómez-Sebastian, S.; Moreno, N.; Nuñez, M.C.; Mulumba-Mfumu, L.K.; Quembo, C.J.; Heath, L.; Etter, E.M.; Jori, F.; Escribano, J.M.; et al. African swine fever virus serodiagnosis: A general review with a focus on the analyses of African serum samples. Virus Res. 2013, 173, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Petrovan, V.; Gimenez-Lirola, L.G.; Zimmerman, J.J.; Rowland, R.R.R.; Fang, Y. Development of a Blocking Enzyme-Linked Immunosorbent Assay for Detection of Antibodies against African Swine Fever Virus. Pathogens 2021, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Indrabalan, U.B.; Suresh, K.P.; Shivamallu, C.; Patil, S.S. An extensive evaluation of codon usage pattern and bias of structural proteins p30, p54 and, p72 of the African swine fever virus (ASFV). VirusDisease 2021, 32, 810–822. [Google Scholar] [CrossRef]

| Fragments | Primers | Sequences (5′-3′) | Position (Amino Acid) |
|---|---|---|---|
| pI215L | PF | CGCGGATCCATGGTTTCCAGGTTTTTAAT | 1–212 aa |
| PR | CCGCTCGAGCTCATCATCCTCCTCTTCTT | ||
| L1 | L1-PF | CGCGGATCCATGGTTTCCAGGTTTTTAAT | 1–80 aa |
| L1-PR | CCGCTCGAGAGAGTAAATATTAGGATGCC | ||
| R1 | R1-PF | CGCGGATCCAAAAGCTACCGTAAACTC | 133–212 aa |
| R1-PR | CCGCTCGAGCTCATCATCCTCCTCTTCTT | ||
| C1 | C1-PF | CGCGGATCCTTAACATTCACCTCTGAAAT | 67–146 aa |
| C1-PR | CCGCTCGAGTGATTCCAAATCCTCTTTAT | ||
| C2 | C2-PF | CGCGGATCCTTAACATTCACCTCTGAAAT | 67–156 aa |
| C2-PR | CCGCTCGAGTTTGACAGTCCTTTTAACT | ||
| C3 | C3-PF | CGCGGATCCTTAACATTCACCTCTGAAAT | 67–166 aa |
| C3-PR | CCGCTCGAGGTCTTCTGGTGAACACT | ||
| C4 | C4-PF | CGCGGATCCTTAACATTCACCTCTGAAAT | 67–176 aa |
| C4-PR | CCGCTCGAGATTGGATGCAGCATTTT | ||
| C5 | C5-PF | CGCGGATCCTTAACATTCACCTCTGAAAT | 67–186 aa |
| C5-PR | CCGCTCGAGTTCATAAGCATCACTGG | ||
| C6 | C6-PF | CGCGGATCCTTAACATTCACCTCTGAAAT | 67–206 aa |
| C6-PR | CCGCTCGAGTTCATCCTCATCATCAT |
| Fragments | Primers | Sequences (5′-3′) |
|---|---|---|
| S80P-I215L | PF | CTAATATTTACCCTGATGGAAAAC |
| PR | CATAGTTTTCCATCAGGGTAAATA | |
| L67I/F69Y/P76R/ S80P-I215L | PF | CCCAGAATAACATACACCTCTGAAATGTGGCATCGTAATATTTACCCTGATGGA |
| PR | CCATCAGGGTAAATATTACGATGCCACATTTCAGAGGTGTATGTTATTCTGGGTG | |
| T70I/S80A-I215L | PF | GATTAACATTCATCTCTGAAATGTGGCATCCTAATATTTACGCTGATGGAAA |
| PR | GTTTTCCATCAGCGTAAATATTAGGATGCCACATTTCAGAGATGAATGTTA | |
| L67I/F69Y/P76R-I215L | PF | CACCCAGAATAACATACACCTCTGAAATGTGGCATCGTAATATTTAC |
| PR | GAGTAAATATTACGATGCCACATTTCAGAGGTGTATGTTATTCTGG | |
| F170C/A174V-I215L | PF | CATAGAATATTGTAAAAATGCTGTATCCAATG |
| PR | GGTGGAACATTGGATACAGCATTTTTACAATATTC | |
| F170C/A173V-I215L | PF | CATAGAATATTGTAAAAATGTTGCATCCAA |
| PR | GAACATTGGATGCAACATTTTTACAATATTCT | |
| A174T/S175F-I215L | PF | GAATATTTTAAAAATGCTACATTCAATGTTCCAC |
| PR | GTATTGGTGGAACATTGAATGTAGCATTTTTAA | |
| F170C-I215L | PF | CATAGAATATTGTAAAAATG |
| PR | GCAGCATTTTTACAATATTC |
| Monoclonal Antibody | Hybridoma Cell Supernatant Antibody Titers | Ascites Titers | |||
|---|---|---|---|---|---|
| P5 | P10 | P15 | P20 | ||
| 1C4 | 1:12,800 | 1:12,800 | 1:12,800 | 1:12,800 | 1:12,800 |
| 2F6 | 1:12,800 | 1:12,800 | 1:25,600 | 1:25,600 | 1:25,600 |
| 2F10 | 1:12,800 | 1:12,800 | 1:12,800 | 1:12,800 | 1:25,600 |
| 3C8 | 1:12,800 | 1:12,800 | 1:12,800 | 1:12,800 | 1:25,600 |
| 5E1 | 1:12,800 | 1:12,800 | 1:12,800 | 1:12,800 | 1:25,600 |
| 5B3 | 1:12,800 | 1:12,800 | 1:12,800 | 1:12,800 | 1:25,600 |
| Monoclonal Antibody | Hybridoma Cell Supernatant Antibody Titers | |||||||
|---|---|---|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG2b | IgG2c | IgG3 | IgM | Igκ | Igλ | |
| 1C4 | 3.186 | 0.208 | 0.205 | 0.105 | 0.126 | 0.135 | 0.130 | 2.026 |
| 2F6 | 0.225 | 2.693 | 0.230 | 0.245 | 0.196 | 0.301 | 1.951 | 0.246 |
| 2F10 | 2.042 | 0.196 | 0.154 | 0.201 | 0.186 | 0.215 | 1.704 | 0.231 |
| 3C8 | 3.012 | 0.241 | 0.235 | 0.199 | 0.261 | 0.186 | 2.013 | 0.195 |
| 5E1 | 2.986 | 0.199 | 0.216 | 0.236 | 0.208 | 0.215 | 0.227 | 1.987 |
| 5B3 | 2.045 | 0.241 | 0.237 | 0.236 | 0.202 | 0.189 | 0.256 | 2.154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Jiang, X.; Yang, X.; Zhang, K.; Jiang, P.; Bai, J. Novel Epitope Mapping of African Swine Fever Virus pI215L Protein Using Monoclonal Antibodies. Viruses 2023, 15, 2081. https://doi.org/10.3390/v15102081
Gao Y, Jiang X, Yang X, Zhang K, Jiang P, Bai J. Novel Epitope Mapping of African Swine Fever Virus pI215L Protein Using Monoclonal Antibodies. Viruses. 2023; 15(10):2081. https://doi.org/10.3390/v15102081
Chicago/Turabian StyleGao, Yanni, Xiaolin Jiang, Xing Yang, Keshan Zhang, Ping Jiang, and Juan Bai. 2023. "Novel Epitope Mapping of African Swine Fever Virus pI215L Protein Using Monoclonal Antibodies" Viruses 15, no. 10: 2081. https://doi.org/10.3390/v15102081
APA StyleGao, Y., Jiang, X., Yang, X., Zhang, K., Jiang, P., & Bai, J. (2023). Novel Epitope Mapping of African Swine Fever Virus pI215L Protein Using Monoclonal Antibodies. Viruses, 15(10), 2081. https://doi.org/10.3390/v15102081





