RSL3 Inhibits Porcine Epidemic Diarrhea Virus Replication by Activating Ferroptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses and Reagents
2.2. Cell Viability Assay
2.3. Lipid Peroxidation Assay
2.4. Quantitative Real-Time PCR
2.5. Indirect Immunofluorescence
2.6. Detection of Reactive Oxygen Species
2.7. Western Blot
2.8. Statistical Analysis
3. Results
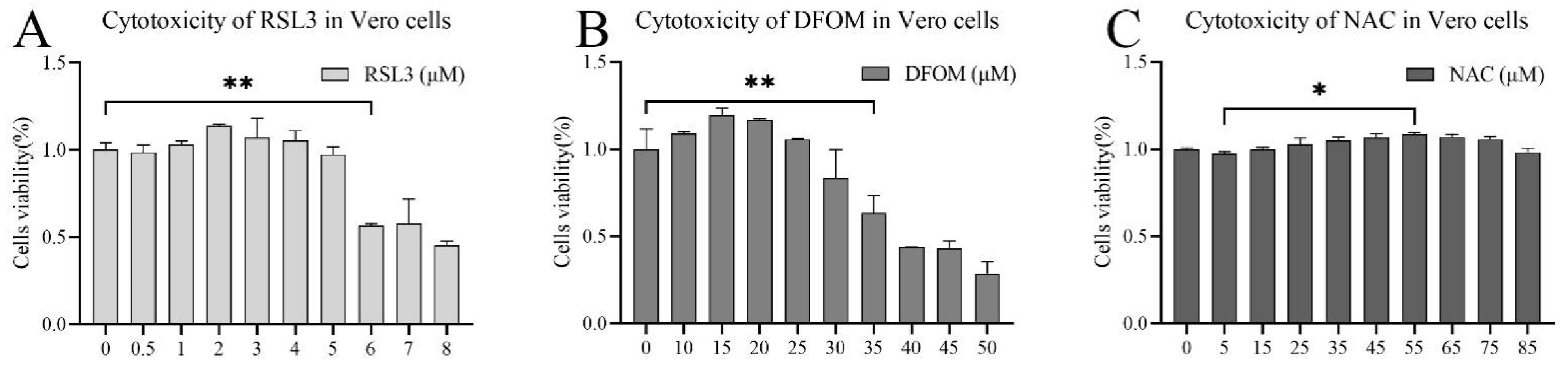
3.1. Evaluation of the Cytotoxicity of (1S,3R)-RSL3, Deferoxine Mestlate and NAC
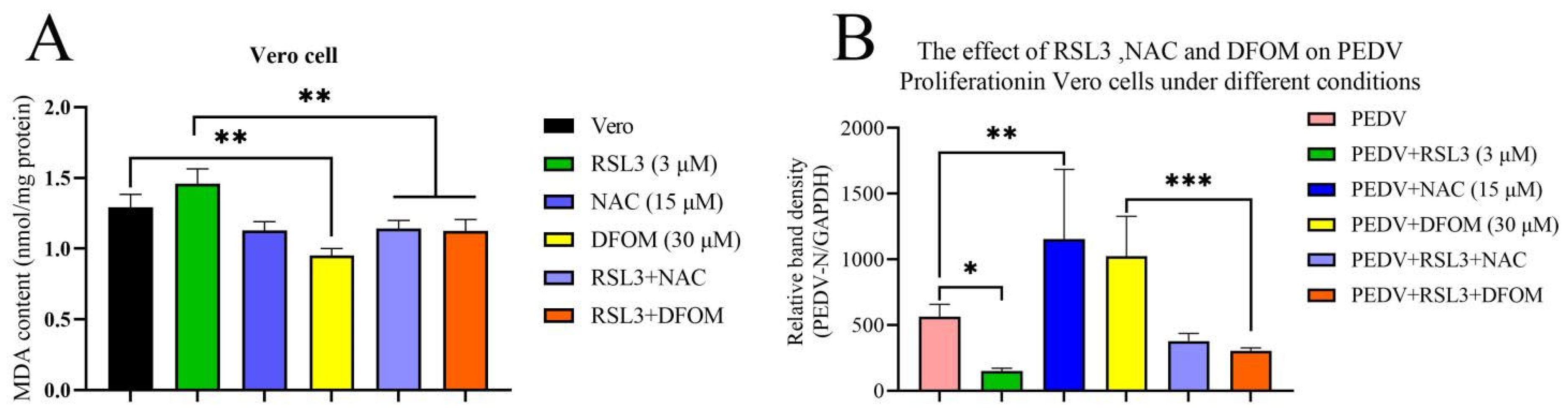
3.2. Validation of the Effects of RSL3, NAC and DFOM on Lipid Oxidation in Vero Cells
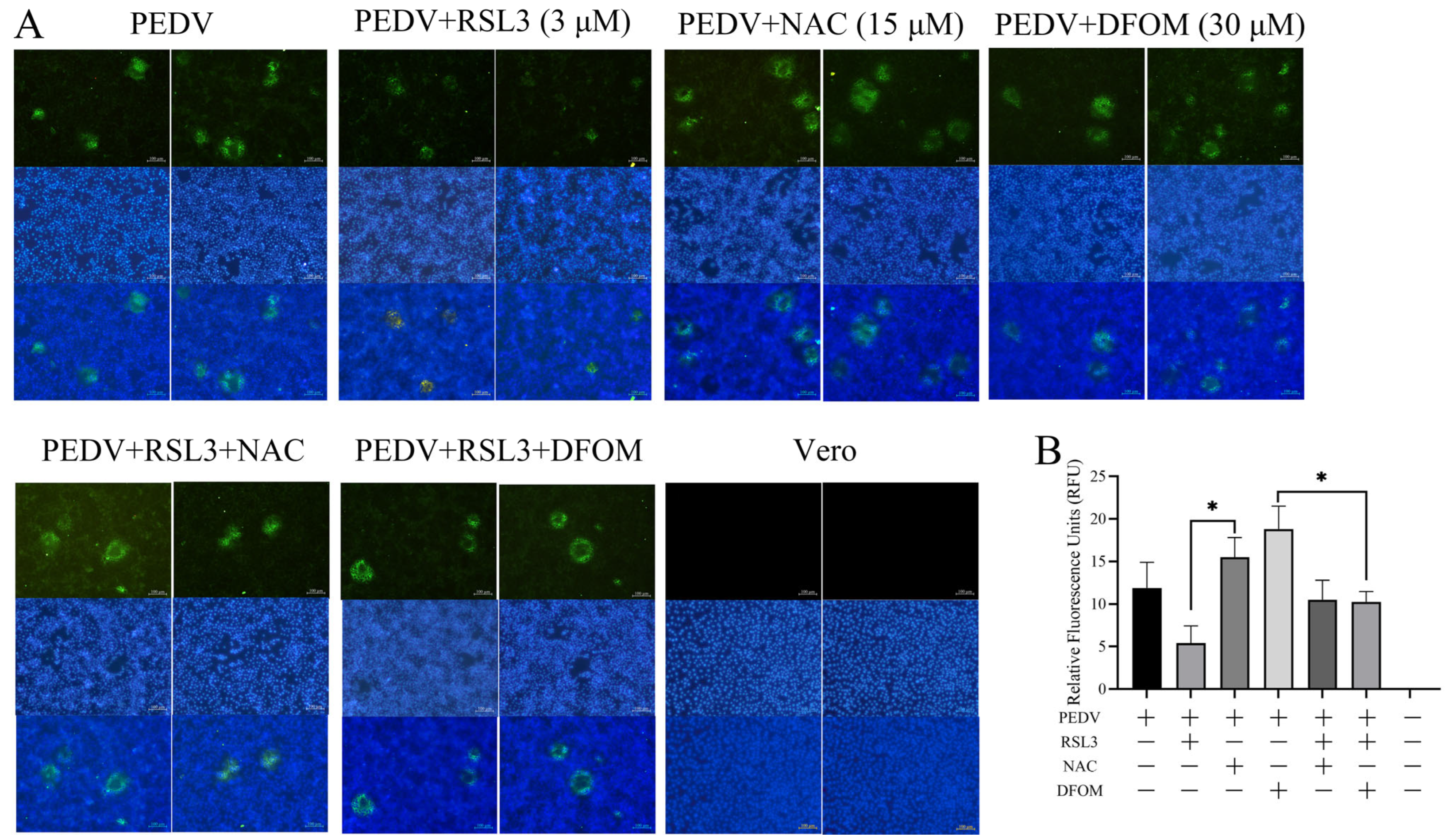
3.3. Effects of RSL3, NAC and DFOM on PEDV Proliferation in Vero Cells
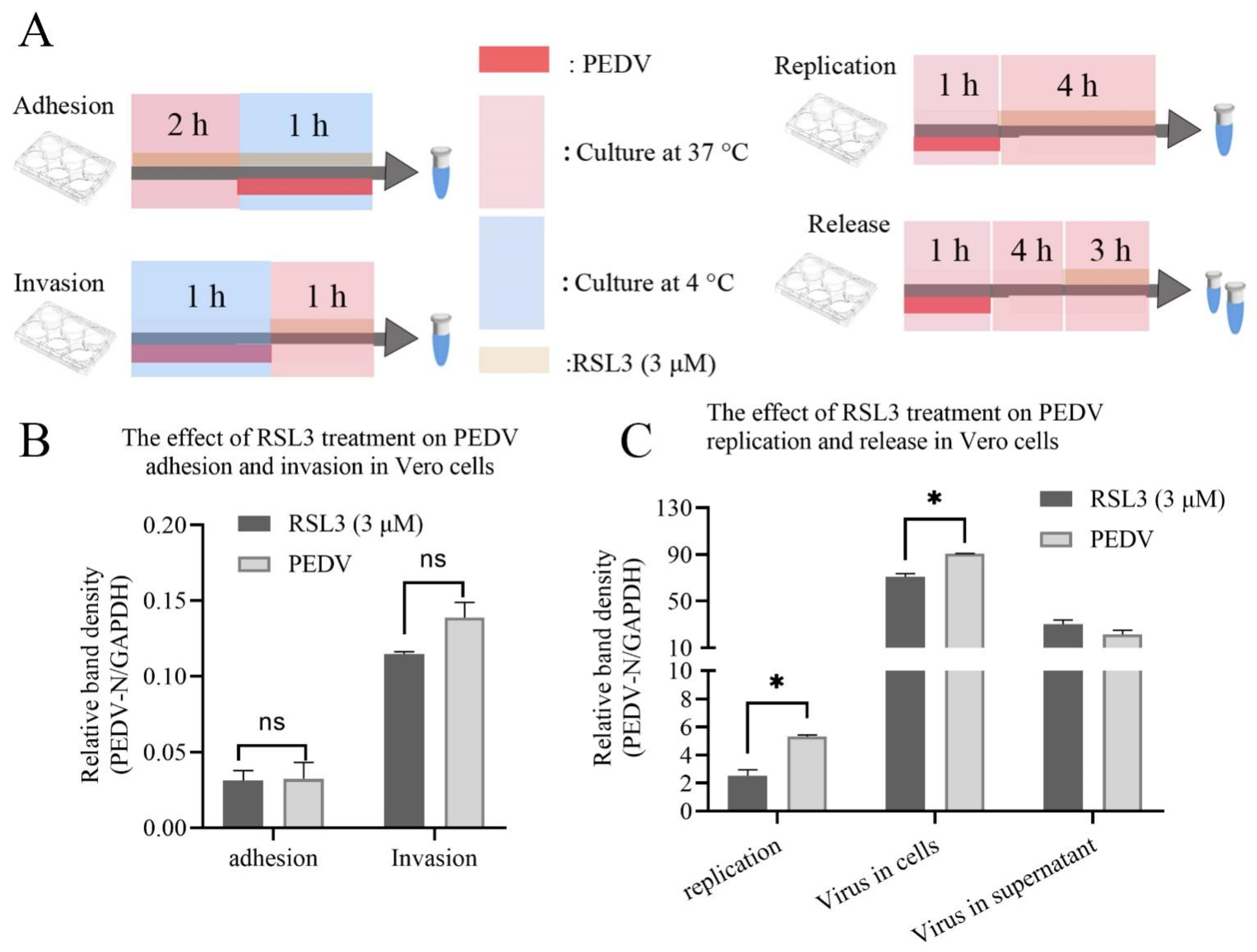
3.4. RSL3 Affects the Adhesion, Invasion, Replication and Release of PEDV on Vero Cells
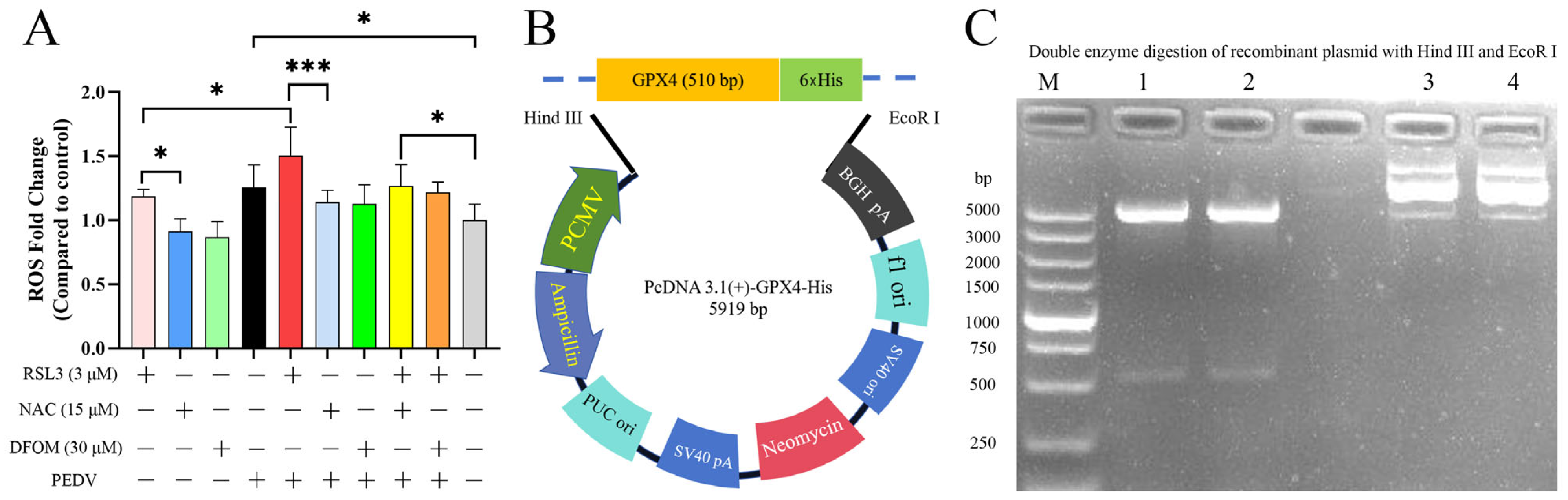
3.5. Effects of RSL3, NAC, DFOM and PEDV Interactions on ROS
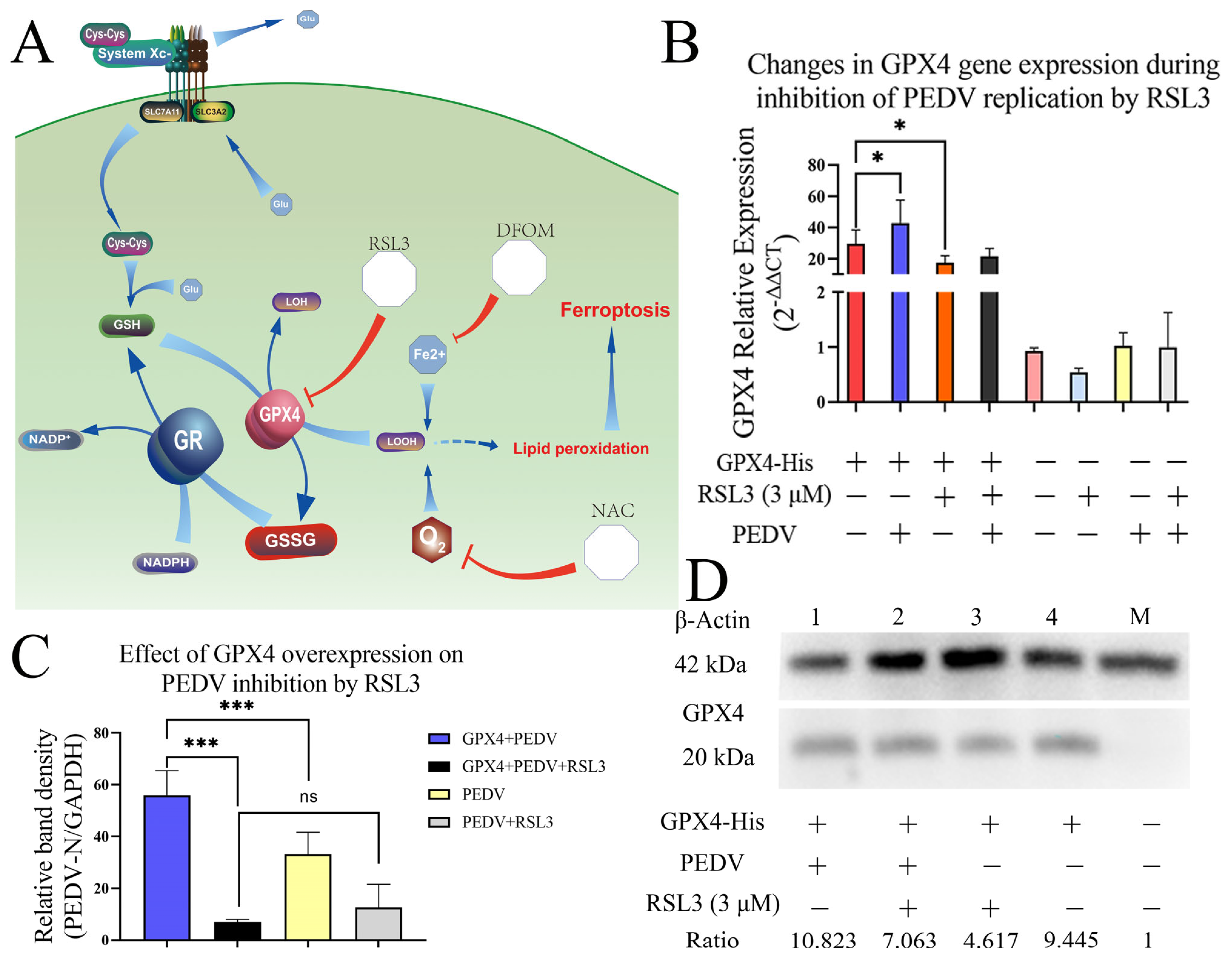
3.6. Overexpression of GPX4-His Affects the Interaction between PEDV and RSL3 during Replication
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, D.; Wang, X.; Wei, S.; Chen, J.; Feng, L. Epidemiology and vaccine of porcine epidemic diarrhea virus in China: A mini-review. J. Vet. Med. Sci. 2016, 78, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, G.W.; Hoang, H.; Schwartz, K.J.; Burrough, E.R.; Sun, D.; Madson, D.; Cooper, V.L.; Pillatzki, A.; Gauger, P.; Schmitt, B.J.; et al. Emergence of Porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Investig. 2013, 25, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Thakor, J.C.; Dinesh, M.; Manikandan, R.; Bindu, S.; Sahoo, M.; Sahoo, D.; Dhawan, M.; Pandey, M.K.; Tiwari, R.; Emran, T.B.; et al. Swine coronaviruses (SCoVs) and their emerging threats to swine population, inter-species transmission, exploring the susceptibility of pigs for SARS-CoV-2 and zoonotic concerns. Vet. Q. 2022, 42, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, C.; Peng, O.; Ashraf, U.; Xu, Q.; Gong, L.; Fan, B.; Zhang, Y.; Xu, Z.; Xue, C.; et al. Global Dynamics of Porcine Enteric Coronavirus PEDV Epidemiology, Evolution, and Transmission. Mol. Biol. Evol. 2023, 40, msad052. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pan, Y.; Xi, Y.; Wang, M.; Zeng, Q. Insights and progress on epidemic characteristics, genotyping, and preventive measures of PEDV in China: A review. Microb. Pathog. 2023, 181, 106185. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Nocera, F.P.; Longobardi, C.; Ciarcia, R.; Fioretti, A.; Damiano, S.; Iovane, G.; Pagnini, U.; Montagnaro, S. Retrospective Serosurvey of Three Porcine Coronaviruses among the Wild Boar (Sus scrofa) Population in the Campania Region of Italy. J. Wildl. Dis. 2022, 58, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Marfil, M.J.; La Sala, L.F.; Suarez, M.; Maidana, C.; Rodriguez, C.; Mesplet, M.; Abate, S.; Rosas, C.; Pena Martinez, J.; et al. Serological survey suggests circulation of coronavirus on wild Suina from Argentina, 2014–2017. Ecohealth 2022, 19, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; D’Anza, E.; Rossi, A.; Improda, E.; Iovane, V.; Pagnini, U.; Iovane, G.; Montagnaro, S. A Serological Investigation of Porcine Reproductive and Respiratory Syndrome and Three Coronaviruses in the Campania Region, Southern Italy. Viruses 2023, 15, 300. [Google Scholar] [CrossRef]
- Ming, X.; Chen, H.; Yang, Y.; Zhao, P.; Sun, L.; Zhang, C.; Shin, H.J.; Lee, J.S.; Jung, Y.S.; Qian, Y. Porcine Enteric Coronavirus PEDV Induces the ROS-ATM and Caspase7-CAD-gammaH2AX Signaling Pathways to Foster Its Replication. Viruses 2022, 14, 1782. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Dong, W.; Gan, S.; Du, J.; Zhou, X.; Fang, W.; Wang, X.; Song, H. Porcine epidemic diarrhea virus activates PERK-ROS axis to benefit its replication in Vero E6 cells. Vet. Res. 2023, 54, 9. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Zhang, Q.; Yang, F.; Yin, Z.; Wang, L.; Li, Q. Porcine epidemic diarrhea virus infections induce apoptosis in Vero cells via a reactive oxygen species (ROS)/p53, but not p38 MAPK and SAPK/JNK signalling pathways. Vet. Microbiol. 2019, 232, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. 2020, 30, R1292–R1297. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Matsuoka, M.; Kumagai, T.; Sakamoto, T.; Koumura, T. Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis. Curr. Top. Microbiol. Immunol. 2017, 403, 143–170. [Google Scholar] [CrossRef] [PubMed]
- Bellmann-Weiler, R.; Lanser, L.; Barket, R.; Rangger, L.; Schapfl, A.; Schaber, M.; Fritsche, G.; Woll, E.; Weiss, G. Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection. J. Clin. Med. 2020, 9, 2429. [Google Scholar] [CrossRef]
- Zhao, K.; Huang, J.; Dai, D.; Feng, Y.; Liu, L.; Nie, S. Serum Iron Level as a Potential Predictor of Coronavirus Disease 2019 Severity and Mortality: A Retrospective Study. Open Forum Infect. Dis. 2020, 7, ofaa250. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, Y.; Yang, Q. Transferrin receptor 1 levels at the cell surface influence the susceptibility of newborn piglets to PEDV infection. PLoS Pathog. 2020, 16, e1008682. [Google Scholar] [CrossRef]
- Yuan, C.; Huang, X.; Zhai, R.; Ma, Y.; Xu, A.; Zhang, P.; Yang, Q. In Vitro Antiviral Activities of Salinomycin on Porcine Epidemic Diarrhea Virus. Viruses 2021, 13, 580. [Google Scholar] [CrossRef]
- Zeng, W.; Ren, J.; Li, Z.; Jiang, C.; Sun, Q.; Li, C.; Li, W.; Li, W.; He, Q. Levistolide A Inhibits PEDV Replication via Inducing ROS Generation. Viruses 2022, 14, 258. [Google Scholar] [CrossRef]
- Cochrane, R.A.; Dritz, S.S.; Woodworth, J.C.; Stark, C.R.; Saensukjaroenphon, M.; Gebhardt, J.T.; Bai, J.; Hesse, R.A.; Poulsen, E.G.; Chen, Q.; et al. Assessing the effects of medium-chain fatty acids and fat sources on PEDV infectivity. Transl. Anim. Sci. 2020, 4, txz179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Yang, R.; Xiao, L.; Dong, S.; Lin, J.; Liu, G.; Shan, H. Erastin inhibits porcine epidemic diarrhea virus replication in Vero cells. Front. Cell Infect. Microbiol. 2023, 13, 1142173. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Yin, Y.; Song, C.; Tan, L.; Qiu, X.; Liao, Y.; Liu, W.; Meng, S.; Sun, Y.; Ding, C. Newcastle-disease-virus-induced ferroptosis through nutrient deprivation and ferritinophagy in tumor cells. iScience 2021, 24, 102837. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Fan, W.; Bai, J.; Tang, Y.; Wang, L.; Jiang, Y.; Tang, L.; Liu, M.; Cui, W.; Xu, Y.; et al. TMPRSS2 and MSPL Facilitate Trypsin-Independent Porcine Epidemic Diarrhea Virus Replication in Vero Cells. Viruses 2017, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, Y.; Peng, P.; Liu, Y.; Huang, M.; Ma, Y.; Xue, C.; Cao, Y. Aloe extract inhibits porcine epidemic diarrhea virus in vitro and in vivo. Vet. Microbiol. 2020, 249, 108849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Zhou, J.; Wang, X.; Ma, L.; Li, J.; Yang, L.; Yuan, H.; Pang, D.; Ouyang, H. Porcine Epidemic Diarrhea Virus: An Updated Overview of Virus Epidemiology, Virulence Variation Patterns and Virus-Host Interactions. Viruses 2022, 14, 2434. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Lin, C.Y.; Chen, C.Y.; Hsueh, C.W.; Chang, Y.W.; Wang, C.C.; Chu, P.Y.; Tai, S.K.; Yang, M.H. Ferroptosis Signature Shapes the Immune Profiles to Enhance the Response to Immune Checkpoint Inhibitors in Head and Neck Cancer. Adv. Sci. 2023, 10, e2204514. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Kar, M.; Panwar, A.; Wangchuk, J.; Kumar, S.; Das, A.; Pandey, A.K.; Lodha, R.; Medigeshi, G.R. Oxidative stress specifically inhibits replication of dengue virus. J. Gen. Virol. 2021, 102, 001596. [Google Scholar] [CrossRef]
- Meuren, L.M.; Prestes, E.B.; Papa, M.P.; de Carvalho, L.R.P.; Mustafa, Y.M.; da Costa, L.S.; Da Poian, A.T.; Bozza, M.T.; Arruda, L.B. Infection of Endothelial Cells by Dengue Virus Induces ROS Production by Different Sources Affecting Virus Replication, Cellular Activation, Death and Vascular Permeability. Front. Immunol. 2022, 13, 810376. [Google Scholar] [CrossRef]
- Wang, L.F.; Lin, Y.S.; Huang, N.C.; Yu, C.Y.; Tsai, W.L.; Chen, J.J.; Kubota, T.; Matsuoka, M.; Chen, S.R.; Yang, C.S.; et al. Hydroxychloroquine-inhibited dengue virus is associated with host defense machinery. J. Interferon Cytokine Res. 2015, 35, 143–156. [Google Scholar] [CrossRef]
- Zhang, Z.; Rong, L.; Li, Y.P. Flaviviridae Viruses and Oxidative Stress: Implications for Viral Pathogenesis. Oxid. Med. Cell Longev. 2019, 2019, 1409582. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, Y.; Lin, X.; Ren, C.; Zhao, J.; Wang, F.; Gao, X.; Xiao, R.; Zhao, L.; Chen, H.; et al. Influenza M2 protein regulates MAVS-mediated signaling pathway through interacting with MAVS and increasing ROS production. Autophagy 2019, 15, 1163–1181. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ge, Y.; Ding, L.; Zhang, Z.; Qu, Y.; Jin, C.; Wang, X.N.; Wang, Z. Synthesis and evaluation of alkoxy-substituted enamides against influenza A virus in vitro and in vivo. Bioorg. Chem. 2023, 139, 106712. [Google Scholar] [CrossRef]
- Haque, M.M.; Murale, D.P.; Lee, J.S. Role of microRNA and Oxidative Stress in Influenza A Virus Pathogenesis. Int. J. Mol. Sci. 2020, 21, 8962. [Google Scholar] [CrossRef] [PubMed]
- van de Sand, L.; Bormann, M.; Schmitz, Y.; Heilingloh, C.S.; Witzke, O.; Krawczyk, A. Antiviral Active Compounds Derived from Natural Sources against Herpes Simplex Viruses. Viruses 2021, 1, 13863. [Google Scholar] [CrossRef] [PubMed]
- Merkel, M.; Goebel, B.; Boll, M.; Adhikari, A.; Maurer, V.; Steinhilber, D.; Culmsee, C. Mitochondrial Reactive Oxygen Species Formation Determines ACSL4/LPCAT2-Mediated Ferroptosis. Antioxidants 2023, 12, 1590. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Shim, J.K.; Choi, H.J. Quercetin 7-rhamnoside reduces porcine epidemic diarrhea virus replication via independent pathway of viral induced reactive oxygen species. Virol. J. 2011, 8, 460. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, P.; Li, H.; Yi, D.; Guo, S.; Wang, L.; Zhao, D.; Wang, C.; Wu, T.; Hou, Y. Multifaceted Effects and Mechanisms of N-Acetylcysteine on Intestinal Injury in a Porcine Epidemic Diarrhea Virus-Infected Porcine Model. Mol. Nutr. Food Res. 2022, 66, e2200369. [Google Scholar] [CrossRef]
- He, H.; Fan, X.; Shen, H.; Gou, H.; Zhang, C.; Liu, Z.; Zhang, B.; Wuri, N.; Zhang, J.; Liao, M.; et al. Butyrate limits the replication of porcine epidemic diarrhea virus in intestine epithelial cells by enhancing GPR43-mediated IFN-III production. Front. Microbiol. 2023, 14, 1091807. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Liu, D.; Wang, Z.; Qiu, J.; Zhang, J.; Chen, P.; Zeng, G.; Guo, Y.; Wang, X.; et al. Glutathione Peroxidase 3 induced mitochondria-mediated apoptosis via AMPK/ERK1/2 pathway and resisted autophagy-related ferroptosis via AMPK/mTOR pathway in hyperplastic prostate. J. Transl. Med. 2023, 21, 575. [Google Scholar] [CrossRef]
- Fu, X.; Wu, H.; Li, C.; Deng, G.; Chen, C. YAP1 inhibits RSL3-induced castration-resistant prostate cancer cell ferroptosis by driving glutamine uptake and metabolism to GSH. Mol. Cell Biochem. 2023. [Google Scholar] [CrossRef]
| Primer Name | Nucleotide Sequence (5′-3′) | Product (bp) | GenBank Accession Number or Citation Source: PMID |
|---|---|---|---|
| PEDV N | F:ACTAATAAAGGGAATAAGGACCAG | 207 | OP894120 PMID: 36936772 |
| R:GTTAGTGGGTTCAGTCTTTGC | |||
| GPX4 | F:CAGTGAGGCAAGACCGAAGTGAAC | 125 | NM_001039847.3 PMID:36936772 |
| R:TTACTCCCTGGCTCCTGCTTCC | |||
| GAPDH | F:CCCACTCCTCCACCTTTGAC | 113 | NM_002046.7 PMID: 36936772 |
| R:TCCACCACCCTGTTGCTGTAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Bao, Y.; Li, Y.; Duan, X.; Dong, S.; Lin, J.; Chang, X.; Tan, Y.; Zhang, H.; Shan, H. RSL3 Inhibits Porcine Epidemic Diarrhea Virus Replication by Activating Ferroptosis. Viruses 2023, 15, 2080. https://doi.org/10.3390/v15102080
Li Y, Bao Y, Li Y, Duan X, Dong S, Lin J, Chang X, Tan Y, Zhang H, Shan H. RSL3 Inhibits Porcine Epidemic Diarrhea Virus Replication by Activating Ferroptosis. Viruses. 2023; 15(10):2080. https://doi.org/10.3390/v15102080
Chicago/Turabian StyleLi, Yingguang, Yuwei Bao, Yan Li, Xiaoxiao Duan, Shaoming Dong, Jiaxu Lin, Xiaoyun Chang, Yue Tan, Hongliang Zhang, and Hu Shan. 2023. "RSL3 Inhibits Porcine Epidemic Diarrhea Virus Replication by Activating Ferroptosis" Viruses 15, no. 10: 2080. https://doi.org/10.3390/v15102080
APA StyleLi, Y., Bao, Y., Li, Y., Duan, X., Dong, S., Lin, J., Chang, X., Tan, Y., Zhang, H., & Shan, H. (2023). RSL3 Inhibits Porcine Epidemic Diarrhea Virus Replication by Activating Ferroptosis. Viruses, 15(10), 2080. https://doi.org/10.3390/v15102080







