Crossroads between Autoimmunity and COVID-19 in Lung Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Post-Transplant Management Protocol
2.3. Autoantigen Microarray Analysis
2.4. Statistical Changes
3. Results
3.1. Clinical Variables Related to COVID-19
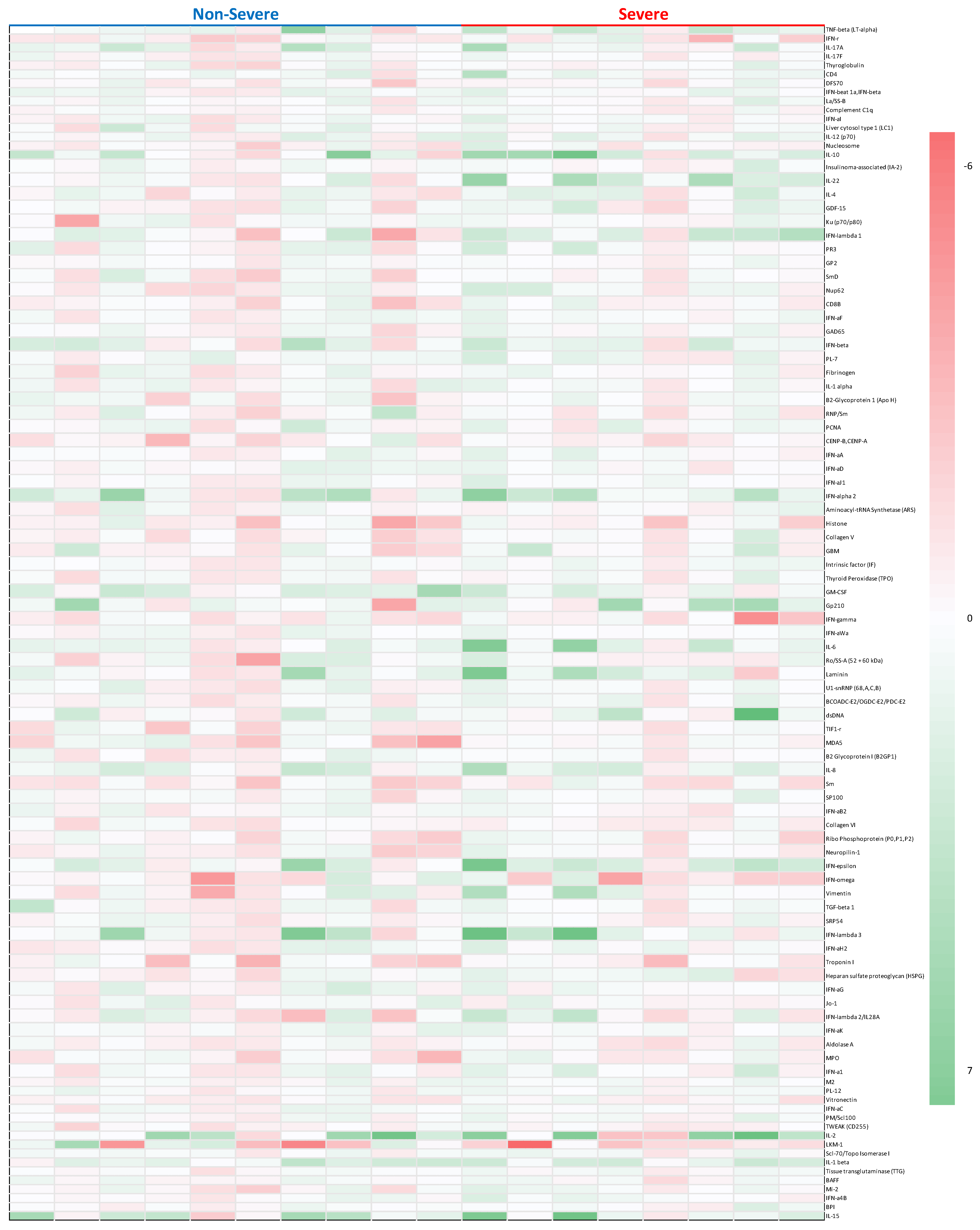
3.2. Auto-Antibodies and Characteristics
3.3. IFN-Related AAbs
3.4. IL-Related AAbs
3.5. Other AAbs
4. Discussion
4.1. Interferon-Related AAbs Leading to Severe COVID-19 Disease
4.2. Interleukin-Related AAbs Leading to Severe COVID-19
4.3. Challenges with Lung Transplant Recipients
4.4. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saez-Giménez, B.; Berastegui, C.; Barrencheguren, M.; Revilla-López, E.; Los Arcos, I.; Alonso, R.; Aguilar, M.; Mora, V.M.; Otero, I.; Reig, J.P.; et al. COVID-19 in lung transplant recipients: A multicenter study. Am. J. Transplant. 2021, 21, 1816–1824. [Google Scholar] [CrossRef]
- Messika, J.; Eloy, P.; Roux, A.; Hirschi, S.; Nieves, A.; Le Pavec, J.; Senechal, A.; Saint Raymond, C.; Carlier, N.; Demant, X.; et al. COVID-19 in lung transplant recipients. Transplantation 2021, 105, 177–186. [Google Scholar] [CrossRef]
- McQuiston, A.; Emtiazjoo, A.; Angel, P.; Machuca, T.; Christie, J.; Atkinson, C. Set up for failure: Pre-existing autoantibodies in lung transplant. Front. Immunol. 2021, 12, 711102. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Fleming, T.; Mohanakumar, T. Autoantibodies in lung transplantation. Transpl. Int. 2020, 33, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Beziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Q.; Sun, B.Q.; Fang, Z.F.; Zhao, J.C.; Liu, X.Y.; Li, Y.M.; Sun, X.Z.; Liang, H.F.; Zhong, B.; Huang, Z.F.; et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur. Respir. J. 2020, 56, 2001526. [Google Scholar] [CrossRef] [PubMed]
- Mohanka, M.R.; Mahan, L.D.; Joerns, J.; Lawrence, A.; Bollineni, S.; Kaza, V.; La Hoz, R.M.; Kershaw, C.D.; Terada, L.S.; Torres, F.; et al. Clinical characteristics, management practices, and outcomes among lung transplant patients with COVID-19. J. Heart Lung Transplant. 2021, 40, 936–947. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, H.; Yan, M.; Zuo, X.; Li, Q.Z. Autoantigen microarray for high-throughput autoantibody profiling in systemic lupus erythematosus. Genom. Proteom. Bioinform. 2015, 13, 210–218. [Google Scholar] [CrossRef]
- Luo, H.; Wang, L.; Bao, D.; Wang, L.; Zhao, H.; Lian, Y.; Yan, M.; Mohan, C.; Li, Q.Z. Novel autoantibodies related to cell death and DNA repair pathways in systemic lupus erythematosus. Genom. Proteom. Bioinform. 2019, 17, 248–259. [Google Scholar] [CrossRef]
- Mukherjee, M.; Thomas, S.R.; Radford, K.; Dvorkin-Gheva, A.; Davydchenko, S.; Kjarsgaard, M.; Svenningsen, S.; Almas, S.; Felix, L.C.; Stearns, J.; et al. Sputum antineutrophil cytoplasmic antibodies in serum antineutrophil cytoplasmic antibody-negative eosinophilic granulomatosis with polyangiitis. Am. J. Respir. Crit. Care Med. 2019, 199, 158–170. [Google Scholar] [CrossRef]
- Sboner, A.; Karpikov, A.; Chen, G.; Smith, M.; Mattoon, D.; Freeman-Cook, L.; Schweitzer, B.; Gerstein, M.B. Robust-linear- model normalization to reduce technical variability in functional protein microarrays. J. Proteome Res. 2009, 8, 5451–5464. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wu, J.; Wu, F.; Guo, D.; Chen, L.; Fang, Z.; Li, C. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Investig. Radiol. 2020, 55, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Huang, Y.; Guo, Y.; Yin, M.; Chen, X.; Xiao, L.; Deng, G. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int. J. Infect. Dis. 2020, 96, 467–474. [Google Scholar] [CrossRef]
- Kaza, V.; Zhu, C.; Feng, L.; Torres, F.; Bollineni, S.; Mohanka, M.; Banga, A.; Joerns, J.; Mohanakumar, T.; Terada, L.S.; et al. Pre-existing self-reactive IgA antibodies associated with primary graft dysfunction after lung transplantation. Transpl. Immunol. 2020, 59, 101271. [Google Scholar] [CrossRef]
- Smatti, M.K.; Cyprian, F.S.; Nasrallah, G.K.; Al Thani, A.A.; Almishal, R.O.; Yassine, H.M. Viruses and autoimmunity: A review on the potential interaction and molecular mechanisms. Viruses 2019, 11, 762. [Google Scholar] [CrossRef]
- Tang, K.T.; Hsu, B.C.; Chen, D.Y. Autoimmune and rheumatic manifestations associated with COVID-19 in adults: An updated systematic review. Front. Immunol. 2021, 12, 645013. [Google Scholar] [CrossRef]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef]
- Knight, J.S.; Caricchio, R.; Casanova, J.L.; Combes, A.J.; Diamond, B.; Fox, S.E.; Hanauer, D.A.; James, J.A.; Kanthi, Y.; Ladd, V.; et al. The intersection of COVID-19 and autoimmunity. J. Clin. Investig. 2021, 131, e154886. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef]
- de Prost, N.; Bastard, P.; Arrestier, R.; Fourati, S.; Mahévas, M.; Burrel, S.; Dorgham, K.; Gorochov, G.; Tandjaoui-Lambiotte, Y.; Azzaoui, I.; et al. Plasma Exchange to Rescue Patients with Autoantibodies Against Type I Interferons and Life-Threatening COVID-19 Pneumonia. J. Clin. Immunol. 2021, 41, 536–544. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Abers, M.S.; Rosen, L.B.; Delmonte, O.M.; Shaw, E.; Bastard, P.; Imberti, L.; Quaresima, V.; Biondi, A.; Bonfanti, P.; Castagnoli, R.; et al. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol. Cell Biol. 2021, 99, 917–921. [Google Scholar] [CrossRef]
- Goncalves, D.; Mezidi, M.; Bastard, P.; Perret, M.; Saker, K.; Fabien, N.; Pescarmona, R.; Lombard, C.; Walzer, T.; Casanova, J.L.; et al. Antibodies against type I interferon: Detection and association with severe clinical outcome in COVID-19 patients. Clin. Transl. Immunol. 2021, 10, e1327. [Google Scholar] [CrossRef] [PubMed]
- Solanich, X.; Rigo-Bonnin, R.; Gumucio, V.D.; Bastard, P.; Rosain, J.; Philippot, Q.; Perez Fernandez, X.L.; Fuset-Cabanes, M.P.; Gordillo-Benitez, M.A.; Suarez-Cuartin, G.; et al. Pre-existing autoantibodies neutralizing high concentrations of type i interferons in almost 10% of COVID-19 patients admitted to intensive care in Barcelona. J. Clin. Immunol. 2021, 41, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Mommert, M.; Mouton, W.; Pizzorno, A.; Brengel-Pesce, K.; Mezidi, M.; Villard, M.; Lina, B.; Richard, J.C.; Fassier, J.B.; et al. Early nasal type I IFN immunity against SARS-CoV-2 is compromised in patients with autoantibodies against type I IFNs. J. Exp. Med. 2021, 218, e20211211. [Google Scholar] [CrossRef] [PubMed]
- van der Wijst, M.G.P.; Vazquez, S.E.; Hartoularos, G.C.; Bastard, P.; Grant, T.; Bueno, R.; Lee, D.S.; Greenland, J.R.; Sun, Y.; Perez, R.; et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci. Transl. Med. 2021, 13, eabh2624. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.G.K.; Miao, V.N.; Owings, A.H.; Navia, A.W.; Tang, Y.; Bromley, J.D.; Lotfy, P.; Sloan, M.; Laird, H.; Williams, H.B.; et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell 2021, 184, 4713–4733. [Google Scholar] [CrossRef]
- Khamsi, R. Rogue antibodies could be driving severe COVID-19. Nature 2021, 590, 29–31. [Google Scholar] [CrossRef]
- Ramakrishnan, R.K.; Kashour, T.; Hamid, Q.; Halwani, R.; Tleyjeh, I.M. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front. Immunol. 2021, 12, 686029. [Google Scholar] [CrossRef]
- Kaklamanos, A.; Belogiannis, K.; Skendros, P.; Gorgoulis, V.G.; Vlachoyiannopoulos, P.G.; Tzioufas, A.G. COVID-19 immunobiology: Lessons learned, new questions arise. Front. Immunol. 2021, 12, 719023. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, Y.; Liu, M.; Shi, S.; Tian, J. Impacts of immunosuppression and immunodeficiency on COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, e93–e95. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated type i interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef]
- Acharya, D.; Liu, G.; Gack, M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020, 20, 397–398. [Google Scholar] [CrossRef]
- Andreakos, E.; Tsiodras, S. COVID-19: Lambda interferon against viral load and hyperinflammation. EMBO Mol. Med. 2020, 12, e12465. [Google Scholar] [CrossRef]
- Rodríguez, Y.; Novelli, L.; Rojas, M.; De Santis, M.; Acosta-Ampudia, Y.; Monsalve, D.M.; Ramirez-Santana, C.; Costanzo, A.; Ridgway, W.M.; Ansari, A.A.; et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020, 114, 102506. [Google Scholar] [CrossRef]
- El Aoud, S.; Morin, C.; Lorriaux, P.; Obert, J.; Sorial, D.; Chaabouni, T.; Thomas, L. COVID-19 presenting as lupus erythematosus-like syndrome. Disaster Med. Public Health Prep. 2021, 15, e12–e15. [Google Scholar]
- Fang, S.; Ju, D.; Lin, Y.; Chen, W. The role of interleukin-22 in lung health and its therapeutic potential for COVID-19. Front. Immunol. 2022, 13, 951107. [Google Scholar] [CrossRef]
- Pociask, D.A.; Scheller, E.V.; Mandalapu, S.; McHugh, K.J.; Enelow, R.I.; Fattman, C.L.; Kolls, J.K.; Alcorn, J.F. IL-22 is essential for lung epithelial repair following influenza infection. Am. J. Pathol. 2013, 182, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Thakar, M.S.; Ouyang, W.; Malarkannan, S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 2013, 6, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Cagan, E.; Tezcan, G.; Simsek, A.; Kizmaz, M.A.; Dombaz, F.; Asan, A.; Demir, H.I.; Bal, H.; Yoyen Ermis, D.; Gorek Dilektasli, A.; et al. The age-dependent role of Th22, Tc22, and Tc17 cells in the severity of pneumonia in COVID-19 immunopathogenesis. Viral Immunol. 2022, 35, 318–327. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study to Evaluate the Safety and Efficacy of mstt1041a (Astegolimab) or uttr1147a in Patients with Severe COVID-19 Pneumonia (Covastil) 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04386616 (accessed on 13 May 2020).
- Mu, H.J.; Xie, P.; Chen, J.Y.; Gao, F.; Zou, J.; Zhang, J.; Zhang, B. Association of TNF-α, TGF-β1, IL-10, IL-6, and IFN-γ gene polymorphism with acute rejection and infection in lung transplant recipients. Clin. Transplant. 2014, 28, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.; Mahan, L.D.; Mohanka, M.R.; Bollineni, S.; Kaza, V.; La Hoz, R.M.; Zhang, S.; Kershaw, C.D.; Terada, L.S.; Torres, F.; et al. Predictors and outcomes of respiratory failure among lung transplant patients with COVID-19. Clin. Transplant. 2022, 36, e14540. [Google Scholar] [CrossRef] [PubMed]
- Sweet, S.C. Community-acquired respiratory viruses post-lung transplant. Semin. Respir. Crit. Care Med. 2021, 42, 449–459. [Google Scholar] [CrossRef]
| Variable | Non-Severe (N = 10) | Severe (N = 8) | All (N = 18) | p-Value |
|---|---|---|---|---|
| Age at Transplant | ||||
| mean ± sd | 61 ± 9 | 61 ± 5 | 0.86 | |
| median (max, min) 95% CI | 63 (42, 71) (54, 67) | 60 (55, 72) (57, 66) | ||
| BMI | ||||
| mean ± sd | 28 ± 4 | 29 ± 6 | 0.73 | |
| median (max, min) 95% CI | 28 (21, 36) (25, 31) | 30 (17, 39) (24, 35) | ||
| Gender | ||||
| Male | 8 (80%) | 6 (75%) | 4 (22%) | 0.80 |
| Female | 2 (20%) | 2 (25%) | 14 (78%) | |
| Race | ||||
| African American | 0 | 2 (25%) | 2 (11%) | 1 |
| Asian | 1 (10%) | 0 | 1 (6%) | |
| Caucasian | 6 (60%) | 5 (63%) | 11 (61%) | |
| Hispanic | 3 (30%) | 1 (13%) | 4 (22%) | |
| Transplant Indication | ||||
| Obstructive | 3 (30%) | 0 | 3 (17%) | 0.21 |
| Restrictive | 7 (70%) | 8 (100%) | 15 (83%) | |
| Type of Transplant | ||||
| Double | 8 (80%) | 5 (63%) | 13 (72%) | 0.61 |
| Single | 2 (20%) | 3 (38%) | 5 (28%) | |
| Time Since Transplant (days) | ||||
| mean ± sd | 1557 ± 1287 | 1388 ± 987 | 0.77 | |
| median (max, min) 95% CI | 1145 (115, 4182) (636, 2478) | 1110 (229, 2979) (563, 2213) | ||
| Hypertension (Y) | 9 (90%) | 8 (100%) | 17 (94%) | 1 |
| DM (Y) | 6 (60%) | 4 (50%) | 10 (56%) | 1 |
| Hyperlipidemia (Y) | 8 (80%) | 7 (87%) | 15 (83%) | 1 |
| OSA (Y) | 2 (20%) | 2 (25%) | 4 (22%) | 1 |
| CAD (Y) | 4 (40%) | 4 (50%) | 8 (44%) | 1 |
| CHF (Y) | 0 | 1 (12%) | 1 (6%) | 0.44 |
| Variable | Non-Severe (N = 10) | Severe (N = 8) | All (N = 18) | p-Value |
|---|---|---|---|---|
| Immunosuppression | ||||
| Prograf, Cyclosporin, Sirolimus | 10 (100%) | 7 (100%) | 17 (100%) | 1.00 |
| Prograf (missing = 1) | 10 | 7 | 17 | |
| Immunosuppression | ||||
| Imuran, Cellcept, Myfortic | 10 (100.00%) | 8 (100.00%) | 18 (100.00%) | 1.000 |
| Azathioprine | 2 | 1 | 3 | |
| Cellcept | 8 | 5 | 13 | |
| Myfortic | 0 | 2 | 2 | |
| Prednisone dose at COVID DX (mg) | ||||
| mean ± sd | 7.500 ± 3.118 | 8.750 ± 3.273 | 0.4205 | |
| median (max, min) 95% CI | 7.50 (5, 15) (5.270, 9.731) | 8.75 (5, 15) (6.014, 11.487) | ||
| History of CMV PCR+ | 1 (10.00%) | 4 (50.00%) | 5 (27.78%) | 0.1176 |
| History of EBV PCR+ | 3 (30.00%) | 4 (50.00%) | 7 (38.89%) | 0.6305 |
| Cumulative Acute Rejection Score | ||||
| mean ± sd | 1.500 ± 1.780 | 0.250 ± 0.463 | 0.0575 | |
| median (max, min) 95% CI | 1 (0, 6) (0.227, 2.773) | 0 (0, 1) (-0.137,0.637) | ||
| History of DSA Positive Pre-COVID | 5 (50.00%) | 3 (37.50%) | 8 (44.44%) | 0.6641 |
| DSA MFI | ||||
| mean ± sd | 3622.4 ± 4692.2 | 4767.0 ± 442.4 | 0.6162 | |
| median (max, min) 95% CI | 1600 (1143, 11,978) (−2203.7, 9448.5) | 4720 (4350, 5231) (3368.1, 5865.9) | ||
| DSA at COVID | 3 (30.00%) | 0 | 3 (16.67%) | 0.2157 |
| Pre-COVID CARV | 8 (80.00%) | 4 (50.00%) | 12 (66.67%) | 0.3213 |
| Pre-COVID CLAD | 3 (30.00%) | 3 (37.50%) | 6 (33.33%) | 1.0000 |
| CLAD TYPE | ||||
| NA | 6 (60.00%) | 4 (50.00%) | 10 (55.56%) | 1.0000 |
| Obstructive | 2 (20.00%) | 2 (25.00%) | 4 (22.22%) | |
| Restrictive | 2 (20.00%) | 2 (25.00%) | 4 (22.22%) | |
| Symptom duration before COVID DX | ||||
| mean ± sd | 2.200 ± 1.033 | 3.250 ± 2.376 | 0.2735 | |
| median (max, min) 95% CI | 2 (1, 4) (1.4312, 2.9388) | 3 (0, 7) (1.264, 5.236) |
| Variable | NS (N = 10) | S (N = 8) | All (N = 18) | p-Value |
|---|---|---|---|---|
| Ferritin | ||||
| mean ± sd | 375.0 ± 416.9 | 1337.0 ± 1036.5 | 0.0361 | |
| median (max, min) 95% CI | 257 (46, 1262) (76.8, 673.2) | 1287 (201, 3083) (470.5, 2203.5) | ||
| D-Dimer | ||||
| mean ± sd | 0.566 ± 0.410 | 1.145 ± 0.883 | 0.1195 | |
| median (max, min) 95% CI | 0.410 (0.250, 1.510) (0.273, 0.859) | 0.710 (0.310, 2.880) (0.406, 1.884) | ||
| ALC | ||||
| mean ± sd | 0.548 ± 0.346 | 0.520 ± 0.388 | 0.8736 | |
| median (max, min) 95% CI | 0.405 (0.300, 1.450) (0.300, 0.756) | 0.435 (0.190, 1.390) (0.196, 0.844) | ||
| Co-morbid kidney dysfunction (Y) | 4 (40.00%) | 5 (62.50%) | 9 (50.00%) | 0.6372 |
| Spirometry Decline > 10% at COVID (Y) | 3 (42.86%) | 1 (33.33%) | 4 (40.00%) | NA |
| Variable | NS (N = 10) | S (N = 8) | All (N = 18) | p-Value |
|---|---|---|---|---|
| Acute Respiratory Failure (Y) | 0 | 8 (100.00%) | 8 (44.44%) | <0.0001 |
| Hospital Length of Stay | ||||
| mean ± sd | 7.000 ± 2.582 | 9.714 ± 4.923 | 0.1570 | |
| median (max, min) 95% CI | 6.5 (4, 11) (5.153, 8.847) | 10.0 (2, 18) (5.161, 14.268) | ||
| Readmission within 30 Days (Y) | 3 (30.00%) | 3 (37.50%) | 6 (33.33%) | 1.0000 |
| Death (Y) | 0 | 3 (37.50%) | 3 (16.67%) | 0.0686 |
| All Patients (N = 18) | NS (N = 10) | S (N = 8) | p < 0.05 | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | p-Value | Mean | SD | Mean | SD | p-Value | |
| IFN-alpha2 (Pre–Post) | 1.686 | 1.955 | 0.002 | 1.332 | 2.089 | 2.13 | 1.807 | 0.406 |
| IFN-beta-1a (Pre–Post) | 0.399 | 0.687 | 0.025 | 0.352 | 0.782 | 0.458 | 0.595 | 0.755 |
| IFN-beta (Pre–Post) | 0.806 | 1.384 | 0.024 | 0.693 | 1.599 | 0.947 | 1.15 | 0.712 |
| IFN-epsilon (Pre–Post) | 1.52 | 1.898 | 0.003 | 0.891 | 1.66 | 2.305 | 1.984 | 0.119 |
| IFN-gamma (Pre–Post) | −0.804 | 1.294 | 0.017 | −0.609 | 0.827 | −1.048 | 1.75 | 0.528 |
| IFN-lambda 1 (Pre–Post) | 0.633 | 1.907 | 0.177 | −0.197 | 1.843 | 1.671 | 1.499 | 0.034 |
| IFN-lambda 3 (Pre–Post) | 1.667 | 2.738 | 0.019 | 1.147 | 2.537 | 2.317 | 3.01 | 0.384 |
| IFNR (Pre–Post) | −0.639 | 1.168 | 0.033 | −0.704 | 0.917 | -0.557 | 1.49 | 0.8 |
| All Patients (N = 18) | NS (N = 10) | S (N = 8) | p < 0.05 | |||||
|---|---|---|---|---|---|---|---|---|
| AAb | Mean | SD | p-Value | Mean | SD | Mean | SD | p-Value |
| IL-1 beta (Pre–Post) | 1.384 | 1.025 | 0 | 1.269 | 1.077 | 1.527 | 1.01 | 0.611 |
| IL-2 (Pre–Post) | 2.303 | 3.14 | 0.006 | 1.958 | 2.64 | 2.734 | 3.822 | 0.617 |
| IL-6 (Pre–Post) | 1.176 | 1.811 | 0.014 | 0.52 | 0.844 | 1.996 | 2.38 | 0.131 |
| IL-8 (Pre–Post) | 1.086 | 1.177 | 0.001 | 0.903 | 1.099 | 1.315 | 1.306 | 0.477 |
| IL-10 (Pre–Post) | 1.657 | 2.441 | 0.01 | 0.911 | 2.267 | 2.588 | 2.465 | 0.153 |
| IL-15 (Pre–Post) | 1.741 | 2.396 | 0.007 | 1.563 | 2.111 | 1.962 | 2.847 | 0.737 |
| IL-17A (Pre–Post) | 0.911 | 1.461 | 0.017 | 0.861 | 1.491 | 0.973 | 1.521 | 0.877 |
| IL-22 (Pre–Post) | 0.993 | 1.8 | 0.032 | -0.081 | 0.932 | 2.335 | 1.744 | 0.002 |
| All Patients (N = 18) | NS (N = 10) | S (N = 8) | p < 0.05 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | AAb | Mean | SD | p-Value | Mean | SD | Mean | SD | p-Value |
| Extracellular matrix protein | Collagen-VI (Pre–Post) | −0.375 | 0.61 | 0.018 | −0.349 | 0.725 | −0.407 | 0.475 | 0.847 |
| Laminin (Pre–Post) | 1.041 | 2.016 | 0.043 | 0.6 | 1.549 | 1.592 | 2.482 | 0.314 | |
| Vitronectin (Pre–Post) | −0.332 | 0.628 | 0.039 | −0.463 | 0.564 | −0.167 | 0.704 | 0.336 | |
| TNF- related | TNF-β (LT-alpha) (Pre–Post) | 1.168 | 1.588 | 0.006 | 0.887 | 1.831 | 1.519 | 1.249 | 0.418 |
|
TWEAK (CD255) (Pre–Post) | −0.319 | 0.596 | 0.036 | −0.367 | 0.701 | −0.259 | 0.474 | 0.716 | |
| Centromere related |
CENP-B, CENP-A (Pre–Post) | −0.611 | 0.954 | 0.015 | −0.734 | 1.196 | −0.458 | 0.569 | 0.557 |
|
Histone (Pre–Post) | −0.747 | 1.301 | 0.026 | −0.91 | 1.476 | −0.544 | 1.108 | 0.57 | |
| Other miscellaneous |
CD-4 (Pre–Post) | 0.533 | 0.953 | 0.03 | 0.325 | 0.723 | 0.794 | 1.181 | 0.315 |
|
GM-CSF (Pre–Post) | 1.11 | 1.29 | 0.002 | 1.406 | 1.425 | 0.74 | 1.072 | 0.29 | |
|
Sm (Pre–Post) | −0.714 | 1.01 | 0.008 | −0.9 | 1.047 | −0.481 | 0.978 | 0.399 | |
|
Troponin I (Pre–Post) | −0.737 | 1.248 | 0.023 | −0.903 | 1.444 | −0.531 | 1.009 | 0.546 | |
| Antibody | Variable | Estimate ± SE | 96% CI | p-Value |
|---|---|---|---|---|
| BCOADC-E2/OGDC-E2/ PDC-E2 | COVID-19 Severity | 0.7112 ± 0.2959 | (0.0766, 1.3458) | 0.0307 |
| Time Post-Transplant | 0.0004 ± 0.0001 | (0.0001, 0.0006) | 0.0173 | |
| History of DSA Prior to COVID | −0.0490 ± 0.2956 | (−0.6830, 0.5851) | 0.8708 | |
| Collagen V | COVID-19 Severity | 0.8743 ± 0.3571 | (0.1085, 1.6401) | 0.0281 |
| Time Post-Transplant | 0.0001 ± 0.0002 | (−0.0002, 0.0005) | 0.4296 | |
| History of DSA Prior to COVID | 0.7489 ± 0.3567 | (−0.0162, 1.5139) | 0.0544 | |
| IFN-lambda 1 | COVID Severe | 2.0666 ± 0.7962 | (0.3589, 3.7742) | 0.0212 |
| Time Post-Transplant | 0.0003 ± 0.0004 | (−0.0005, 0.0010) | 0.4931 | |
| History of DSA Prior to COVID | 1.2483 ± 0.7954 | (−0.4577, 2.9544) | 0.1389 | |
| IL-22 | COVID Severe | 2.5713 ± 0.6348 | (1.2098, 3.9328) | 0.0012 |
| Time Post-Transplant | 0.0003 ± 0.0003 | (−0.0003, 0.0009) | 0.3656 | |
| History of DSA Prior to COVID | 0.8818 ± 0.6342 | (−0.4784, 2.2420) | 0.1861 | |
| Mi-2 | COVID Severe | 0.8121 ± 0.3819 | (−0.0070, 1.6311) | 0.0517 |
| Time Post-Transplant | 0.0004 ± 0.0002 | (0.0000, 0.0007) | 0.0576 | |
| History of DSA Prior to COVID | 0.3298 ± 0.3815 | (−0.4885, 1.1480) | 0.4020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narasimhan, M.; Muthukumar, A.; Sataranatarajan, K.; Mahimainathan, L.; Mahan, L.; Timofte, I.; Bollineni, S.; Joerns, J.; Zhang, S.; Gorman, A.; et al. Crossroads between Autoimmunity and COVID-19 in Lung Transplant Recipients. Viruses 2023, 15, 2045. https://doi.org/10.3390/v15102045
Narasimhan M, Muthukumar A, Sataranatarajan K, Mahimainathan L, Mahan L, Timofte I, Bollineni S, Joerns J, Zhang S, Gorman A, et al. Crossroads between Autoimmunity and COVID-19 in Lung Transplant Recipients. Viruses. 2023; 15(10):2045. https://doi.org/10.3390/v15102045
Chicago/Turabian StyleNarasimhan, Madhusudhanan, Alagarraju Muthukumar, Kavithalakshmi Sataranatarajan, Lenin Mahimainathan, Luke Mahan, Irina Timofte, Srinivas Bollineni, John Joerns, Song Zhang, April Gorman, and et al. 2023. "Crossroads between Autoimmunity and COVID-19 in Lung Transplant Recipients" Viruses 15, no. 10: 2045. https://doi.org/10.3390/v15102045
APA StyleNarasimhan, M., Muthukumar, A., Sataranatarajan, K., Mahimainathan, L., Mahan, L., Timofte, I., Bollineni, S., Joerns, J., Zhang, S., Gorman, A., Banga, A., Mohanka, M., Torres, F., Lawrence, A., Thalachallour, M., & Kaza, V. (2023). Crossroads between Autoimmunity and COVID-19 in Lung Transplant Recipients. Viruses, 15(10), 2045. https://doi.org/10.3390/v15102045





