The Magic Staff: A Comprehensive Overview of Baculovirus-Based Technologies Applied to Human and Animal Health
Abstract
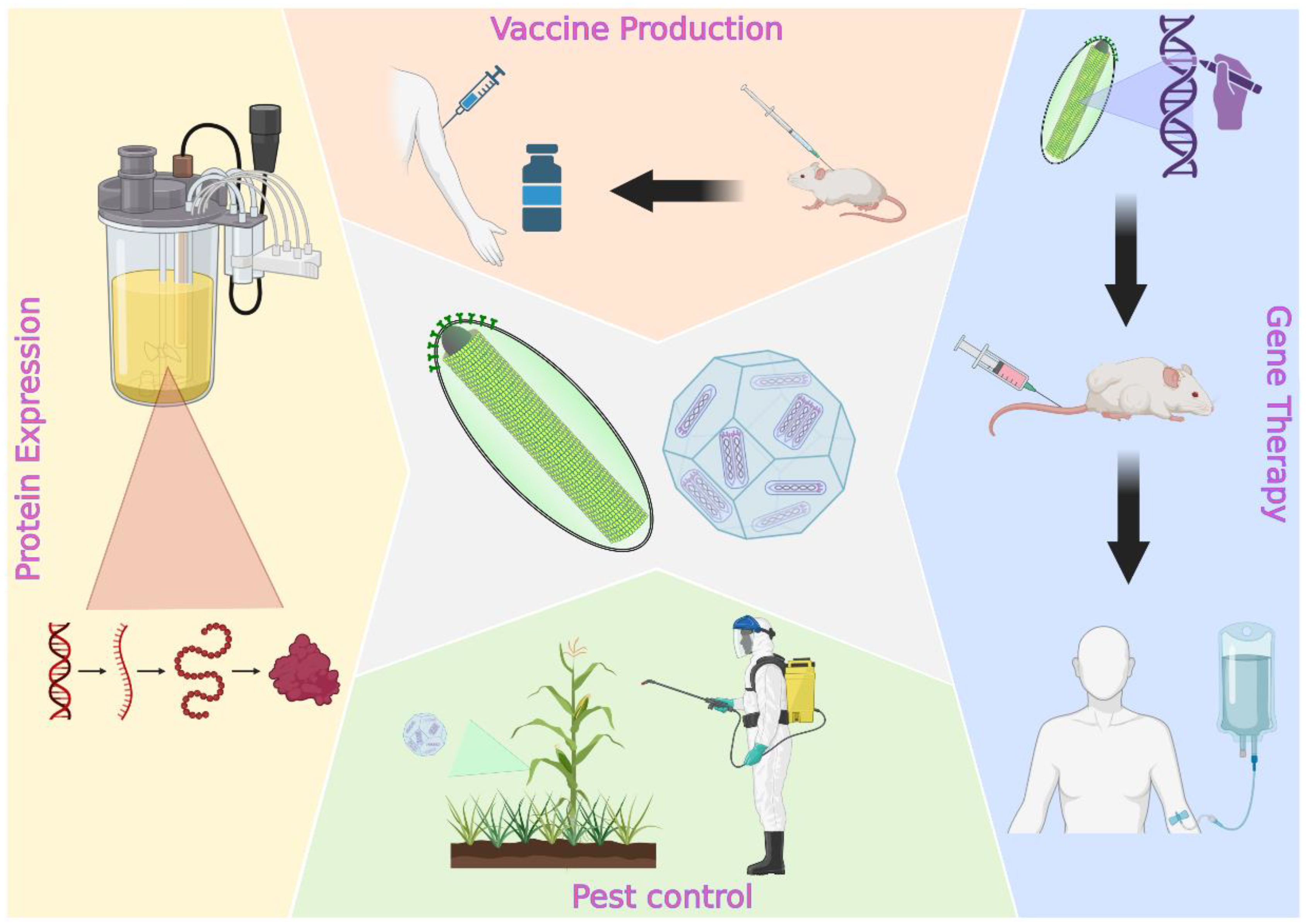
1. Introduction
2. Baculovirus in Nature
3. Budded Viruses and Cell Lines
4. Recombinant Baculovirus Production
5. Baculovirus-Derived Immunogens for Vaccine Development
5.1. Recombinant Proteins
5.2. Virus-like Particles
5.3. Baculovirus Surface and Capsid Display
6. Baculovirus Entry into Mammalian Cells: Transduction
Strategies to Improve Baculovirus Transduction Efficiency
7. Gene Therapy
7.1. Baculoviral Gene Delivery Vectors
7.2. Protein Expression
7.3. shRNA and ncRNA Expression
7.4. CRISPR/Cas Systems
7.5. recAAV Produced in BEVS
8. Enhancing Baculovirus Transduction Efficiency in Gene Delivery
8.1. Promoter Selection
8.2. Extending Baculovirus GOI Expression In Vivo and In Vitro
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AcMNPV | Autographa californica Multiple Nucleopolyhedrovirus |
| BEVS | baculovirus-based protein expression system |
| ODV | occlusion-derived virus |
| OB | occlusion bodies |
| BV | budded virus |
| HA | hemagglutinin |
| ZIKV | Zika virus |
| VSV | Vesicular stomatitis virus |
| VSV-G | Vesicular stomatitis virus glycoprotein |
| TBV | transmission-blocking vaccines |
| VLP | virus-like particles |
| MOI | multiplicity of infection |
| HIV | Human immunodeficiency virus |
| SP | signal peptide |
| TM | transmembrane |
| CTD | cytoplasmic terminal domain |
| MHC | major histocompatibility complex |
| OVA | ovalbumin |
| PEI | polyethylenimine |
| PEG | polyethylene glycol |
| RIPK1 | receptor interaction protein kinase 1 |
| AAV | adeno-associated virus |
| FDA | U.S. Food and Drug Administration |
| CMV | cytomegalovirus |
| SV40 | simian virus 40 |
| CAG | chicken beta-actin |
| HBV | Hepatitis B virus |
| GOI | gene of interest |
| lncRNA | long non-coding RNA |
| shRNA | short hairpin RNA |
| gRNA | guide RNA |
| ncRNA | Non-coding RNA |
| RNAi | interference RNA |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| EF1α | elongation factor-1 alpha |
| DAF | decay accelerating factor |
| EBV | Epstein-Barr virus |
References
- Smith, G.E.; Summers, M.D.; Fraser, M.J. Production of Human Beta Interferon in Insect Cells Infected with a Baculovirus Expression Vector. Mol. Cell. Biol. 1983, 3, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.L.; Herniou, E.A.; Jehle, J.A.; Theilmann, D.A.; Burand, J.P.; Becnel, J.J.; Krell, P.J.; van Oers, M.M.; Mowery, J.D.; Bauchan, G.R.; et al. ICTV Virus Taxonomy Profile: Baculoviridae. J. Gen. Virol. 2018, 99, 1185–1186. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chang, P.; Liu, X.; Lü, P.; Tang, Q.; Guo, Z.; Qiu, J.; Chen, K.; Yao, Q. Bombyx mori Pupae Efficiently Produce Recombinant AAV2/HBoV1 Vectors with a Bombyx mori Nuclear Polyhedrosis Virus Expression System. Viruses 2021, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, G.F. Baculovirus Molecular Biology, 4th ed.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2019. [Google Scholar]
- Haase, S.; Sciocco-Cap, A.; Romanowski, V. Baculovirus Insecticides in Latin America: Historical Overview, Current Status and Future Perspectives. Viruses 2015, 7, 2230–2267. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.; Mabashi-Asazuma, H.; Jarvis, D.L. An Overview and History of Glyco-Engineering in Insect Expression Systems. Methods Mol. Biol. Clifton. NJ 2015, 1321, 131–152. [Google Scholar] [CrossRef]
- Palmberger, D.; Wilson, I.B.H.; Berger, I.; Grabherr, R.; Rendic, D. SweetBac: A New Approach for the Production of Mammalianised Glycoproteins in Insect Cells. PloS ONE 2012, 7, e34226. [Google Scholar] [CrossRef]
- Maghodia, A.B.; Geisler, C.; Jarvis, D.L. A New Bacmid for Customized Protein Glycosylation Pathway Engineering in the Baculovirus-Insect Cell System. ACS Chem. Biol. 2021, 16, 1941–1950. [Google Scholar] [CrossRef]
- Pelosse, M.; Crocker, H.; Gorda, B.; Lemaire, P.; Rauch, J.; Berger, I. MultiBac: From Protein Complex Structurto Synthetic Viral Nanosystems. BMC Biol. 2017, 15, 99. [Google Scholar] [CrossRef]
- Neuhold, J.; Radakovics, K.; Lehner, A.; Weissmann, F.; Garcia, M.Q.; Romero, M.C.; Berrow, N.S.; Stolt-Bergner, P. GoldenBac: A Simple, Highly Efficient, and Widely Applicable System for Construction of Multi-Gene Expression Vectors for Use with the Baculovirus Expression Vector System. BMC Biotechnol. 2020, 20, 26. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, D.; Yu, L.; Sun, F.; Sun, F. SmartBac, a New Baculovirus System for Large Protein Complex Production. J. Struct. Biol. X 2019, 1, 100003. [Google Scholar] [CrossRef]
- Jacob, A.; Brun, L.; Jiménez Gil, P.; Ménard, L.; Bouzelha, M.; Broucque, F.; Roblin, A.; Vandenberghe, L.H.; Adjali, O.; Robin, C.; et al. Homologous Recombination Offers Advantages over Transposition-Based Systems to Generate Recombinant Baculovirus for Adeno-Associated Viral Vector Production. Biotechnol. J. 2021, 16, e2000014. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Hu, H.; Wang, X.; Wang, H.; Deng, F.; Wang, M.; Hu, Z. Construction and Characterization of a Novel Bacmid AcBac-Syn Based on a Synthesized Baculovirus Genome. Virol. Sinica. 2022, 36, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Fedson, D.S.; Dunnill, P. New Approaches to Confronting an Imminent Influenza Pandemic. Perm. J. 2007, 11, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.J.; Patriarca, P.A.; Treanor, J. FluBlok, a Recombinant Hemagglutinin Influenza Vaccine. Influenza Other Respir. Viruses 2008, 2, 211–219. [Google Scholar] [CrossRef]
- Shin, M.; Kim, K.; Lee, H.-J.; Lee, R.; Jung, Y.-J.; Park, J.; Hahn, T.-W. Zika Virus Baculovirus-Expressed Envelope Protein Elicited Humoral and Cellular Immunity in Immunocompetent Mice. Sci. Rep. 2022, 12, 660. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Chen, T.; Mi, L.; Sun, X.; Zhou, X.; Miao, F.; Zhang, S.; Liu, Y.; Hu, R. The Mink Circovirus Capsid Subunit Expressed by Recombinant Baculovirus Protects Minks against Refractory Diarrhea in Field. Viruses 2021, 13, 606. [Google Scholar] [CrossRef]
- Chang, D.; Liu, Y.; Chen, Y.; Hu, X.; Burov, A.; Puzyr, A.; Bondar, V.; Yao, L. Study of the Immunogenicity of the VP2 Protein of Canine Parvovirus Produced Using an Improved Baculovirus Expression System. BMC Vet. Res. 2020, 16, 202. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Vakharia, V.N.; Zhou, Y.; Jiang, N.; Liu, W.; Meng, Y.; Li, Y.; Xue, M.; Zhang, J.; Zeng, L.; et al. A Novel Subunit Vaccine Based on Outer Capsid Proteins of Grass Carp Reovirus (GCRV) Provides Protective Immunity against GCRV Infection in Rare Minnow (Gobiocypris Rarus). Pathog. Basel Switz. 2020, 9, 945. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Park, S.-Y.; Kim, A.-Y.; Lee, S.-O.; Kim, J.-S.; Kim, H.; Youn, H.-J.; Ko, Y.-J. Recombinant Vesicular Stomatitis Virus Glycoprotein Carrying a Foot-and-Mouth Disease Virus Epitope as a Vaccine Candidate. J. Vet. Med. Sci. 2020, 82, 1155–1159. [Google Scholar] [CrossRef]
- Lee, S.-M.; Hickey, J.M.; Miura, K.; Joshi, S.B.; Volkin, D.B.; King, C.R.; Plieskatt, J.L. A C-Terminal Pfs48/45 Malaria Transmission-Blocking Vaccine Candidate Produced in the Baculovirus Expression System. Sci. Rep. 2020, 10, 395. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhao, Y.; Liu, F.; Ye, B.; Zhao, Z.; Thongpoon, S.; Roobsoong, W.; Sattabongkot, J.; Cui, L.; Fan, Q.; et al. Evaluation of Plasmodium Vivax HAP2 as a Transmission-Blocking Vaccine Candidate. Vaccine 2020, 38, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Forti, K.; Cagiola, M.; Pellegrini, M.; Anzalone, L.; Di Paolo, A.; Corneli, S.; Severi, G.; De Giuseppe, A. Generation of Recombinant Baculovirus Expressing Atoxic C-Terminal CPA Toxin of Clostridium Perfringens and Production of Specific Antibodies. BMC Biotechnol. 2020, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Z.; Zhang, Z.; Qiao, J.; Rong, R.; Zhang, Y.; Yao, Q.; Li, Z.; Shen, H.; Huang, F.; et al. Characterization of Native-like HIV-1 Gp140 Glycoprotein Expressed in Insect Cells. Vaccine 2019, 37, 1418–1427. [Google Scholar] [CrossRef]
- Huo, Y.; Ma, J.; Zheng, L.; Liu, J.; Yang, Z.; Wang, C.; Zhao, Q. Expression of Chimeric Proteins Based on a Backbone of the GII.4 Norovirus VP1 and Their Application in the Study of a GII.6 Norovirus-Specific Blockade Epitope. Arch. Virol. 2022, 167, 819–827. [Google Scholar] [CrossRef]
- Chen, W.; Kang, T.; Yuan, R.; Shao, C.; Jing, S. Immunogenicity and Protective Potency of Norovirus GII.17 Virus-like Particle-Based Vaccine. Biotechnol. Lett. 2020, 42, 1211–1218. [Google Scholar] [CrossRef]
- Watanabe, S.; Shibahara, T.; Andoh, K.; Hatama, S.; Mase, M. Production of Immunogenic Recombinant L1 Protein of Bovine Papillomavirus Type 9 Causing Teat Papillomatosis. Arch. Virol. 2020, 165, 1441–1444. [Google Scholar] [CrossRef]
- Hassine, I.H.; Gharbi, J.; Hamrita, B.; Almalki, M.A.; Rodríguez, J.F.; Ben M’hadheb, M. Characterization of Coxsackievirus B4 Virus-like Particles VLP Produced by the Recombinant Baculovirus-Insect Cell System Expressing the Major Capsid Protein. Mol. Biol. Rep. 2020, 47, 2835–2843. [Google Scholar] [CrossRef]
- Bernardino, T.C.; Astray, R.M.; Pereira, C.A.; Boldorini, V.L.; Antoniazzi, M.M.; Jared, S.G.S.; Núñez, E.G.F.; Jorge, S.A.C. Production of Rabies VLPs in Insect Cells by Two Monocistronic Baculoviruses Approach. Mol. Biotechnol. 2021, 63, 1068–1080. [Google Scholar] [CrossRef]
- Zhang, N.; Zheng, T.; Chen, Y.; Zhu, H.; Qu, Y.; Zheng, H.; Liu, H.; Liu, Q. Coxsackievirus B5 Virus-like Particle Vaccine Exhibits Greater Immunogenicity and Immunoprotection than Its Inactivated Counterpart in Mice. Vaccine 2021, 39, 5699–5705. [Google Scholar] [CrossRef]
- Utomo, D.I.S.; Hirono, I.; Kato, T.; Park, E.Y. Formation of Virus-Like Particles of the Dengue Virus Serotype 2 Expressed in Silkworm Larvae. Mol. Biotechnol. 2019, 61, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Son, H.S.; Lee, S.W.; Yoon, Y.; Hyeon, J.-Y.; Chung, G.T.; Lee, J.-W.; Yoo, J.S. Efficient Expression of Enterovirus 71 Based on Virus-like Particles Vaccine. PloS ONE 2019, 14, e0210477. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Takahashi, H.; Hamazaki, H.; Miyano-Kurosaki, N.; Matsuura, Y.; Takaku, H. Baculovirus Induces an Innate Immune Response and Confers Protection from Lethal Influenza Virus Infection in Mice. J. Immunol. Baltim. 2003, 171, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Gronowski, A.M.; Hilbert, D.M.; Sheehan, K.C.; Garotta, G.; Schreiber, R.D. Baculovirus Stimulates Antiviral Effects in Mammalian Cells. J. Virol. 1999, 73, 9944–9951. [Google Scholar] [CrossRef] [PubMed]
- Khanefard, N.; Sapavee, S.; Akeprathumchai, S.; Mekvichitsaeng, P.; Poomputsa, K. Production of Neuraminidase Virus Like Particles by Stably Transformed Insect Cells: A Simple Process for NA-Based Influenza Vaccine Development. Mol. Biotechnol. 2022, 64, 1409–1418. [Google Scholar] [CrossRef]
- Cox, M.M. Recombinant protein vaccines produced in insect cells. Vaccine 2012, 30, 1759–17566. [Google Scholar] [CrossRef]
- Schiller, J.T.; Castellsagué, X.; Garland, S.M. A Review of Clinical Trials of Human Papillomavirus Prophylactic Vaccines. Vaccine 2012, 30, F123–F138. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Zhang, H.; Han, Y.; Song, G.; Xu, X. Encapsidating Artificial Human Papillomavirus-16 ME7 Protein in Human Papillomavirus-6b L1/L2 Virus like Particles. Chin. Med. J. (Engl.) 2007, 120, 503–508. [Google Scholar] [CrossRef]
- Fruscalzo, A.; Londero, A.P.; Bertozzi, S.; Lellè, R.J. Second-Generation Prophylactic HPV Vaccines: Current Options and Future Strategies for Vaccines Development. Minerva Med. 2016, 107, 26–38. [Google Scholar]
- Yong, C.Y.; Liew WP, P.; Ong, H.K.; Poh, C.L. Development of virus-like particles-based vaccines against coronaviruses. Biotechnol. Prog. 2022, 38, e3292. [Google Scholar] [CrossRef]
- Boublik, Y.; Di Bonito, P.; Jones, I.M. Eukaryotic Virus Display: Engineering the Major Surface Glycoprotein of the Autographa Californica Nuclear Polyhedrosis Virus (AcNPV) for the Presentation of Foreign Proteins on the Virus Surface. Biotechnol. Nat. Publ. Co. 1995, 13, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.-W.; Chu, K.-B.; Kang, H.-J.; Kim, M.-J.; Eom, G.-D.; Lee, S.-H.; Moon, E.-K.; Quan, F.-S. Mucosal Administration of Recombinant Baculovirus Displaying Toxoplasma Gondii ROP4 Confers Protection Against T. Gondii Challenge Infection in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 735191. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Li, T.; Zhang, S.; Wang, Y.; Hong, M.; Cui, L.; Wang, H.; Zhang, Y.; Chen, T.; Zhu, R.; et al. Baculovirus Display of Varicella-Zoster Virus Glycoprotein E Induces Robust Humoral and Cellular Immune Responses in Mice. Viruses 2022, 14, 1785. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Yang, W.-C.; Chang, Y.-K.; Wang, C.-Y.; Huang, W.-R.; Li, J.-Y.; Chuang, K.-P.; Wu, H.-Y.; Chang, C.-D.; Nielsen, B.L.; et al. Construction of Polycistronic Baculovirus Surface Display Vectors to Express the PCV2 Cap(D41) Protein and Analysis of Its Immunogenicity in Mice and Swine. Vet. Res. 2020, 51, 112. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Miao, Y.; Ke, X.; Tan, Z.; Hu, C.; Li, P.; Wang, T.; Zhang, Y.; Sun, J.; Liu, Y.; et al. Baculovirus Surface Display of Zika Virus Envelope Protein Protects against Virus Challenge in Mouse Model. Virol. Sin. 2020, 35, 637–650. [Google Scholar] [CrossRef]
- Chapple, S.D.J.; Jones, I.M. Non-Polar Distribution of Green Fluorescent Protein on the Surface of Autographa Californica Nucleopolyhedrovirus Using a Heterologous Membrane Anchor. J. Biotechnol. 2002, 95, 269–275. [Google Scholar] [CrossRef]
- Ojala, K.; Koski, J.; Ernst, W.; Grabherr, R.; Jones, I.; Oker-Blom, C. Improved Display of Synthetic IgG-Binding Domains on the Baculovirus Surface. Technol. Cancer Res. Treat. 2004, 3, 77–84. [Google Scholar] [CrossRef]
- Molinari, P.; Crespo, M.I.; Gravisaco, M.J.; Taboga, O.; Morón, G. Baculovirus Capsid Display Potentiates OVA Cytotoxic and Innate Immune Responses. PloS ONE 2011, 6, e24108. [Google Scholar] [CrossRef]
- Kukkonen, S.P.; Airenne, K.J.; Marjomäki, V.; Laitinen, O.H.; Lehtolainen, P.; Kankaanpää, P.; Mähönen, A.J.; Räty, J.K.; Nordlund, H.R.; Oker-Blom, C.; et al. Baculovirus Capsid Display: A Novel Tool for Transduction Imaging. Mol. Ther. J. Am. Soc. Gene Ther. 2003, 8, 853–862. [Google Scholar] [CrossRef]
- Tavarone, E.; Molina, G.N.; Amalfi, S.; Peralta, A.; Molinari, P.; Taboga, O. The Localization of a Heterologous Displayed Antigen in the Baculovirus-Budded Virion Determines the Type and Strength of Induced Adaptive Immune Response. Appl. Microbiol. Biotechnol. 2017, 101, 4175–4184. [Google Scholar] [CrossRef]
- Duisit, G.; Saleun, S.; Douthe, S.; Barsoum, J.; Chadeuf, G.; Moullier, P. Baculovirus Vector Requires Electrostatic Interactions Including Heparan Sulfate for Efficient Gene Transfer in Mammalian Cells. J. Gene Med. 1999, 1, 93–102. [Google Scholar] [CrossRef]
- Makkonen, K.-E.; Turkki, P.; Laakkonen, J.P.; Ylä-Herttuala, S.; Marjomäki, V.; Airenne, K.J. 6-O- and N-Sulfated Syndecan-1 Promotes Baculovirus Binding and Entry into Mammalian Cells. J. Virol. 2013, 87, 11148–11159. [Google Scholar] [CrossRef]
- Hu, L.; Li, Y.; Ning, Y.-J.; Deng, F.; Vlak, J.M.; Hu, Z.; Wang, H.; Wang, M. The Major Hurdle for Effective Baculovirus Transduction into Mammalian Cells Is Passing Early Endosomes. J. Virol. 2019, 93, e00709-19. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Pan, X.; Kormelink, R.; Vlak, J.M. Functional Entry of Baculovirus into Insect and Mammalian Cells Is Dependent on Clathrin-Mediated Endocytosis. J. Virol. 2006, 80, 8830–8833. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Lin, C.-Y.; Chen, G.-Y.; Hu, Y.-C. Baculovirus as a Gene Delivery Vector: Recent Understandings of Molecular Alterations in Transduced Cells and Latest Applications. Biotechnol. Adv. 2011, 29, 618–631. [Google Scholar] [CrossRef] [PubMed]
- van Loo, N.D.; Fortunati, E.; Ehlert, E.; Rabelink, M.; Grosveld, F.; Scholte, B.J. Baculovirus Infection of Nondividing Mammalian Cells: Mechanisms of Entry and Nuclear Transport of Capsids. J. Virol. 2001, 75, 961–970. [Google Scholar] [CrossRef]
- Wu, C.; Wang, S. A PH-Sensitive Heparin-Binding Sequence from Baculovirus Gp64 Protein Is Important for Binding to Mammalian Cells but Not to Sf9 Insect Cells. J. Virol. 2012, 86, 484–491. [Google Scholar] [CrossRef]
- Ohkawa, T.; Volkman, L.E.; Welch, M.D. Actin-Based Motility Drives Baculovirus Transit to the Nucleus and Cell Surface. J. Cell Biol. 2010, 190, 187–195. [Google Scholar] [CrossRef]
- Laakkonen, J.P.; Kaikkonen, M.U.; Ronkainen, P.H.A.; Ihalainen, T.O.; Niskanen, E.A.; Häkkinen, M.; Salminen, M.; Kulomaa, M.S.; Ylä-Herttuala, S.; Airenne, K.J.; et al. Baculovirus-Mediated Immediate-Early Gene Expression and Nuclear Reorganization in Human Cells. Cell. Microbiol. 2008, 10, 667–681. [Google Scholar] [CrossRef]
- Yang, Y.; Lo, S.-L.; Yang, J.; Yang, J.; Goh, S.S.L.; Wu, C.; Feng, S.-S.; Wang, S. Polyethylenimine Coating to Produce Serum-Resistant Baculoviral Vectors for in Vivo Gene Delivery. Biomaterials 2009, 30, 5767–5774. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Park, I.-K.; Jiang, H.-L.; Choi, J.-Y.; Je, Y.-H.; Jin, H.; Kim, H.-W.; Cho, M.-H.; Cho, C.-S. Regulation of Transduction Efficiency by Pegylation of Baculovirus Vector in Vitro and in Vivo. J. Biotechnol. 2006, 125, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Kolangath, S.M.; Basagoudanavar, S.H.; Hosamani, M.; Saravanan, P.; Tamil Selvan, R.P. Baculovirus Mediated Transduction: Analysis of Vesicular Stomatitis Virus Glycoprotein Pseudotyping. Virusdisease 2014, 25, 441–446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borg, J.; Nevsten, P.; Wallenberg, R.; Stenstrom, M.; Cardell, S.; Falkenberg, C.; Holm, C. Amino-Terminal Anchored Surface Display in Insect Cells and Budded Baculovirus Using the Amino-Terminal End of Neuraminidase. J. Biotechnol. 2004, 114, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Liangbo, H.; Li, Y.; Deng, F.; Hu, Z.; Wang, H.; Wang, M. Improving Baculovirus Transduction of Mammalian Cells by Incorporation of Thogotovirus Glycoproteins. Virol. Sin. 2019, 34, 454–466. [Google Scholar] [CrossRef]
- Mäkelä, A.R.; Matilainen, H.; White, D.J.; Ruoslahti, E.; Oker-Blom, C. Enhanced Baculovirus-Mediated Transduction of Human Cancer Cells by Tumor-Homing Peptides. J. Virol. 2006, 80, 6603–6611. [Google Scholar] [CrossRef]
- Räty, J.K.; Airenne, K.J.; Marttila, A.T.; Marjomäki, V.; Hytönen, V.P.; Lehtolainen, P.; Laitinen, O.H.; Mähönen, A.J.; Kulomaa, M.S.; Ylä-Herttuala, S. Enhanced Gene Delivery by Avidin-Displaying Baculovirus. Mol. Ther. 2004, 9, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Kawabata, C.; Matsushita, M.; Sakaguchi, M.; Yoshida, S. Malaria Sporozoite Protein Expression Enhances Baculovirus-Mediated Gene Transfer to Hepatocytes. J. Gene Med. 2016, 18, 75–85. [Google Scholar] [CrossRef]
- Wang, C.-H.; Naik, N.G.; Liao, L.-L.; Wei, S.-C.; Chao, Y.-C. Global Screening of Antiviral Genes That Suppress Baculovirus Transgene Expression in Mammalian Cells. Mol. Ther. Methods Clin. Dev. 2017, 6, 194–206. [Google Scholar] [CrossRef]
- Verma, I.M.; Somia, N. Gene Therapy—Promises, Problems and Prospects. Nature 1997, 389, 239–242. [Google Scholar] [CrossRef]
- Pidre, M.L.; Ferrelli, M.L.; Haase, S.; Romanowski, V. Baculovirus Display: A Novel Tool for Vaccination; IntechOpen: London, UK, 2013. [Google Scholar]
- Shahryari, A.; Saghaeian Jazi, M.; Mohammadi, S.; Razavi Nikoo, H.; Nazari, Z.; Hosseini, E.S.; Burtscher, I.; Mowla, S.J.; Lickert, H. Development and Clinical Translation of Approved Gene Therapy Products for Genetic Disorders. Front. Genet. 2019, 10, 868. [Google Scholar] [CrossRef]
- Vanin, E.F.; Kaloss, M.; Broscius, C.; Nienhuis, A.W. Characterization of Replication-Competent Retroviruses from Nonhuman Primates with Virus-Induced T-Cell Lymphomas and Observations Regarding the Mechanism of Oncogenesis. J. Virol. 1994, 68, 4241–4250. [Google Scholar] [CrossRef] [PubMed]
- Kost, T.A.; Condreay, J.P. Recombinant Baculoviruses as Mammalian Cell Gene-Delivery Vectors; Cell Press: Cambridge, MA, USA, 2002; Volume 20, ISBN 0167-7799. [Google Scholar]
- Kost, T.A.; Condreay, J.P. Innovations—Biotechnology: Baculovirus Vectors as Gene Transfer Vectors for Mammalian Cells: Biosafety Considerations. Appl. Biosaf. 2002, 7, 167–169. [Google Scholar] [CrossRef]
- Schaly, S.; Ghebretatios, M.; Prakash, S. Baculoviruses in Gene Therapy and Personalized Medicine. Biol. Targets Ther. 2021, 15, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Monie, A.; Hung, C.-F.; Roden, R.; Wu, T.-C. Cervarix: A Vaccine for the Prevention of HPV 16, 18-Associated Cervical Cancer. Biol. Targets Ther. 2008, 2, 97–105. [Google Scholar]
- Muzzarelli, R.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef]
- Gao, R.; McCormick, C.J.; Arthur, M.J.P.; Ruddell, R.; Oakley, F.; Smart, D.E.; Murphy, F.R.; Harris, M.P.G.; Mann, D.A. High Efficiency Gene Transfer into Cultured Primary Rat and Human Hepatic Stellate Cells Using Baculovirus Vectors. Liver 2002, 22, 15–22. [Google Scholar] [CrossRef]
- Hofmann, C.; Sandig, V.; Jennings, G.; Rudolph, M.; Schlag, P.; Strauss, M. Efficient Gene Transfer into Human Hepatocytes by Baculovirus Vectors. Proc. Natl. Acad. Sci. USA 1995, 92, 10099–10103. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Yao, K.; Wu, T.-Y. Baculovirus as an Expression and/or Delivery Vehicle for Vaccine Antigens. Expert Rev. Vaccines 2008, 7, 363–371. [Google Scholar] [CrossRef]
- Airenne, K.J.; Hu, Y.-C.; Kost, T.A.; Smith, R.H.; Kotin, R.M.; Ono, C.; Matsuura, Y.; Wang, S.; Ylä-Herttuala, S. Baculovirus: An Insect-Derived Vector for Diverse Gene Transfer Applications. Mol. Ther. 2013, 21, 739–749. [Google Scholar] [CrossRef]
- Kaikkonen, M.U.; Ylä-Herttuala, S.; Airenne, K.J. How to Avoid Complement Attack in Baculovirus-Mediated Gene Delivery. J. Invertebr. Pathol. 2011, 107, S71–S79. [Google Scholar] [CrossRef]
- Blissard, G.W.; Wenz, J.R. Baculovirus Gp64 Envelope Glycoprotein Is Sufficient to Mediate PH-Dependent Membrane Fusion. J. Virol. 1992, 66, 6829–6835. [Google Scholar] [CrossRef] [PubMed]
- Haase, S.; Ferrelli, L.; Pidre, M.L.; Romanowski, V. Genetic Engineering of Baculoviruses; IntechOpen: London, UK, 2013. [Google Scholar]
- Via, S.T.; zu Altenschildesche, G.M.; Doerfler, W. Autographa Californica Nuclear Polyhedrosis Virus (AcNPV) DNA Does Not Persist in Mass Cultures of Mammalian Cells. Virology 1983, 125, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Boyce, F.M.; Bucher, N.L.R. Baculovirus-Mediated Gene Transfer into Mammalian Cells. Proc. Natl. Acad. Sci. USA 1996, 93, 2348–2352. [Google Scholar] [CrossRef] [PubMed]
- Mehalko, J.L.; Esposito, D. Engineering the Transposition-Based Baculovirus Expression Vector System for Higher Efficiency Protein Production from Insect Cells. J. Biotechnol. 2016, 238, 1–8. [Google Scholar] [CrossRef]
- Berger, I.; Garzoni, F.; Chaillet, M.; Haffke, M.; Gupta, K.; Aubert, A. The MultiBac Protein Complex Production Platform at the EMBL. J. Vis. Exp. 2013, 50159. [Google Scholar] [CrossRef]
- Fabre, M.; Arrías, P.; Massón, T.; Pidre, M.; Romanowski, V. Baculovirus-Derived Vectors for Immunization and Therapeutic Applications. In Emerging and Reemerging Viral Pathogens; Ennaji, M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 197–224. [Google Scholar]
- Pijlman, G.P.; Van Den Born, E.; Martens, D.E.; Vlak, J.M. Autographa Californica Baculoviruses with Large Genomic Deletions Are Rapidly Generated in Infected Insect Cells. Virology 2001, 283, 132–138. [Google Scholar] [CrossRef]
- Pan, Y.; Fang, L.; Fan, H.; Luo, R.; Zhao, Q.; Chen, H.; Xiao, S. Antitumor Effects of a Recombinant Pseudotype Baculovirus Expressing Apoptin in Vitro and in Vivo. Int. J. Cancer 2010, 126, 2741–2751. [Google Scholar]
- Bak, X.Y.; Lam, D.H.; Yang, J.; Ye, K.; Wei, E.L.X.; Lim, S.K.; Wang, S. Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells as Cellular Delivery Vehicles for Prodrug Gene Therapy of Glioblastoma. Hum. Gene Ther. 2011, 22, 1365–1377. [Google Scholar] [CrossRef]
- Luo, W.-Y.; Lin, S.-Y.; Lo, K.-W.; Lu, C.-H.; Hung, C.-L.; Chen, C.-Y.; Chang, C.-C.; Hu, Y.-C. Adaptive immune responses elicited by baculovirus and impacts on subsequent transgene expression in vivo. J. Virol. 2013, 87, 4965–4973. [Google Scholar] [CrossRef]
- Ang, W.X.; Zhao, Y.; Kwang, T.; Wu, C.; Chen, C.; Toh, H.C.; Mahendran, R.; Esuvaranathan, K.; Wang, S. Local Immune Stimulation by Intravesical Instillation of Baculovirus to Enable Bladder Cancer Therapy. Sci. Rep. 2016, 6, 27455. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.; Chen, X.; Huang, R.; Wang, S.; Dong, P. Human Bone Marrow Mesenchymal Stem Cells Functionalized by Hybrid Baculovirus-Adeno-Associated Viral Vectors for Targeting Hypopharyngeal Carcinoma. Stem. Cells Dev. 2019, 28, 543–553. [Google Scholar] [CrossRef]
- Wang, J.; Kong, D.; Zhu, L.; Wang, S.; Sun, X. Human Bone Marrow Mesenchymal Stem Cells Modified Hybrid Baculovirus-Adeno-Associated Viral Vectors Targeting 131i Therapy of Hypopharyngeal Carcinoma. Hum. Gene Ther. 2020, 31, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vega, M.A.; Vargas-Jerónimo, R.Y.; Montiel-Martínez, A.G.; Munõz-Fuentes, R.M.; Zamorano-Carrillo, A.; Pastor, A.R.; Palomares, L.A. Delivery of Glutamine Synthetase Gene by Baculovirus Vectors: A Proof of Concept for the Treatment of Acute Hyperammonemia. Gene Ther. 2015, 22, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Binsalamah, Z.M.; Khan, A.A.; Abbasia, S.; Elias, C.B.; Shum-Tim, D.; Prakash, S. A Nanobiohybrid Complex of Recombinant Baculovirus and Tat/DNA Nanoparticles for Delivery of Ang-1 Transgene in Myocardial Infarction Therapy. Biomaterials 2011, 32, 8304–8318. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Nayan, M.; Khan, A.A.; Shum-Tim, D.; Prakash, S. Angiopoietin-1-Expressing Adipose Stem Cells Genetically Modified with Baculovirus Nanocomplex: Investigation in Rat Heart with Acute Infarction. Int. J. Nanomed. 2012, 7, 663–682. [Google Scholar]
- Paul, A.; Elias, C.B.; Shum-Tim, D.; Prakash, S. Bioactive baculovirus nanohybrids for stent based rapid vascular re-endothelialization. Sci. Rep. 2013, 3, 2366. [Google Scholar] [CrossRef]
- Heikura, T.; Nieminen, T.; Roschier, M.; Karvinen, H.; Kaikkonen, M.; Mähönen, A.; Laitinen, O.; Lesch, H.; Airenne, K.; Rissanen, T.; et al. Pro-Opiomelanocortin Gene Delivery Suppresses the Growth of Established Lewis Lung Carcinoma through a Melanocortin-1 Receptor-Independent Pathway. J. Gene Med. 2012, 14, 44–53. [Google Scholar]
- Yeh, T.S.; Dean Fang, Y.H.; Lu, C.H.; Chiu, S.C.; Yeh, C.L.; Yen, T.C.; Parfyonova, Y.; Hu, Y.C. Baculovirus-Transduced, VEGF-Expressing Adipose-Derived Stem Cell Sheet for the Treatment of Myocardium Infarction. Biomaterials 2014, 35, 174–184. [Google Scholar] [CrossRef]
- Makarevich, P.I.; Boldyreva, M.A.; Gluhanyuk, E.V.; Efimenko, A.Y.; Dergilev, K.V.; Shevchenko, E.K.; Sharonov, G.V.; Gallinger, J.O.; Rodina, P.A.; Sarkisyan, S.S.; et al. Enhanced Angiogenesis in Ischemic Skeletal Muscle after Transplantation of Cell Sheets from Baculovirus-Transduced Adipose-Derived Stromal Cells Expressing Vegf165. Stem. Cell Res. Ther. 2015, 6, 204. [Google Scholar] [CrossRef]
- Hitchman, E.; Hitchman, R.B.; King, L.A. BacMam Delivery of a Protective Gene to Reduce Renal Ischemia-Reperfusion Injury. Hum. Gene Ther. 2017, 28, 747–756. [Google Scholar] [CrossRef]
- Giménez, C.S.; Castillo, M.G.; Simonin, J.A.; Núñez Pedrozo, C.N.; Pascuali, N.; Rosario Bauzá, M.; Locatelli, P.; López, A.E.; Belaich, M.N.; Mendiz, A.O.; et al. Effect of Intramuscular Baculovirus Encoding Mutant Hypoxia-Inducible Factor 1-Alpha on Neovasculogenesis and Ischemic Muscle Protection in Rabbits with Peripheral Arterial Disease. Cytotherapy 2020, 22, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lin, K.J.; Kao, C.Y.; Chen, M.C.; Lo, W.H.; Yen, T.C.; Chang, Y.H.; Hu, Y.C. The Role of Adipose-Derived Stem Cells Engineered with the Persistently Expressing Hybrid Baculovirus in the Healing of Massive Bone Defects. Biomaterials 2011, 32, 6505–6514. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chang, Y.H.; Sung, L.Y.; Chen, C.L.; Lin, S.Y.; Li, K.C.; Yen, T.C.; Lin, K.J.; Hu, Y.C. Long-Term Tracking of Segmental Bone Healing Mediated by Genetically Engineered Adipose-Derived Stem Cells: Focuses on Bone Remodeling and Potential Side Effects. Tissue Eng. Part A 2014, 20, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Wang, Y.H.; Li, K.C.; Sung, L.Y.; Yeh, C.L.; Lin, K.J.; Yen, T.C.; Chang, Y.H.; Hu, Y.C. Healing of Massive Segmental Femoral Bone Defects in Minipigs by Allogenic ASCs Engineered with FLPo/Frt-Based Baculovirus Vectors. Biomaterials 2015, 50, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C. Bone Marrow Mesenchymal Stem Cells Expressing Baculovirus-Engineered Bone Morphogenetic Protein-7 Enhance Rabbit Posterolateral Fusion. Int. J. Mol. Sci. 2016, 17, 1073. [Google Scholar] [CrossRef]
- Liao, J.C. Cell Therapy Using Bone Marrow-Derived Stem Cell Overexpressing BMP-7 for Degenerative Discs in a Rat Tail Disc Model. Int. J. Mol. Sci. 2016, 17, 147. [Google Scholar] [CrossRef]
- Hong, S.S.; Marotte, H.; Courbon, G.; Firestein, G.S.; Boulanger, P.; Miossec, P. PUMA Gene Delivery to Synoviocytes Reduces Inflammation and Degeneration of Arthritic Joints. Nat. Commun. 2017, 8, 146. [Google Scholar] [CrossRef]
- Nicholson, L.J.; Philippe, M.; Paine, A.J.; Mann, D.A.; Dolphin, C.T. RNA Interference Mediated in Human Primary Cells via Recombinant Baculoviral Vectors. Mol. Ther. 2005, 11, 638–644. [Google Scholar] [CrossRef]
- Ong, S.T.; Li, F.; Du, J.; Tan, Y.W.; Wang, S. Hybrid Cytomegalovirus Enhancer–H1 Promoter-Based Plasmid and Baculovirus Vectors Mediate Effective RNA Interference. Hum. Gene Ther. 2005, 16, 1404–1412. [Google Scholar] [CrossRef]
- Gottardo, M.F.; Pidre, M.L.; Zuccato, C.; Asad, A.S.; Imsen, M.; Jaita, G.; Candolfi, M.; Romanowski, V.; Seilicovich, A. Baculovirus-Based Gene Silencing of Humanin for the Treatment of Pituitary Tumors. Apoptosis 2018, 23, 143–151. [Google Scholar] [CrossRef]
- Marvaldi, C.; Martin, D.; Conte, J.G.; Gottardo, M.F.; Pidre, M.L.; Imsen, M.; Irizarri, M.; Manuel, S.L.; Duncan, F.E.; Romanowski, V.; et al. Mitochondrial Humanin Peptide Acts as a Cytoprotective Factor in Granulosa Cell Survival. Reproduction 2021, 161, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Tseng, Y.W.; Wu, J.C.; Chen, G.Y.; Lin, K.C.; Hwang, S.M.; Hu, Y.C. Suppression of Hepatocellular Carcinoma by Baculovirus-Mediated Expression of Long Non-Coding RNA PTENP1 and MicroRNA Regulation. Biomaterials 2015, 44, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Li, K.C.; Chang, Y.H.; Yeh, C.L.; Hu, Y.C. Healing of Osteoporotic Bone Defects by Baculovirus-Engineered Bone Marrow-Derived MSCs Expressing MicroRNA Sponges. Biomaterials 2016, 74, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Li, K.C.; Chang, Y.H.; Hsu, M.N.; Lo, S.C.; Li, W.H.; Hu, Y.C. Baculovirus-Mediated MiR-214 Knockdown Shifts Osteoporotic ASCs Differentiation and Improves Osteoporotic Bone Defects Repair. Sci. Rep. 2017, 7, 16225. [Google Scholar] [CrossRef] [PubMed]
- Hindriksen, S.; Bramer, A.J.; Truong, M.A.; Vromans, M.J.M.; Post, J.B.; Verlaan-Klink, I.; Snippert, H.J.; Lens, S.M.A.; Hadders, M.A. Baculoviral Delivery of CRISPR/Cas9 Facilitates Efficient Genome Editing in Human Cells. PLoS ONE 2017, 12, e0179514. [Google Scholar] [CrossRef]
- Aulicino, F.; Pelosse, M.; Toelzer, C.; Capin, J.; Ilegems, E.; Meysami, P.; Rollarson, R.; Berggren, P.-O.; Dillingham, M.S.; Schaffitzel, C.; et al. Highly Efficient CRISPR-Mediated Large DNA Docking and Multiplexed Prime Editing Using a Single Baculovirus. Nucleic Acids Res. 2022, 50, 7783–7799. [Google Scholar] [CrossRef]
- Hsu, M.N.; Liao, H.T.; Truong, V.A.; Huang, K.L.; Yu, F.J.; Chen, H.H.; Nguyen, T.K.N.; Makarevich, P.; Parfyonova, Y.; Hu, Y.C. CRISPR-Based Activation of Endogenous Neurotrophic Genes in Adipose Stem Cell Sheets to Stimulate Peripheral Nerve Regeneration. Theranostics 2019, 9, 6099–6111. [Google Scholar] [CrossRef]
- Sun, W.; Wang, J.; Hu, Q.; Zhou, X.; Khademhosseini, A.; Gu, Z. CRISPR-Cas12a Delivery by DNA-Mediated Bioresponsive Editing for Cholesterol Regulation. Sci. Adv. 2020, 6, eaba2983. [Google Scholar] [CrossRef]
- Mabashi-Asazuma, H.; Jarvis, D.L. CRISPR-Cas9 Vectors for Genome Editing and Host Engineering in the Baculovirus–Insect Cell System. Proc. Natl. Acad. Sci. USA 2017, 114, 9068–9073. [Google Scholar] [CrossRef]
- Pazmiño-Ibarra, V.; Mengual-Martí, A.; Targovnik, A.M.; Herrero, S. Improvement of Baculovirus as Protein Expression Vector and as Biopesticide by CRISPR/Cas9 Editing. Biotechnol. Bioeng. 2019, 116, 2823–2833. [Google Scholar] [CrossRef]
- Keeler, A.M.; Flotte, T.R. Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here? Annu. Rev. Virol. 2019, 6, 601–621. [Google Scholar] [CrossRef] [PubMed]
- Keeler, A.M.; ElMallah, M.K.; Flotte, T.R. Gene Therapy 2017: Progress and Future Directions; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2017; Volume 10. [Google Scholar]
- Targovnik, A.M.; Simonin, J.A.; Mc Callum, G.J.; Smith, I.; Cuccovia Warlet, F.U.; Nugnes, M.V.; Miranda, M.V.; Belaich, M.N. Solutions against Emerging Infectious and Noninfectious Human Diseases through the Application of Baculovirus Technologies. Appl. Microbiol. Biotechnol. 2021, 105, 8195–8226. [Google Scholar] [CrossRef] [PubMed]
- Galibert, L.; Merten, O.W. Latest Developments in the Large-Scale Production of Adeno-Associated Virus Vectors in Insect Cells toward the Treatment of Neuromuscular Diseases. J. Inver.-Tebr. Pathol. 2011, 107, S80–S93. [Google Scholar] [CrossRef] [PubMed]
- Aponte-Ubillus, J.J.; Barajas, D.; Peltier, J.; Bardliving, C.; Shamlou, P.; Gold, D. Molecular Design for Recombinant Adeno-Associated Virus (RAAV) Vector Production. Appl. Microbiol. Biotechnol. 2018, 102, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Urabe, M.; Ding, C.; Kotin, R.M. Insect Cells as a Factory to Produce Adeno-Associated Virus Type 2 Vectors. Hum. Gene Ther. 2002, 13, 1935–1943. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, L.; Geng, H.; Yang, T.; Han, Z.; He, X.; Lin, K.; Xu, F. A Recombinant Baculovirus Efficiently Generates Recombinant Adeno-Associated Virus Vectors in Cultured Insect Cells and Larvae. Mol. Ther. Methods Clin. Dev. 2018, 10, 38–47. [Google Scholar] [CrossRef]
- Wu, Y.; Mei, T.; Jiang, L.; Han, Z.; Dong, R.; Yang, T.; Xu, F. Development of Versatile and Flexible Sf9 Packaging Cell Line-Dependent Onebac System for Large-Scale Recombinant Adeno-Associated Virus Production. Hum. Gene Ther. Methods 2019, 30, 172–183. [Google Scholar] [CrossRef]
- Mietzsch, M.; Casteleyn, V.; Weger, S.; Zolotukhin, S.; Heilbronn, R. OneBac 2.0: Sf9 Cell Lines for Production of AAV5 Vectors with Enhanced Infectivity and Minimal Encapsidation of Foreign DNA. Hum. Gene Ther. 2015, 26, 688–697. [Google Scholar] [CrossRef]
- Joshi, P.R.H.; Cervera, L.; Ahmed, I.; Kondratov, O.; Zolotukhin, S.; Schrag, J.; Chahal, P.S.; Kamen, A.A. Achieving High-Yield Production of Functional AAV5 Gene Delivery Vectors via Fedbatch in an Insect Cell-One Baculovirus System. Mol. Ther. Methods Clin. Dev. 2019, 13, 279–289. [Google Scholar] [CrossRef]
- Jarvis, D.L.; Fleming, J.-A.G.W.; Kovacs, G.R.; Summers, M.D.; Guarino, L.A. Use of Early Baculovirus Promoters for Continuous Expression and Efficient Processing of Foreign Gene Products in Stably Transformed Lepidopteran Cells. Nat. Biotechnol. 1990, 8, 950–955. [Google Scholar] [CrossRef]
- López-Vidal, J.; Gómez-Sebastián, S.; Sánchez-Ramos, I.; Escribano, J.M. Characterization of a Trichoplusia Ni Hexamerin-Derived Promoter in the AcMNPV Baculovirus Vector. J. Biotechnol. 2013, 165, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Sprick, G.; Weidner, T.; Salzig, D.; Czermak, P. Baculovirus-Induced Recombinant Protein Expression in Human Mesenchymal Stromal Stem Cells: A Promoter Study. New Biotechnol. 2017, 39, 161–166. [Google Scholar] [CrossRef]
- Gwak, W.-S.; Kim, H.-S.; Bae, J.-S.; Kim, T.-H.; Bae, S.-M.; Woo, S.-D. Development of a Novel Enhanced Baculovirus Expression Vector via Promoter Combination. J. Asia-Pac. Entomol. 2020, 23, 909–914. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Tsai, C.-T.; Chang, Y.-J.; Huang, J.-H. Enhancement and Prolongation of Baculovirus-Mediated Expression in Mammalian Cells: Focuses on Strategic Infection and Feeding. Biotechnol. Prog. 2003, 19, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Hoare, J.; Waddington, S.; Thomas, H.C.; Coutelle, C.; McGarvey, M.J. Complement Inhibition Rescued Mice Allowing Observation of Transgene Expression Following Intraportal Delivery of Baculovirus in Mice. J. Gene Med. 2005, 7, 325–333. [Google Scholar] [CrossRef]
- Hüser, A.; Rudolph, M.; Hofmann, C. Incorporation of Decay-Accelerating Factor into the Baculovirus Envelope Generates Complement-Resistant Gene Transfer Vectors. Nat. Biotechnol. 2001, 19, 451–455. [Google Scholar] [CrossRef]
- Kaikkonen, M.U.; Maatta, A.I.; Ylä-Herttuala, S.; Airenne, K.J. Screening of Complement Inhibitors: Shielded Baculoviruses Increase the Safety and Efficacy of Gene Delivery. Mol. Ther. 2010, 18, 987–992. [Google Scholar] [CrossRef]
- Kawai, Y.; Kawabata, C.; Sakaguchi, M.; Tamura, T. Protection of Baculovirus Vectors Expressing Complement Regulatory Proteins against Serum Complement Attack. Biol. Pharm. Bull. 2018, 41, 1600–1605. [Google Scholar] [CrossRef]
- Pieroni, L.; Maione, D.; La Monica, N. In Vivo Gene Transfer in Mouse Skeletal Muscle Mediated by Baculovirus Vectors. Hum. Gene Ther. 2001, 12, 871–881. [Google Scholar] [CrossRef]
- Pieroni, L.; La Monica, N. Towards the Use of Baculovirus as a Gene Therapy Vector. Curr. Opin. Mol. Ther. 2001, 3, 464–467. [Google Scholar]
- Sung, L.-Y.; Chen, C.-L.; Lin, S.-Y.; Li, K.-C.; Yeh, C.-L.; Chen, G.-Y.; Lin, C.-Y.; Hu, Y.-C. Efficient Gene Delivery into Cell Lines and Stem Cells Using Baculovirus. Nat. Protoc. 2014, 9, 1882–1899. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.H.; Hwang, S.M.; Chuang, C.K.; Chen, C.Y.; Hu, Y.C. Development of a Hybrid Baculoviral Vector for Sustained Transgene Expression. Mol. Ther. 2009, 17, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Sung, L.Y.; Chen, C.L.; Lin, S.Y.; Hwang, S.M.; Lu, C.H.; Li, K.C.; Lan, A.S.; Hu, Y.C. Enhanced and Prolonged Baculovirus-Mediated Expression by Incorporating Recombinase System and in Cis Elements: A Comparative Study. Nucleic Acids Res. 2013, 41, e139. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, M.; Xiao, G.; Wang, X.; Hou, D.; Pan, K.; Liu, S.; Li, J.; Wang, J.; Arif, B.M.; et al. Construction and Rescue of a Functional Synthetic Baculovirus. ACS Synth. Biol. 2017, 6, 1393–1402. [Google Scholar] [CrossRef]
- Aulicino, F.; Capin, J.; Berger, I. Synthetic Virus-Derived Nanosystems (Svns) for Delivery and Precision Docking of Large Multifunctional DNA Circuitry in Mammalian Cells. Pharmaceutics 2020, 12, 759. [Google Scholar] [CrossRef]
- Kumar, N.; Pandey, N.; Halder, A. Preventive, Diagnostic and Therapeutic Application of Baculovirus Expression Vector System. In Trends in Insect Molecular Biology and Biotechnology; Kumar, D., Gong, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 163–191. [Google Scholar]
- Rawat, K.; Kumari, P.; Saha, L. COVID-19 Vaccine: A Recent Update in Pipeline Vaccines, Their Design and Development Strategies. Eur. J. Pharmacol. 2021, 892, 173751. [Google Scholar] [CrossRef] [PubMed]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene Therapy Clinical Trials Worldwide to 2017: An Update. J. Gene Med. 2018, 20, e3015. [Google Scholar] [CrossRef]
- Paavonen, J.; Jenkins, D.; Bosch, F.X.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.-N.; Apter, D.L.; Kitchener, H.C.; Castellsague, X.; et al. Efficacy of a Prophylactic Adjuvanted Bivalent L1 Virus-like-Particle Vaccine against Infection with Human Papillomavirus Types 16 and 18 in Young Women: An Interim Analysis of a Phase III Double-Blind, Randomised Controlled Trial. Lancet Lond. Engl. 2007, 369, 2161–2170. [Google Scholar] [CrossRef]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.A.; Goldenthal, K.L.; Muse, D.; Cox, M.M.J. Randomized Comparison of Immunogenicity and Safety of Quadrivalent Recombinant Versus Inactivated Influenza Vaccine in Healthy Adults 18–49 Years of Age. J. Infect. Dis. 2017, 216, 1219–1226. [Google Scholar] [CrossRef]
- Guebre-Xabier, M.; Patel, N.; Tian, J.-H.; Zhou, B.; Maciejewski, S.; Lam, K.; Portnoff, A.D.; Massare, M.J.; Frieman, M.B.; Piedra, P.A.; et al. NVX-CoV2373 Vaccine Protects Cynomolgus Macaque Upper and Lower Airways against SARS-CoV-2 Challenge. Vaccine 2020, 38, 7892–7896. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y.; et al. A Vaccine Targeting the RBD of the S Protein of SARS-CoV-2 Induces Protective Immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Pavot, V.; Berry, C.; Kishko, M.; Anosova, N.G.; Huang, D.; Tibbitts, T.; Raillard, A.; Gautheron, S.; Gutzeit, C.; Koutsoukos, M.; et al. Protein-Based SARS-CoV-2 Spike Vaccine Booster Increases Cross-Neutralization against SARS-CoV-2 Variants of Concern in Non-Human Primates. Nat. Commun. 2022, 13, 1699. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Liu, Y.; Flyer, D.; Massare, M.J.; Zhou, B.; Patel, N.; Ellingsworth, L.; Lewis, M.; Cummings, J.F.; Glenn, G. Novel Hemagglutinin Nanoparticle Influenza Vaccine with Matrix-MTM Adjuvant Induces Hemagglutination Inhibition, Neutralizing, and Protective Responses in Ferrets against Homologous and Drifted A(H3N2) Subtypes. Vaccine 2017, 35, 5366–5372. [Google Scholar] [CrossRef] [PubMed]
- Massare, M.J.; Patel, N.; Zhou, B.; Maciejewski, S.; Flores, R.; Guebre-Xabier, M.; Tian, J.-H.; Portnoff, A.D.; Fries, L.; Shinde, V.; et al. Combination Respiratory Vaccine Containing Recombinant SARS-CoV-2 Spike and Quadrivalent Seasonal Influenza Hemagglutinin Nanoparticles with Matrix-M Adjuvant. bioRxiv 2021. [Google Scholar]
- van Oirschot, J.T. Vaccinology of Classical Swine Fever: From Lab to Field. Vet. Microbiol. 2003, 96, 367–384. [Google Scholar] [CrossRef]
- Venegas-Vargas, C.; Taylor, L.P.; Foss, D.L.; Godbee, T.K.; Philip, R.; Bandrick, M. Cellular and Humoral Immunity Following Vaccination with Two Different PCV2 Vaccines (Containing PCV2a or PCV2a/PCV2b) and Challenge with Virulent PCV2d. Vaccine 2021, 39, 5615–5625. [Google Scholar] [CrossRef]
- Depner, K.R.; Bouma, A.; Koenen, F.; Klinkenberg, D.; Lange, E.; de Smit, H.; Vanderhallen, H. Classical Swine Fever (CSF) Marker Vaccine. Trial II. Challenge Study in Pregnant Sows. Vet. Microbiol. 2001, 83, 107–120. [Google Scholar] [CrossRef]
- Fachinger, V.; Bischoff, R.; Jedidia, S.B.; Saalmüller, A.; Elbers, K. The Effect of Vaccination against Porcine Circovirus Type 2 in Pigs Suffering from Porcine Respiratory Disease Complex. Vaccine 2008, 26, 1488–1499. [Google Scholar] [CrossRef]
- Segalés, J. Best Practice and Future Challenges for Vaccination against Porcine Circovirus Type 2. Expert Rev. Vaccines 2015, 14, 473–487. [Google Scholar] [CrossRef]
- Blanchard, P.; Mahé, D.; Cariolet, R.; Keranflec’h, A.; Baudouard, M.A.; Cordioli, P.; Albina, E.; Jestin, A. Protection of Swine against Post-Weaning Multisystemic Wasting Syndrome (PMWS) by Porcine Circovirus Type 2 (PCV2) proteins. Vaccine 2003, 21, 4565–4575. [Google Scholar] [CrossRef]
- Yasukawa, K.; Saito, S.; Kubo, T.; Shibasaki, Y.; Yamaoka, K.; Hachimura, H.; Kuyama, T.; Amimoto, A.; Kumata, T.; Kitahara, Y.; et al. Low-Dose Recombinant Canine Interferon-Gamma for Treatment of Canine Atopic Dermatitis: An Open Randomized Comparative Trial of Two Doses. Vet. Dermatol. 2010, 21, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Sakurai, T.; Yanai, A. Homogeneous Production of Feline Interferon in Silkworm by Replacing Single Amino Acid Code in Signal Peptide Region in Recombinant Baculovirus and Characterization of the Product. J. Vet. Med. Sci. 1993, 55, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Steinhagen-Thiessen, E.; Stroes, E.; Soran, H.; Johnson, C.; Moulin, P.; Iotti, G.; Zibellini, M.; Ossenkoppele, B.; Dippel, M.; Averna, M.R.; et al. The Role of Registries in Rare Genetic Lipid Disorders: Review and Introduction of the First Global Registry in Lipoprotein Lipase Deficiency. Atherosclerosis 2017, 262, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Beam, C.A.; MacCallum, C.; Herold, K.C.; Wherrett, D.K.; Palmer, J.; Ludvigsson, J. Type 1 Diabetes TrialNet Study Group GAD Vaccine Reduces Insulin Loss in Recently Diagnosed Type 1 Diabetes: Findings from a Bayesian Meta-Analysis. Diabetologia 2017, 60, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA-Approved Therapeutic Cancer Vaccine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [PubMed]

| Table | Commercial Name | Manufacturer | Target (Organism/Disease) | Composition | Clinical Stage | Year of Approval */Phase III Trial | Reference |
|---|---|---|---|---|---|---|---|
| Human vaccines | Cervarix® | GlaxoSmithKline | human papillomaviruses 16 and 18 | VLPs of L1 proteins from HPV 16 and 18 | Approved | 2007 * | [155] |
| Flublok® Quadrivalent | Sanofi Pasteur | influenza virus | HA from 4 different strains of influenza A and B | Approved | 2016 * | [156] | |
| Nuvaxoid™/Covovax™ | Novavax | SARS-CoV-2 | S protein | Approved | 2022 * | [157] | |
| N/A | West China Hospital of Sichuan University | SARS-CoV-2 | S RBD (residues 319-545) | Phase III | 2021 | [158] | |
| N/A | Sanofi Pasteur/GlaxoSmithKline | SARS-CoV-2 | S (pre-fusion state) without its transmembrane domain | Phase III | 2021 | [159] | |
| NanoFlu™ | Novavax | Influenza virus | HA, NA and M1 proteins | Phase III | 2019 | [160] | |
| N/A | Novavax | Influenza viruses and SARS-CoV-2 | HA and Spike (recombinant) nanoparticles | Phase I/II | N/A | [161] | |
| Veterinary products | Porcilis Pesti® | MSD Animal Health | Pestivirus C (CSFV) (Classical swine fever) | E2 protein | Approved (withdrawn from UE market) | 2009 * | [162] |
| Circumvent® PCV-M G2 | MSD Animal Health | Porcine circovirus 2, Mycoplasma hyopneumoniae | ORF2, non replicative antigen of Mycoplasma hyopneumoniae | Approved | 2013 * | [163] | |
| Bayovac® CSF-E2 | Bayer AG/Pfizer Animal Health | Pestivirus C (CSFV) (Classical swine fever) | E2 protein | Approved | 2000 * | [164] | |
| Ingelvac CircoFLEX® | Boehringer Ingelheim | Porcine circovirus 2 | ORF2 | Approved | 2008 * | [165] | |
| Circumvent® PCV A | Merck Animal Health | Porcine circovirus 2 | ORF2 | Approved | 2005 * | [166] | |
| Porcilis PCV® A | MSD Animal Health | Porcine circovirus 2 | ORF2 | Approved | 2005 * | [167] | |
| INTERDOG™ | TORAY Industries, Inc. | Treatment of atopic dermatitis in dogs | Canine gamma interferon | Approved in Japan | 2005 * | [168] | |
| Virbagen® Omega | Virbac | Treatment of infections with feline leukemia virus (FeLV) and/or feline immunodeficiency virus (FIV) | Feline omega interferon | Approved | 2001 * | [169] | |
| Gene Therapy | Glybera® | uniQure | Lipoprotein lipase deficiency | Adeno-associated vector with LPL gene | Approved (withdrawn from market in 2017 due to high cost) | 2012 * | [170] |
| Others | Diamyd® | Diamyd | Type I diabetes | GAD65 | Phase III | 2021 | [171] |
| Provenge® | Dendreon Pharmaceuticals | Prostate cancer | PAP-GM-CSF fusion protein | Approved | 2010 * | [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pidre, M.L.; Arrías, P.N.; Amorós Morales, L.C.; Romanowski, V. The Magic Staff: A Comprehensive Overview of Baculovirus-Based Technologies Applied to Human and Animal Health. Viruses 2023, 15, 80. https://doi.org/10.3390/v15010080
Pidre ML, Arrías PN, Amorós Morales LC, Romanowski V. The Magic Staff: A Comprehensive Overview of Baculovirus-Based Technologies Applied to Human and Animal Health. Viruses. 2023; 15(1):80. https://doi.org/10.3390/v15010080
Chicago/Turabian StylePidre, Matías L., Paula N. Arrías, Leslie C. Amorós Morales, and Víctor Romanowski. 2023. "The Magic Staff: A Comprehensive Overview of Baculovirus-Based Technologies Applied to Human and Animal Health" Viruses 15, no. 1: 80. https://doi.org/10.3390/v15010080
APA StylePidre, M. L., Arrías, P. N., Amorós Morales, L. C., & Romanowski, V. (2023). The Magic Staff: A Comprehensive Overview of Baculovirus-Based Technologies Applied to Human and Animal Health. Viruses, 15(1), 80. https://doi.org/10.3390/v15010080






