Development of an ELISA Assay for the Determination of SARS-CoV-2 Protein Subunit Vaccine Antigen Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ATP
2.3. mAb Screening
2.3.1. mAb Preparation
2.3.2. RBD Binding Activity and Neutralization Assay
2.3.3. Challenge Trials in Hamsters
2.3.4. Western Blot Analysis
2.3.5. Epitope Mapping
2.4. Polyclonal Antibody Preparation
2.5. National Standard
2.6. Establishment and Optimization
2.7. ELISA Validation
2.7.1. Range
2.7.2. Accuracy and Precision
2.8. ELISA Suitability Evaluation
2.9. Statistical Analysis
3. Results
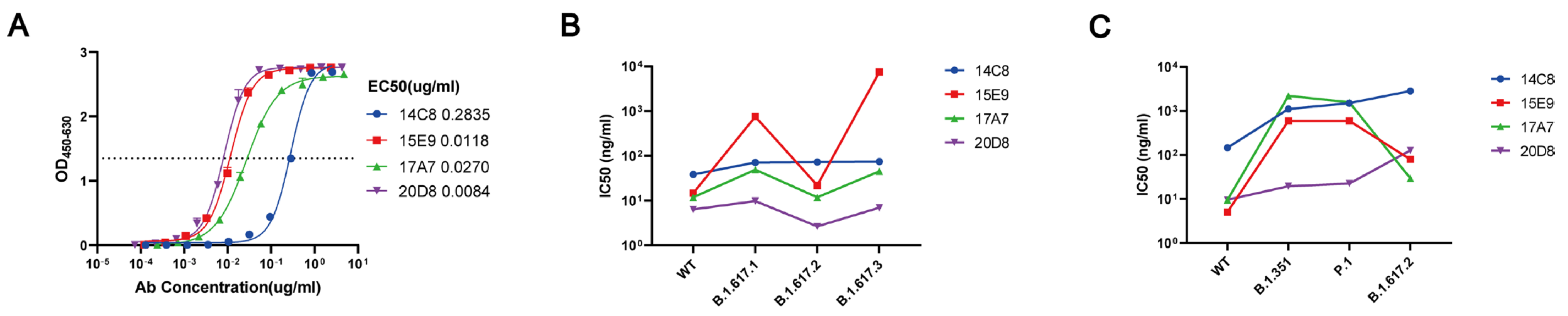
3.1. mAb 20D8 Exhibits Good Neutralizing Capacity against WT and Variant Strains of SARS-CoV-2
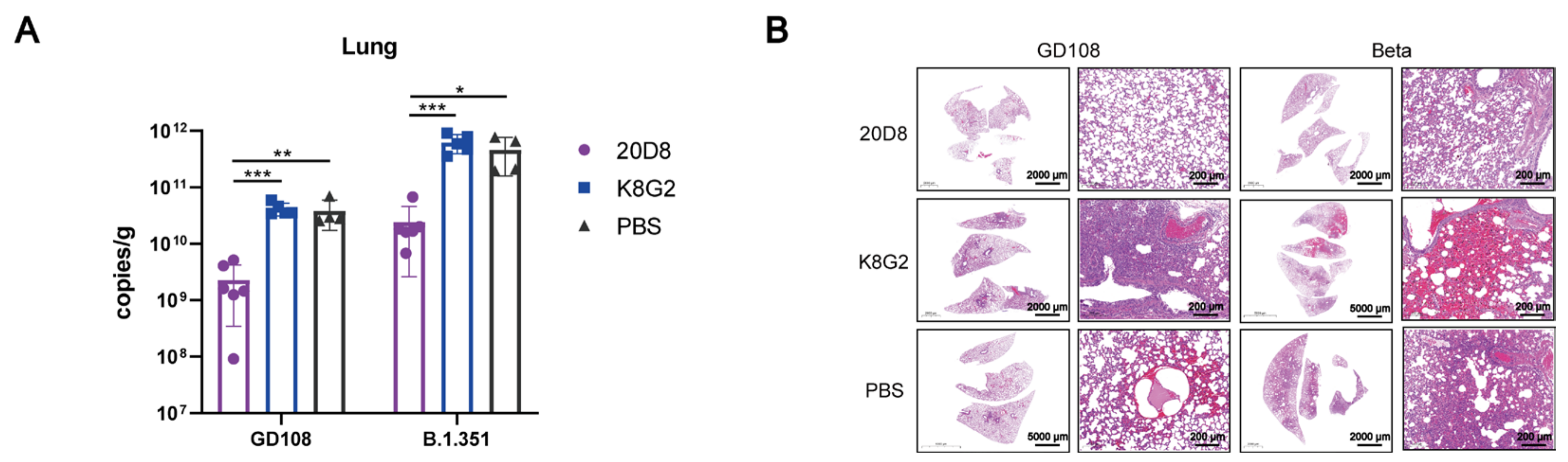
3.2. Protective Effect of mAb 20D8 in a Hamster Model
3.3. mAb 20D8 Recognizes a Linear Epitope (Amino Acids 369–379) of the RBD
3.4. ELISA
3.4.1. Specificity
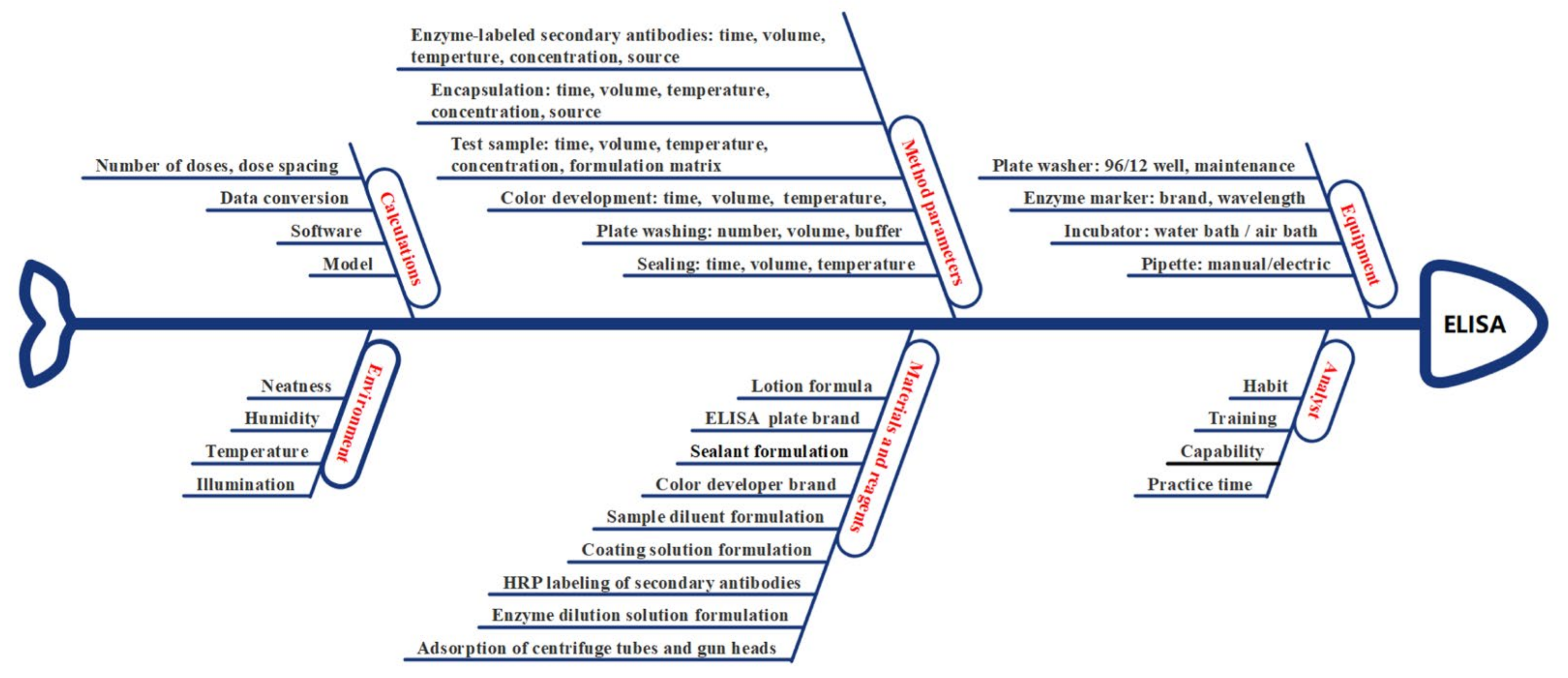
3.4.2. Risk Assessment
3.4.3. Screening of Fixed Factors
3.4.4. Optimization
3.4.5. Model Selection
3.5. Validation of Method
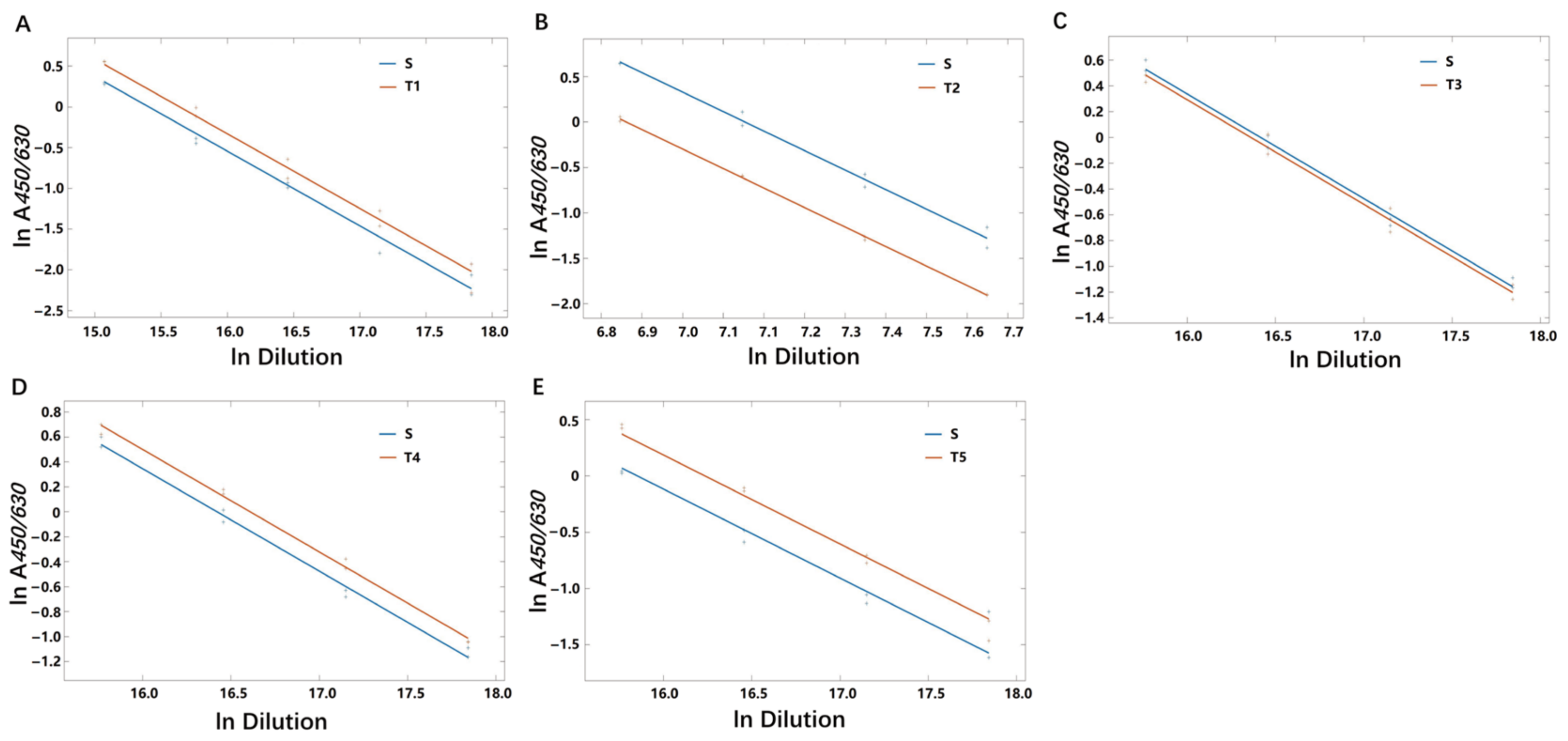
3.5.1. Range
3.5.2. Accuracy and Precision
3.5.3. Sample Size Evaluation
3.5.4. Method Capability Evaluation Indicators of ELISA
3.6. Method Suitability Evaluation of ELISA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.D.; Chi, W.Y.; Su, J.H.; Ferrall, L.; Hung, C.F.; Wu, T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef] [PubMed]
- Awadasseid, A.; Wu, Y.; Tanaka, Y.; Zhang, W. Current advances in the development of SARS-CoV-2 vaccines. Int. J. Biol. Sci. 2021, 17, 8–19. [Google Scholar] [CrossRef] [PubMed]
- The State Food and Drug Administration Approved the Registration Application of Anhui Zhifeilong Kema Biopharmaceutical Co., Ltd. for Recombinant Novel Coronavirus Protein Vaccine (CHO Cells) with Conditions. Available online: https://www.nmpa.gov.cn/yaowen/ypjgyw/20220302143909157.html (accessed on 29 July 2022).
- COVID-19 Vaccines-Authorised for Use in the European Union. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines (accessed on 29 July 2022).
- Coronavirus (COVID-19) Update: FDA Authorizes Emergency Use of Novavax COVID-19 Vaccine, Adjuvanted. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-emergency-use-novavax-covid-19-vaccine-adjuvanted (accessed on 29 July 2022).
- COVID-19 Vaccine Tracker and Landscape. 2022. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 1 November 2022).
- Liu, S.Y.; Bian, L.L.; Gao, F.; Liu, P.; Huo, Y.Q.; Wang, Z.J.; Zhang, Z.Y.; Li, Y.J.; Ge, X.Q.; Gu, M.R.; et al. Development of a universal quantitative detection kit for coxsackievirus A6 antigen. Chin. J. Biol. 2021, 34, 300–306. [Google Scholar]
- Cui, B.P.; Cai, F.; Gao, F.; Bian, L.L.; Wu, R.X.; Du, R.X.; Wu, X.; Liu, P.; Song, L.F.; Cui, L.S.; et al. A uniform quantitative enzyme-linked immunosorbent assay for Coxsackievirus A16 antigen in vaccine. Hum. Vaccin Immunother. 2021, 17, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.M.; Chen, L.; Gao, F.; Yao, X.; Li, G.S.H.; Gu, M.R.; Mao, Q.Y.; Liu, J.K.; Zheng, H.F. Development of quantitative ELISA for recombinant enterovirus 71 vaccine (Hansenula polymorpha) antigen. Chin. J. Viral Dis. 2018, 8, 476–480. [Google Scholar]
- Tome, T.; Žigart, N.; Časar, Z.; Obreza, A. Development and optimization of liquid chromatography analytical methods by using AQbD principles: Overview and recent advances. Org. Process Res. Dev. 2019, 23, 1784–1802. [Google Scholar] [CrossRef]
- Wang, X.J.; Wu, X.; Mao, Q.Y.; Liang, Z.L.; Tan, D.J. Interpretation of USP <1220> Analytical procedure life cycle. Chin. J. Biol. 2022, 35, 626–631. [Google Scholar]
- The United States Pharmacopeial Convention. <1032>Design and development of biological assays. In Pharmacopeia; U S Pharmacopeia43-NF38ed; USA Pharmacopeia Convention: North Bethesda, MD, USA, 2020; pp. 7320–7336. [Google Scholar]
- The United States Pharmacopeial Convention. <1033> Biological assay validation. In Pharmacopeia; U S Pharmacopeia43-NF38ed; USA Pharmacopeia Convention: North Bethesda, MD, USA, 2020; pp. 7337–7350. [Google Scholar]
- The United States pharmacopeial convention. <1210> Statistical tools for procedure validation. In Pharmacopeia; U S Pharmacopeia43-NF38ed; USA Pharmacopeia Convention: North Bethesda, MD, USA, 2020; pp. 8117–8128. [Google Scholar]
- The United States pharmacopeial convention. <1220> Analytical Procedure Life Cycle. In U. S. Pharmacopeia. USPNF Issue 1 ed; USA Pharmacopeia Convention: North Bethesda, MD, USA, 2022; Volume 2022. [Google Scholar]
- International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use. Q14 Analytical Procedure Development. Geneva, CH. 2022. Available online: https://www.ich.org/page/quality-guidelines (accessed on 29 July 2022).
- International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use. Q2, R2 Harmonised Guideline Validation of Analytical Procedure. Geneva, CH. 2022. Available online: https://www.ich.org/page/quality-guidelines (accessed on 29 July 2022).
- Alhakeem, M.A.; Ghica, M.V.; Pîrvu, C.D.; Anuța, V.; Popa, L. Analytical quality by design with the lifecycle approach: A modern epitome for analytical method development. Acta Med. Marisiensis 2019, 65, 37–44. [Google Scholar] [CrossRef]
- Peraman, R.; Bhadraya, K.; Padmanabha Reddy, Y. Analytical quality by design: A tool for regulatory flexibility and robust analytics. Int. J. Anal. Chem. 2015, 2015, 868727. [Google Scholar] [CrossRef]
- Borman, P.; Campa, C.; Delpierre, G.; Hook, E.; Jackson, P.; Kelley, W.; Protz, M.; Vandeputte, O. Selection of analytical technology and development of analytical procedures using the analytical target profile. Anal. Chem. 2022, 94, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Borman, P.; Campa, C.; Chatfield, M.; Godfrey, M.; Hamilton, P.; Hoyer, W.; Norelli, F.; Orr, R.; Schofield, T. Using the analytical target profile to drive the analytical method lifecycle. Anal. Chem. 2019, 91, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Radić, I.; Runje, M.; Babić, S. Development of an analytical method for the determination of pimavanserin and its impurities applying analytical quality by design principles as a risk-based strategy. J. Pharm. Biomed. Anal. 2021, 201, 114091. [Google Scholar] [CrossRef] [PubMed]
- Du, R.X.; Mao, Q.Y.; Hu, Y.L.; Lang, S.H.; Sun, S.Y.; Li, K.L.; Gao, F.; Bian, L.L.; Yang, C.; Cui, B.P.; et al. A potential therapeutic neutralization monoclonal antibody specifically against multi-coxsackievirus A16 strains challenge. Hum. Vaccin Immunother. 2019, 15, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Mao, Q.Y.; Peng, X.Z.; He, Z.L.; Lu, S.Y.; Zhang, J.L.; Gao, F.; Bian, L.L.; An, C.Q.; Yu, W.H.; et al. Immunogenicity and protective efficacy of a recombinant protein subunit vaccine and an inactivated vaccine against SARS-CoV-2 variants in non-human primates. Signal Transduct. Target. Ther. 2022, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Zhao, Y.; Yu, W.H.; Yang, Y.; Gao, J.H.; Wang, J.B.; Kuang, D.X.; Yang, M.L.; Yang, J.; Ma, C.X.; et al. Comparison of nonhuman primates identified the suitable model for COVID-19. Signal Transduct. Target. Ther. 2020, 5, 157. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wei, Q.; Lin, Q.Q.; Fang, J.; Wang, H.B.; Kwok, H.; Tang, H.Y.; Nishiura, K.; Peng, J.; Tan, Z.W.; et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARA-CoV infection. JCI Insight 2019, 4, e123158. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; An, C.Q.; Bian, L.L.; Wang, Y.P.; Zhang, J.L.; Cui, B.P.; He, Q.; Yuan, Y.D.; Song, L.F.; Yang, J.H.; et al. Establishment of the first Chinese national standard for protein subunit SARS-CoV-2 vaccine. Vaccine 2022, 40, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Molnár, I.; Rieger, H.J.; Monks, K.E. Aspects of the “Design Space” in high pressure liquid chromatography method development. J. Chromatogr. A 2010, 1217, 3193–3200. [Google Scholar] [CrossRef] [PubMed]
- Rozet, E.; Lebrun, P.; Hubert, P.; Debrus, B.; Boulanger, B. Design Spaces for analytical methods. TrAC Trends Anal. Chem. 2013, 42, 157–167. [Google Scholar] [CrossRef]
- Han, L.; Sui, S.L.; Tan, D.J.; Duan, L.; Li, N.; Du, Y.; Shi, R.H. Design and function of statistical software for validation of biological methods. Chin. J. Pharm. Anal. 2022, 42, 979–987. [Google Scholar]
- Yarovoi, H.; Frey, T.; Bouaraphan, S.; Retzlaff, M.; Verch, T. Quality by design for a vaccine release immunoassay: A case study. Bioanalysis 2013, 5, 2531–2545. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Han, L.; Du, Y.; Geng, Y.; Li, N.; Xu, H.; Tan, D.J. Discussion on the application of method validation data set. Chin. J. Pharm. Anal. 2022, 42, 972–978. [Google Scholar]
- Liu, H.; Wilson, I.A. Protective neutralizing epitopes in SARS-CoV-2. Immunol Rev. 2022, 310, 76–92. [Google Scholar] [CrossRef]
- Khodadadi, A.; Madani, R.; Hoghooghi, R.N.; Atyabi, N. Development of Nano-ELISA method for serological diagnosis of toxoplasmosis in mice. Arch. Razi Inst. 2021, 75, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Dhanze, H.; Singh, V.; Mehta, D.; Gupta, M.; Singh, M.; Vinod, V.K.; Gulati, B.R. Development of lgM-ELISA for diagnosis of recent infection of Japanese encephalitis virus in equines. Biological 2022, 75, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Bhadricha, H.; Khatkhatay, M.I.; Desai, M. Development of an in house ELISA for human intact osteocalcin and its utility in diagnosis and management of osteoporosis. Clin. Chim. Acta 2019, 489, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Yu, J.S.; Wang, P.J.; Hsiao, Y.C.; Liu, C.H.; Chen, Y.C.; Lai, P.F.; Hsu, C.P.; Fann, W.C.; Lin, C.C. Development of sandwich ELISA and lateral flow strip assays for diagnosing clinically significant snakebite in Taiwan. PLoS Negl. Trop. Dis. 2018, 12, e0007014. [Google Scholar] [CrossRef] [PubMed]
- Verch, T.; Campa, C.; Chéry, C.C.; Frenkel, R.; Graul, T.; Jaya, N.; Nakhle, B.; Springall, J.; Starkey, J.; Wypych, J.; et al. Analytical quality by design, life cycle management, and method control. AAPS J. 2022, 24, 34. [Google Scholar] [CrossRef] [PubMed]
- Vogt, F.G.; Kord, A.S. Development of quality-by-design analytical methods. J. Pharm. Sci. 2011, 100, 797–812. [Google Scholar] [CrossRef] [PubMed]
- ASTM E2709-19; Standard Practice for Demonstrating Capability to Comply with an Acceptance Procedure[S/OL]. 2019. Available online: https://www.astm.org/e2709-19.html (accessed on 22 April 2022).
- Westgard, J.O.; Westgard, S.A. Measuring analytical quality: Total analytical error versus measurement uncertainty. Clin. Lab. Med. 2017, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Westgard, J.O.; Carey, R.N.; Wold, S. Criteria for judging precision and accuracy in method development and evaluation. Clin. Chem. 1974, 20, 825–833. [Google Scholar] [CrossRef]
- Jaffar-Aghaei, M.; Khanipour, F.; Maghsoudi, A.; Sarvestani, R.; Mohammadian, M.; Maleki, M.; Havasi, F.; Rahmani, H.; Karagah, A.H.; Kazemali, M.R. QbD-guided pharmaceutical development of Pembrolizumab biosimilar candidate PSG-024 propelled to industry meeting primary requirements of comparability to Keytruda®. Eur. J. Pharm. Sci. 2022, 173, 106171. [Google Scholar] [CrossRef]


| Rating of Importance (Priority) | 8 | 8 | 10 | |||
|---|---|---|---|---|---|---|
| ID | Process Step | Process Input | Accuracy | Specificity | Precision | Total |
| 1 | Environment | Temperature | 1 | 1 | 1 | 26 |
| 2 | Environment | Illumination | 1 | 1 | 1 | 26 |
| 3 | Environment | Humidity | 1 | 1 | 1 | 26 |
| 4 | Environment | Neatness | 1 | 1 | 1 | 26 |
| 5 | Enzyme markers | Brand | 1 | 1 | 1 | 26 |
| 6 | Plate washer | 96-well/12-well | 1 | 1 | 1 | 26 |
| 7 | Calculation | Software | 1 | 1 | 1 | 26 |
| 8 | Enzyme marker | Wavelength | 2 | 1 | 1 | 34 |
| 9 | Blocking | Volume | 1 | 2 | 1 | 34 |
| 10 | Test sample | Volume | 2 | 1 | 2 | 44 |
| 11 | Enzyme-labeled mAbs | Volume | 2 | 1 | 2 | 44 |
| 12 | Substrate | Volume | 2 | 1 | 2 | 44 |
| 13 | Enzyme labeling plate | Brand | 2 | 1 | 2 | 44 |
| 14 | Substrate | Brand | 2 | 1 | 2 | 44 |
| 15 | Analyst | Habit | 2 | 1 | 2 | 44 |
| 16 | Analyst | Training | 2 | 1 | 2 | 44 |
| 17 | Analyst | Practice time | 2 | 1 | 2 | 44 |
| 18 | Consumable | Absorbance | 2 | 1 | 2 | 44 |
| 19 | Blocking | Volume | 2 | 1 | 2 | 44 |
| 20 | Coating | Volume | 1 | 1 | 3 | 46 |
| 21 | Blocking | Time | 2 | 2 | 2 | 52 |
| 22 | Blocking | Temperature | 2 | 2 | 2 | 52 |
| 23 | Coating | Temperature | 2 | 1 | 3 | 54 |
| 24 | Calculation | Model | 3 | 1 | 3 | 62 |
| 25 | Pipette | Electric/manual | 3 | 1 | 3 | 62 |
| 26 | Washboard | Buffers | 2 | 3 | 3 | 70 |
| 27 | Lotion | Recipe | 2 | 3 | 3 | 70 |
| 28 | Sample dilution | Recipe | 2 | 3 | 3 | 70 |
| 29 | Enzyme secondary antibody dilution | Recipe | 2 | 3 | 3 | 70 |
| 30 | Blocking solution | Recipe | 2 | 3 | 3 | 70 |
| 31 | Coating solution | Recipe | 2 | 3 | 3 | 70 |
| 32 | Test sample | Concentration | 3 | 1 | 4 | 72 |
| 33 | Test sample | Time | 3 | 1 | 4 | 72 |
| 34 | Test sample | Temperature | 3 | 1 | 4 | 72 |
| 35 | Enzyme-labeled mAbs | Concentration | 3 | 1 | 4 | 72 |
| 36 | Enzyme-labeled mAbs | Time | 3 | 1 | 4 | 72 |
| 37 | Enzyme-labeled mAbs | Temperature | 3 | 1 | 4 | 72 |
| 38 | Coating | Concentration | 4 | 1 | 4 | 80 |
| 39 | Coating | Time | 4 | 1 | 4 | 80 |
| 40 | Washboard | Number of times | 2 | 3 | 4 | 80 |
| 41 | Calculation | Concentration point setting | 4 | 1 | 4 | 80 |
| 42 | Incubator | Incubation mode | 4 | 1 | 4 | 80 |
| 43 | Analyst | Capability | 4 | 1 | 4 | 80 |
| 44 | Substrate | Time | 4 | 3 | 5 | 106 |
| 45 | Substrate | Temperature | 4 | 3 | 5 | 106 |
| 46 | Plate washer | Maintenance | 5 | 5 | 5 | 130 |
| Antigen Content (U/mL) | 0.50 | 0.25 | 0.13 | 0.063 | 0.031 | 0.016 |
|---|---|---|---|---|---|---|
| Bias (%) | −8.48 | 4.51 | 6.55 | 4.51 | −1.50 | −4.86 |
| Confidence interval for bias (%) | −11.57−5.28 | 2.30−6.77 | 4.40−8.75 | 2.40−6.66 | −3.03−0.061 | −7.49−2.15 |
| Intermediate precision (%) | 8.25 | 5.50 | 5.12 | 5.39 | 4.45 | 7.29 |
| Upper bound on intermediate precision (%) | 12.01 | 7.66 | 7.19 | 7.44 | 6.01 | 10.18 |
| Antigen Content (U/mL) | Method Variability (%) | 90% Tolerance Interval (%) | 90% Prediction Interval (%) | MCI Under | Misjudgment Probability of Method | Method Level |
|---|---|---|---|---|---|---|
| 0.50 | 11.83 | 70.07−119.54 | 73.83−113.46 | 0.96 | 3.92 × 10−3 | VI |
| 0.25 | 7.11 | 89.43−122.14 | 92.20−118.47 | 1.65 | 7.62 × 10−7 | II |
| 0.13 | 8.31 | 88.88−127.73 | 92.09−123.28 | 1.42 | 2.14 × 10−5 | II |
| 0.063 | 7.03 | 89.59−121.90 | 92.33−118.29 | 1.67 | 5.64 × 10−7 | I |
| 0.031 | 4.70 | 88.81−109.25 | 90.63−107.06 | 2.48 | 1.02 × 10−13 | I |
| 0.016 | 8.76 | 78.39−115.47 | 81.42−111.18 | 1.33 | 6.94 × 10−5 | III |
| Antigen Content (U/mL) | (A) Development Objectives | (B) Development Results |
|---|---|---|
| Intended purpose | Quantifying the antigen content of SARS-CoV-2 recombinant protein vaccines. | |
| Link to critical quality attribute (CQA) | CQA: The antigen content of SARS-CoV-2 recombinant protein vaccines. Experimental principle: The specific antibody was bound to a solid-phase carrier to form a solid-phase antibody. The immune complex was then formed by binding to the corresponding antigen in the sample to be examined. After washing, enzyme-labeled antibodies were added and bound to antigens in the immune complexes to form enzyme-labeled monoclonal antibody–antigen solid-phase complexes. The antigen content was determined by adding substrate for color development. (The shade of color in the microplate was positively correlated with the concentration of the substance to be measured.) | |
| Specificity | No cross-reactivity with SARS-CoV and MERS-CoV recombinant S proteins. | No cross-reactivity with SARS-CoV and MERS-CoV recombinant S proteins. |
| Accuracy | ≥85% | ≥90% |
| Precision | ≥85% | ≥90% |
| Total analytical error | 20% | 11.83% |
| Specification range to be controlled by this method | 63−158% | 70−143% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; An, C.; Liu, D.; Wang, Z.; Bian, L.; He, Q.; Liu, J.; Wang, Q.; Liu, M.; Mao, Q.; et al. Development of an ELISA Assay for the Determination of SARS-CoV-2 Protein Subunit Vaccine Antigen Content. Viruses 2023, 15, 62. https://doi.org/10.3390/v15010062
Han L, An C, Liu D, Wang Z, Bian L, He Q, Liu J, Wang Q, Liu M, Mao Q, et al. Development of an ELISA Assay for the Determination of SARS-CoV-2 Protein Subunit Vaccine Antigen Content. Viruses. 2023; 15(1):62. https://doi.org/10.3390/v15010062
Chicago/Turabian StyleHan, Lu, Chaoqiang An, Dong Liu, Zejun Wang, Lianlian Bian, Qian He, Jianyang Liu, Qian Wang, Mingchen Liu, Qunying Mao, and et al. 2023. "Development of an ELISA Assay for the Determination of SARS-CoV-2 Protein Subunit Vaccine Antigen Content" Viruses 15, no. 1: 62. https://doi.org/10.3390/v15010062
APA StyleHan, L., An, C., Liu, D., Wang, Z., Bian, L., He, Q., Liu, J., Wang, Q., Liu, M., Mao, Q., Hang, T., Wang, A., Gao, F., Tan, D., & Liang, Z. (2023). Development of an ELISA Assay for the Determination of SARS-CoV-2 Protein Subunit Vaccine Antigen Content. Viruses, 15(1), 62. https://doi.org/10.3390/v15010062







