A Systematic Review of Mathematical Models of Dengue Transmission and Vector Control: 2010–2020
Abstract
1. Introduction
2. Methods
2.1. Selection Criteria
- A representation of vectors or vector-host dynamics to control dengue transmission.
- A deterministic (DM), stochastic (SM), or network (NM) modelling approach using systems of ODEs.
- A vector control strategy leading to dengue viral reduction or elimination.
2.2. Data Extraction
2.3. Assessment of Study Quality
3. Results
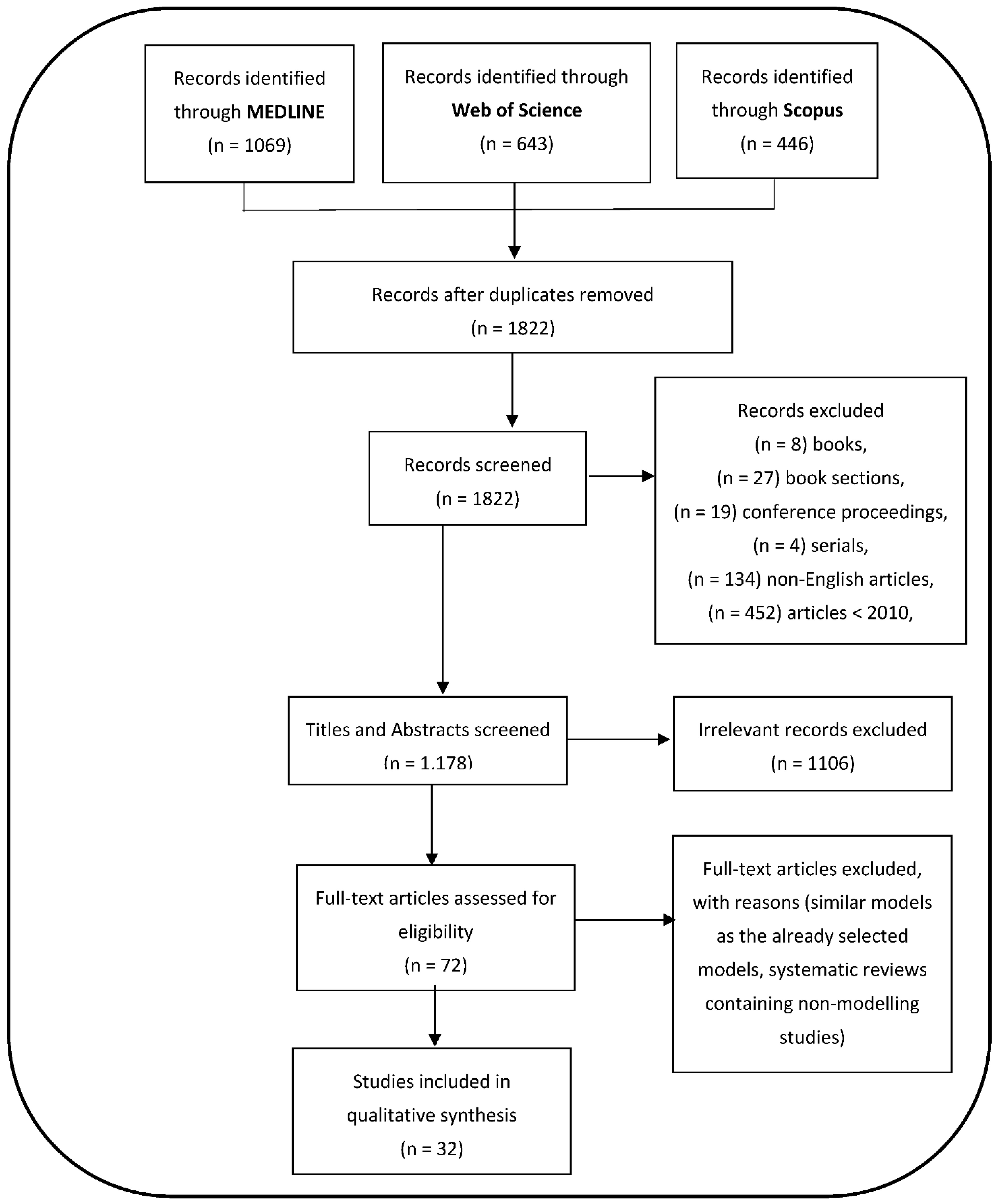
3.1. Search Strategies and General Study Characteristics
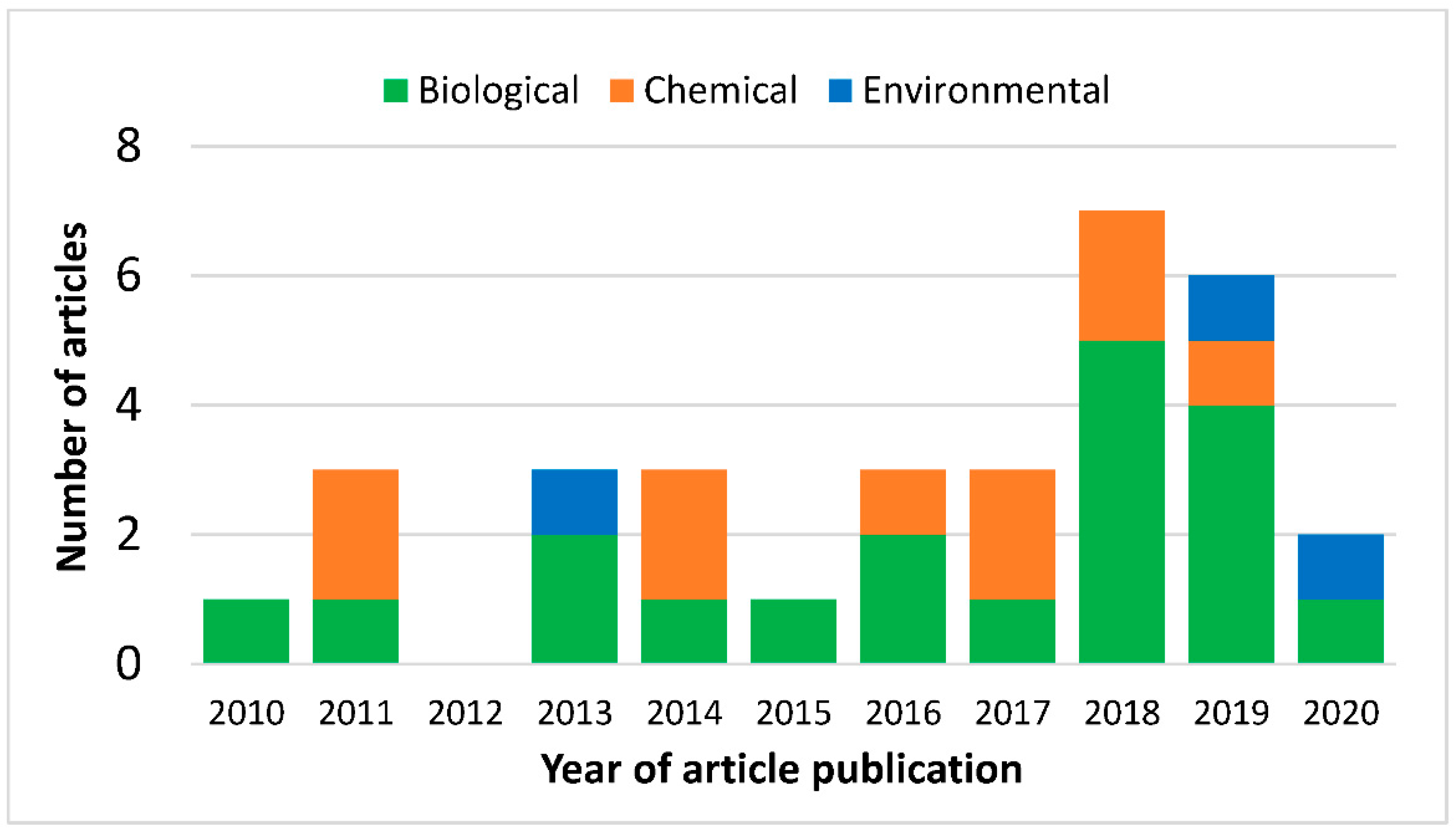
3.2. Distribution of Vector Control Modelling Articles
4. Vector Control Methods
4.1. Chemical Control Methods
4.2. Biological Control Methods
4.3. Environmental Control Methods
4.4. Quality Assessment of Study Results
5. Discussion
Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; De Lamballerie, X. Emerging arboviruses: Why today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Buhler, C.; Winkler, V.; Runge-Ranzinger, S.; Boyce, R.; Horstick, O. Environmental methods for dengue vector control—A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007420. [Google Scholar] [CrossRef]
- Simmons, C.P.; Farrar, J.J.; van Vinh Chau, N.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef]
- Reich, N.G.; Shrestha, S.; King, A.; Rohani, P.; Lessler, J.; Kalayanarooj, S.; Yoon, I.-K.; Gibbons, R.V.; Burke, D.S.; Cummings, D.A.T. Interactions between serotypes of dengue highlight epidemiological impact of cross-immunity. J. R. Soc. Interface 2013, 10, 20130414. [Google Scholar] [CrossRef]
- Fukusumi, M.; Arashiro, T.; Arima, Y.; Matsui, T.; Shimada, T.; Kinoshita, H.; Arashiro, A.; Takasaki, T.; Sunagawa, T.; Oishi, K. Dengue Sentinel Traveler Surveillance: Monthly and Yearly Notification Trends among Japanese Travelers, 2006–2014. PLoS Negl. Trop. Dis. 2016, 10, e0004924. [Google Scholar] [CrossRef]
- Capeding, M.R.; Tran, N.H.; Hadinegoro, S.R.S.; Ismail, H.I.H.M.; Chotpitayasunondh, T.; Chua, M.N.; Luong, C.Q.; Rusmil, K.; Wirawan, D.N.; Nallusamy, R.; et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014, 384, 1358–1365. [Google Scholar] [CrossRef]
- Villar, L.; Dayan, G.H.; Arredondo-García, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramírez, J.O.; Carrasquilla, G.; et al. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Ooi, E.-E.; Horstick, O.; Wills, B. Dengue. Lancet 2019, 393, 350–363. [Google Scholar] [CrossRef]
- Sulistyawati, S.; Astuti, F.D.; Umniyati, S.R.; Satoto, T.B.T.; Lazuardi, L.; Nilsson, M.; Rocklov, J.; Andersson, C.; Holmner, A. Dengue Vector Control through Community Empowerment: Lessons Learned from a Community-Based Study in Yogyakarta, Indonesia. Int. J. Environ. Res. Public Health 2019, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Otu, A.; Ebenso, B.; Etokidem, A.; Chukwuekezie, O. Dengue fever-an update review and implications for Nigeria, and similar countries. Afr. Health Sci. 2019, 19, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.M. Challenges and opportunities in controlling mosquito-borne infections. Nature 2018, 559, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Vannice, K.S.; Hombach, J.; Farrar, J.; Nolan, T. Population Perspectives and World Health Organization Recommendations for CYD-TDV Dengue Vaccine. J. Infect. Dis. 2016, 214, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Tatuene, J.K.; Makepeace, B.L.; Benjamin, L.; Baylis, M.; Solomon, T. The potential role of Wolbachia in controlling the transmission of emerging human arboviral infections. Curr. Opin. Infect. Dis. 2017, 30, 108–116. [Google Scholar] [CrossRef]
- Horstick, O.; Boyce, R.; Runge-Ranzinger, S. Dengue Vector Control: Assessing What Works? Southeast Asian J. Trop Med. 2017, 48, 181–195. [Google Scholar]
- Dusfour, I.; Vontas, J.; David, J.-P.; Weetman, D.; Fonseca, D.M.; Corbel, V.; Raghavendra, K.; Coulibaly, M.B.; Martins, A.J.; Kasai, S.; et al. Management of insecticide resistance in the major Aedes vectors of arboviruses: Advances and challenges. PLoS Negl. Trop. Dis. 2019, 13, e0007615. [Google Scholar] [CrossRef]
- Loroño-Pino, M.A.; Chan-Dzul, Y.N.; Zapata-Gil, R.; Carrillo-Solís, C.; Uitz-Mena, A.; García-Rejón, J.E.; Keefe, T.J.; Beaty, B.J.; Eisen, L. Household use of insecticide consumer products in a dengue-endemic area in México. Trop. Med. Int. Health 2014, 19, 1267–1275. [Google Scholar] [CrossRef]
- Lacroix, R.; McKemey, A.R.; Raduan, N.; Kwee Wee, L.; Hong Ming, W.; Guat Ney, T.; Rahidah, A.A.S.; Salman, S.; Subramaniam, S.; Nordin, O.; et al. Open Field Release of Genetically Engineered Sterile Male Aedes aegypti in Malaysia. PLoS ONE 2012, 7, e42771. [Google Scholar] [CrossRef]
- Carvalho, D.O.; McKemey, A.R.; Garziera, L.; Lacroix, R.; Donnelly, C.A.; Alphey, L.; Malavasi, A.; Capurro, M.L. Suppression of a Field Population of Aedes aegypti in Brazil by Sustained Release of Transgenic Male Mosquitoes. PLoS Negl. Trop. Dis. 2015, 9, e0003864. [Google Scholar] [CrossRef]
- Lebon, C.; Benlali, A.; Atyame, C.; Mavingui, P.; Tortosa, P. Construction of a genetic sexing strain for Aedes albopictus: A promising tool for the development of sterilizing insect control strategies targeting the tiger mosquito. Parasites Vectors 2018, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Ant, T.H.; Herd, C.S.; Geoghegan, V.; Hoffmann, A.A.; Sinkins, S.P. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018, 14, e1006815. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, I.; Rath, A.; Mahapatra, N.; Hazra, R.K. Wolbachia: A biological control strategy against arboviral diseases. J. Vector Borne Dis. 2016, 53, e1006815. [Google Scholar]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbeormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef]
- Barrera, R. New tools for Aedes control: Mass trapping. Curr. Opin. Insect Sci. 2022, 52, 100942. [Google Scholar] [CrossRef] [PubMed]
- Hemme, R.R.; A Smith, E.; Felix, G.; White, B.J.; I Diaz-Garcia, M.; Rodriguez, D.; Ruiz-Valcarcel, J.; Acevedo, V.; Amador, M.; Barrera, R. Multi-Year Mass-Trapping with Autocidal Gravid Ovitraps has Limited Influence on Insecticide Susceptibility in Aedes aegypti (Diptera: Culicidae) from Puerto Rico. J. Med. Èntomol. 2022, 59, 314–319. [Google Scholar] [CrossRef]
- Jahir, A.; Kahamba, N.F.; Knols, T.O.; Jackson, G.; Patty, N.F.A.; Shivdasani, S.; Okumu, F.O.; Knols, B.G.J. Mass Trapping and Larval Source Management for Mosquito Elimination on Small Maldivian Islands. Insects 2022, 13, 805. [Google Scholar] [CrossRef] [PubMed]
- Lana, R.M.; Morais, M.M.; De Lima, T.F.M.; Carneiro, T.; Stolerman, L.M.; Dos Santos, J.P.C.; Cortês, J.J.C.; Eiras, E.; Codeço, C.T. Assessment of a trap based Aedes aegypti surveillance program using mathematical modeling. PLoS ONE 2018, 13, e0190673. [Google Scholar] [CrossRef]
- Ogunlade, S.; Meehan, M.; Adekunle, A.; Rojas, D.; Adegboye, O.; McBryde, E. A Review: Aedes-Borne Arboviral Infections, Controls and Wolbachia-Based Strategies. Vaccines 2021, 9, 32. [Google Scholar] [CrossRef]
- Adekunle, A.I.; Meehan, M.T.; McBryde, E.S. Mathematical analysis of a Wolbachia invasive model with imperfect maternal transmission and loss of Wolbachia infection. Infect. Dis. Model. 2019, 4, 265–285. [Google Scholar] [CrossRef]
- Almeida, L.; Privat, Y.; Strugarek, M.; Vauchelet, N. Optimal Releases for Population Replacement Strategies: Application to Wolbachia. SIAM J. Math. Anal. 2019, 51, 3170–3194. [Google Scholar] [CrossRef]
- Bliman, P.-A.; Cardona-Salgado, D.; Dumont, Y.; Vasilieva, O. Implementation of control strategies for sterile insect techniques. Math. Biosci. 2019, 314, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Marini, G.; Guzzetta, G.; Toledo, C.A.M.; Teixeira, M.; Rosà, R.; Merler, S. Effectiveness of Ultra-Low Volume insecticide spraying to prevent dengue in a non-endemic metropolitan area of Brazil. PLoS Comput. Biol. 2019, 15, e1006831. [Google Scholar] [CrossRef] [PubMed]
- Strugarek, M.; Bossin, H.; Dumont, Y. On the use of the sterile insect release technique to reduce or eliminate mosquito populations. Appl. Math. Model. 2019, 68, 443–470. [Google Scholar] [CrossRef]
- Zhang, H.; Lui, R. Releasing Wolbachia-infected Aedes aegypti to prevent the spread of dengue virus: A mathematical study. Infect. Dis. Model. 2020, 5, 142–160. [Google Scholar] [CrossRef]
- Andraud, M.; Hens, N.; Marais, C.; Beutels, P. Dynamic Epidemiological Models for Dengue Transmission: A Systematic Review of Structural Approaches. PLoS ONE 2012, 7, e49085. [Google Scholar] [CrossRef]
- Agusto, F.; Khan, M. Optimal control strategies for dengue transmission in pakistan. Math. Biosci. 2018, 305, 102–121. [Google Scholar] [CrossRef]
- Alphey, N.; Alphey, L.; Bonsall, M.B. A Model Framework to Estimate Impact and Cost of Genetics-Based Sterile Insect Methods for Dengue Vector Control. PLoS ONE 2011, 6, e25384. [Google Scholar] [CrossRef] [PubMed]
- Andraud, M.; Hens, N.; Beutels, P. A simple periodic-forced model for dengue fitted to incidence data in Singapore. Math. Biosci. 2013, 244, 22–28. [Google Scholar] [CrossRef]
- Cai, L.; Ai, S.; Fan, G. Dynamics of delayed mosquitoes populations models with two different strategies of releasing sterile mosquitoes. Math. Biosci. Eng. 2018, 15, 1181–1202. [Google Scholar] [CrossRef] [PubMed]
- Campo-Duarte, D.E.; Vasilieva, O.; Cardona-Salgado, D.; Svinin, M. Optimal control approach for establishing wMelPop Wolbachia infection among wild Aedes aegypti populations. J. Math. Biol. 2018, 76, 1907–1950. [Google Scholar] [CrossRef] [PubMed]
- Ndii, M.Z.; Allingham, D.; Hickson, R.; Glass, K. The effect of Wolbachia on dengue outbreaks when dengue is repeatedly introduced. Theor. Popul. Biol. 2016, 111, 9–15. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, K.M.; Hendrickx, E.; Kharisma, D.D.; Wilastonegoro, N.N.; Carrington, L.B.; Elyazar, I.R.F.; Kucharski, A.J.; Lowe, R.; Flasche, S.; Pigott, D.M.; et al. Estimating the burden of dengue and the impact of release of wMel Wolbachia-infected mosquitoes in Indonesia: A modelling study. BMC Med. 2019, 17, 172. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Xue, L.; Hyman, J.M. Modeling the Transmission of Wolbachia in Mosquitoes for Controlling Mosquito-Borne Diseases. SIAM J. Appl. Math. 2018, 78, 826–852. [Google Scholar] [CrossRef]
- Ogunlade, S.T.; Adekunle, A.I.; Meehan, M.T.; Rojas, D.P.; McBryde, E.S. Modeling the potential of wAu-Wolbachia strain invasion in mosquitoes to control Aedes-borne arboviral infections. Sci. Rep. 2020, 10, 16812. [Google Scholar] [CrossRef]
- Abad-Franch, F.; Zamora-Perea, E.; Luz, S.L.B. Mosquito-Disseminated Insecticide for Citywide Vector Control and Its Potential to Block Arbovirus Epidemics: Entomological Observations and Modeling Results from Amazonian Brazil. PLoS Med. 2017, 14, e1002213. [Google Scholar] [CrossRef]
- Barmak, D.H.; Dorso, C.O.; Otero, M.; Solari, H.G. Modelling interventions during a dengue outbreak. Epidemiol. Infect. 2014, 142, 545–561. [Google Scholar] [CrossRef]
- Buonomo, B.; Della Marca, R. Optimal bed net use for a dengue disease model with mosquito seasonal pattern. Math. Methods Appl. Sci. 2018, 41, 537–592. [Google Scholar] [CrossRef]
- Chávez, J.P.; Götz, T.; Siegmund, S.; Wijaya, K.P. An SIR-Dengue transmission model with seasonal effects and impulsive control. Math. Biosci. 2017, 289, 29–39. [Google Scholar] [CrossRef]
- Hancock, P.A.; White, V.L.; Ritchie, S.A.; Hoffmann, A.A.; Godfray, H.C.J. Predicting Wolbachia invasion dynamics in Aedes aegypti populations using models of density-dependent demographic traits. BMC Biol. 2016, 14, 96. [Google Scholar] [CrossRef]
- He, S.; Zhang, X.; Liang, J.; Tang, S. Multiscale modelling the effects of CI genetic evolution in mosquito population on the control of dengue fever. Sci. Rep. 2017, 7, 13895. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.; Britton, N.F. Modelling the Use of Wolbachia to Control Dengue Fever Transmission. Bull. Math. Biol. 2013, 75, 796–818. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X. A sex-structured model with birth pulse and release strategy for the spread of Wolbachia in mosquito population. J. Theor. Biol. 2018, 448, 53–65. [Google Scholar] [CrossRef]
- Luz, P.M.; Vanni, T.; Medlock, J.; Paltiel, A.D.; Galvani, A.P. Dengue vector control strategies in an urban setting: An economic modelling assessment. Lancet 2011, 377, 1673–1680. [Google Scholar] [CrossRef]
- Aziz-Alaoui, M.A.; Gakkhar, S.; Ambrosio, B.; Mishra, A. A network model for control of dengue epidemic using sterile insect technique. Math. Biosci. Eng. 2018, 15, 441–460. [Google Scholar] [CrossRef] [PubMed]
- Oki, M.; Sunahara, T.; Hashizume, M.; Yamamoto, T. Optimal Timing of Insecticide Fogging to Minimize Dengue Cases: Modeling Dengue Transmission among Various Seasonalities and Transmission Intensities. PLoS Negl. Trop. Dis. 2011, 5, e1367. [Google Scholar] [CrossRef]
- Pleydell, D.R.J.; Bouyer, J. Biopesticides improve efficiency of the sterile insect technique for controlling mosquito-driven dengue epidemics. Commun. Biol. 2019, 2, 201. [Google Scholar] [CrossRef]
- Robert, M.A.; Okamoto, K.; Lloyd, A.L.; Gould, F. A Reduce and Replace Strategy for Suppressing Vector-Borne Diseases: Insights from a Deterministic Model. PLoS ONE 2013, 8, e73233. [Google Scholar] [CrossRef]
- Salami, D.; Capinha, C.; Sousa, C.A.; Martins, M.D.R.O.; Lord, C. Simulation models of dengue transmission in Funchal, Madeira Island: Influence of seasonality. PLoS Negl. Trop. Dis. 2020, 14, e0008679. [Google Scholar] [CrossRef]
- Senapati, A.; Sardar, T.; Ganguly, K.S.; Chattopadhyay, A.K.; Chattopadhyay, J. Impact of adult mosquito control on dengue prevalence in a multi-patch setting: A case study in Kolkata (2014–2015). J. Theor. Biol. 2019, 478, 139–152. [Google Scholar] [CrossRef]
- Tang, B.; Xiao, Y.; Tang, S.; Wu, J. Modelling weekly vector control against Dengue in the Guangdong Province of China. J. Theor. Biol. 2016, 410, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Thomé, R.C.; Yang, H.M.; Esteva, L. Optimal control of Aedes aegypti mosquitoes by the sterile insect technique and insecticide. Math. Biosci. 2010, 223, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, K.P.; Goetz, T.; Soewono, E. An optimal control model of mosquito reduction management in a dengue endemic region. Int. J. Biomath. 2014, 7, 1450056. [Google Scholar] [CrossRef]
- Winskill, P.; Harris, A.F.; A Morgan, S.; Stevenson, J.; Raduan, N.; Alphey, L.; McKemey, A.R.; A Donnelly, C. Genetic control of Aedes aegypti: Data-driven modelling to assess the effect of releasing different life stages and the potential for long-term suppression. Parasites Vectors 2014, 7, 68. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, S.; Cheke, R.A. Models to assess how best to replace dengue virus vectors with Wolbachia -infected mosquito populations. Math. Biosci. 2015, 269, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef]
- Perkins, T.A.; Reiner, R.C.; Rodriguez-Barraquer, I.; Smith, D.L.; Scott, T.W.; Cummings, D.A.T. A review of transmission models of dengue: A quantitative and qualitative analysis of model features. In Dengue and Dengue Hemorrhagic Fever, 2nd ed.; CAB International: Wallingford, UK, 2014; pp. 99–114. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Fone, D.L.; Hollinghurst, S.; A Temple, M.; Round, A.; Lester, N.; Weightman, A.; Roberts, K.; Coyle, E.; Bevan, G.; Palmer, S. Systematic review of the use and value of computer simulation modelling in population health and health care delivery. J. Public Health Med. 2003, 25, 325–335. [Google Scholar] [CrossRef]
- Harris, R.C.; Sumner, T.; Knight, G.; White, R.G. Systematic review of mathematical models exploring the epidemiological impact of future TB vaccines. Hum. Vaccines Immunother. 2016, 12, 2813–2832. [Google Scholar] [CrossRef]
- WHO. Dengue and Severe Dengue. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 15 June 2022).
- Thomas, M.B. Biological control of human disease vectors: A perspective on challenges and opportunities. Biocontrol 2018, 63, 61–69. [Google Scholar] [CrossRef]
- De Oliveira, S.R.; Caleffe, R.R.T.; Conte, H. Chemical control of aedes aegypti: A review on effects on the environment and human health. Revista Eletrônica Em Gestão Educ. E Tecnol. Ambiental 2017, 21, 240–247. [Google Scholar] [CrossRef]
- Jesus, T.; Wanner, E.; Cardoso, R. A receding horizon control approach for integrated vector management of Aedes aegypti using chemical and biological control: A mono and a multiobjective approach. Math. Methods Appl. Sci. 2019, 43, 3220–3237. [Google Scholar] [CrossRef]
- Lima, E.P.; Goulart, M.; Neto, M.R. Meta-analysis of studies on chemical, physical and biological agents in the control of Aedes aegypti. BMC Public Health 2015, 15, 858. [Google Scholar] [CrossRef] [PubMed]
- Amelia-Yap, Z.H.; Chen, C.D.; Sofian-Azirun, M.; Low, V.L. Pyrethroid resistance in the dengue vector Aedes aegypti in Southeast Asia: Present situation and prospects for management. Parasites Vectors 2018, 11, 332. [Google Scholar] [CrossRef]
- Fulcher, A.; Farooq, M.; Richardson, A.G.; Smith, M.L.; Scott, J.M.; Gaines, M.K.; Xue, R.-D. Characteristics and Efficacy of Three Commercial Handheld Thermal Foggers with Pyrethroid Insecticides Against Three Species of Mosquitoes. J. Am. Mosq. Control. Assoc. 2016, 32, 44–50. [Google Scholar] [CrossRef]
- Samuel, M.; Maoz, D.; Manrique, P.; Ward, T.; Runge-Ranzinger, S.; Toledo, J.; Boyce, R.; Horstick, O. Community effectiveness of indoor spraying as a dengue vector control method: A systematic review. PLoS Negl. Trop. Dis. 2017, 11, e0005837. [Google Scholar] [CrossRef] [PubMed]
- Killeen, G.F. Control of malaria vectors and management of insecticide resistance through universal coverage with next-generation insecticide-treated nets. Lancet 2020, 395, 1394–1400. [Google Scholar] [CrossRef]
- Okumu, F. The fabric of life: What if mosquito nets were durable and widely available but insecticide-free? Malar. J. 2020, 19, 260. [Google Scholar] [CrossRef]
- Manrique-Saide, P.; Che-Mendoza, A.; Barrera-Pérez, M.; Guillermo-May, G.; Herrera-Bojorquez, J.; Dzul-Manzanilla, F.; Gutiérrez-Castro, C.; Lenhart, A.; Vazquez-Prokopec, G.; Sommerfeld, J.; et al. Use of Insecticide-Treated House Screens to Reduce Infestations of Dengue Virus Vectors, Mexico. Emerg. Infect. Dis. 2015, 21, 308–311. [Google Scholar] [CrossRef]
- Lenhart, A.; Morrison, A.C.; Paz-Soldan, V.A.; Forshey, B.M.; Cordova-Lopez, J.J.; Astete, H.; Elder, J.P.; Sihuincha, M.; Gotlieb, E.E.; Halsey, E.S.; et al. The impact of insecticide treated curtains on dengue virus transmission: A cluster randomized trial in Iquitos, Peru. PLoS Negl. Trop. Dis. 2020, 14, e0008097. [Google Scholar] [CrossRef]
- Rather, I.A.; Parray, H.A.; Lone, J.B.; Paek, W.K.; Lim, J.; Bajpai, V.K.; Park, Y.-H. Prevention and Control Strategies to Counter Dengue Virus Infection. Front. Cell Infect. Microbiol. 2017, 7, 336. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Lenhart, A.; Toledo, J.; Lazaro, A.; Han, W.W.; Velayudhan, R.; Ranzinger, S.R.; Horstick, O. Community-Effectiveness of Temephos for Dengue Vector Control: A Systematic Literature Review. PLoS Negl. Trop. Dis. 2015, 9, e0004006. [Google Scholar] [CrossRef] [PubMed]
- Cavany, S.M.; España, G.; Lloyd, A.L.; Waller, L.A.; Kitron, U.; Astete, H.; Elson, W.H.; Vazquez-Prokopec, G.M.; Scott, T.W.; Morrison, A.C.; et al. Optimizing the deployment of ultra-low volume and targeted indoor residual spraying for dengue outbreak response. PLoS Comput. Biol. 2020, 16, e1007743. [Google Scholar] [CrossRef]
- Paiva, C.N.; Lima, J.W.D.O.; Camelo, S.S.; Lima, C.D.F.; Cavalcanti, L.P.D.G. Survival of larvivorous fish used for biological control of Aedes aegypti (Diptera: Culicidae) combined with different larvicides. Trop. Med. Int. Health 2014, 19, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Han, W.W.; Lazaro, A.; McCall, P.; George, L.; Runge-Ranzinger, S.; Toledo, J.; Velayudhan, R.; Horstick, O. Efficacy and community effectiveness of larvivorous fish for dengue vector control. Trop. Med. Int. Health 2015, 20, 1239–1256. [Google Scholar] [CrossRef]
- Morales-Pérez, A.; Nava-Aguilera, E.; Legorreta-Soberanis, J.; Cortés-Guzmán, A.J.; Balanzar-Martínez, A.; Harris, E.; Coloma, J.; Alvarado-Castro, V.M.; Bonilla-Leon, M.V.; Morales-Nava, L.; et al. “Where we put little fish in the water there are no mosquitoes:” A cross-sectional study on biological control of the Aedes aegypti vector in 90 coastal-region communities of Guerrero, Mexico. BMC Public Health 2017, 17, 433. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, A.; Han, W.W.; George, L.; Velayudhan, R.; Toledo, J.; Ranzinger, S.R.; Horstick, O.; Manrique-Saide, P. Community effectiveness of copepods for dengue vector control: Systematic review. Trop. Med. Int. Health 2015, 20, 685–706. [Google Scholar] [CrossRef]
- Udayanga, N.L.; Ranathunge, T.; Iqbal, M.C.M.; Abeyewickreme, W.; Hapugoda, M. Predatory efficacy of five locally available copepods on Aedes larvae under laboratory settings: An approach towards bio-control of dengue in Sri Lanka. PLoS ONE 2019, 14, e0216140. [Google Scholar] [CrossRef]
- Bohari, R.; Hin, C.J.; Matusop, A.; Abdullah, M.R.; Ney, T.G.; Benjamin, S.; Lim, L.H. Wide area spray of bacterial larvicide, Bacillus thuringiensis israelensis strain AM65-52, integrated in the national vector control program impacts dengue transmission in an urban township in Sibu district, Sarawak, Malaysia. PLoS ONE 2020, 15, e0230910. [Google Scholar] [CrossRef]
- Boyce, R.; Lenhart, A.; Kroeger, A.; Velayudhan, R.; Roberts, B.; Horstick, O. Bacillus thuringiensis israelensis (Bti) for the control of dengue vectors: Systematic literature review. Trop. Med. Int. Health 2013, 18, 564–577. [Google Scholar] [CrossRef]
- Carvalho, K.D.; Crespo, M.M.; Araujo, A.P.; da Silva, R.S.; de Melo-Santos, M.A.V.; de Oliveira, C.M.F.; Silva-Filha, M.H.N.L. Long-term exposure of Aedes aegypti to Bacillus thuringiensis svar. israelensis did not involve altered susceptibility to this microbial larvicide or to other control agents. Parasites Vectors 2018, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Kittayapong, P.; Ninphanomchai, S.; Limohpasmanee, W.; Chansang, C.; Chansang, U.; Mongkalangoon, P. Combined sterile insect technique and incompatible insect technique: The first proof-of-concept to suppress Aedes aegypti vector populations in semi-rural settings in Thailand. PLoS Negl. Trop. Dis. 2019, 13, e0007771. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Qsim, M.; Ashfaq, U.A.; Yousaf, M.Z.; Masoud, M.; Rasul, I.; Noor, N.; Hussain, A. Genetically Modified Aedes aegypti to Control Dengue: A Review. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.E.; Franz, A.W. Advances in genetically modified Aedes aegypti to control transmission of dengue viruses. Futur. Virol. 2015, 10, 609–624. [Google Scholar] [CrossRef]
- Golstein, C.; Boireau, P.; Pagès, J.-C. Benefits and limitations of emerging techniques for mosquito vector control. Comptes Rendus Biol. 2019, 342, 270–272. [Google Scholar] [CrossRef]
- Joubert, D.A.; Walker, T.; Carrington, L.B.; De Bruyne, J.T.; Kien, D.H.T.; Hoang, N.L.T.; Chau, N.V.V.; Iturbe-Ormaetxe, I.; Simmons, C.P.; O’Neill, S.L. Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management. PLoS Pathog. 2016, 12, e1005434. [Google Scholar] [CrossRef]
- Jr, R.C.R.; Achee, N.; Barrera, R.; Burkot, T.R.; Chadee, D.D.; Devine, G.; Endy, T.; Gubler, D.; Hombach, J.; Kleinschmidt, I.; et al. Quantifying the Epidemiological Impact of Vector Control on Dengue. PLoS Negl. Trop. Dis. 2016, 10, e0004588. [Google Scholar] [CrossRef]
- Ndii, M.Z.; Hickson, R.; Allingham, D.; Mercer, G. Modelling the transmission dynamics of dengue in the presence of Wolbachia. Math. Biosci. 2015, 262, 157–166. [Google Scholar] [CrossRef]
- Carvalho, S.A.; da Silva, S.O.; Charret, I.D.C. Mathematical modeling of dengue epidemic: Control methods and vaccination strategies. Theory Biosci. 2019, 138, 223–239. [Google Scholar] [CrossRef]
- Perera, S.; Perera, S.S.N. Mathematical modeling and analysis of innate and humoral immune responses to dengue infections. Int. J. Biomath. 2019, 12, 1950077. [Google Scholar] [CrossRef]
- Zhao, S.; Musa, S.S.; Meng, J.; Qin, J.; He, D. The long-term changing dynamics of dengue infectivity in Guangdong, China, from 2008-2018: A modelling analysis. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 62–71. [Google Scholar] [CrossRef] [PubMed]
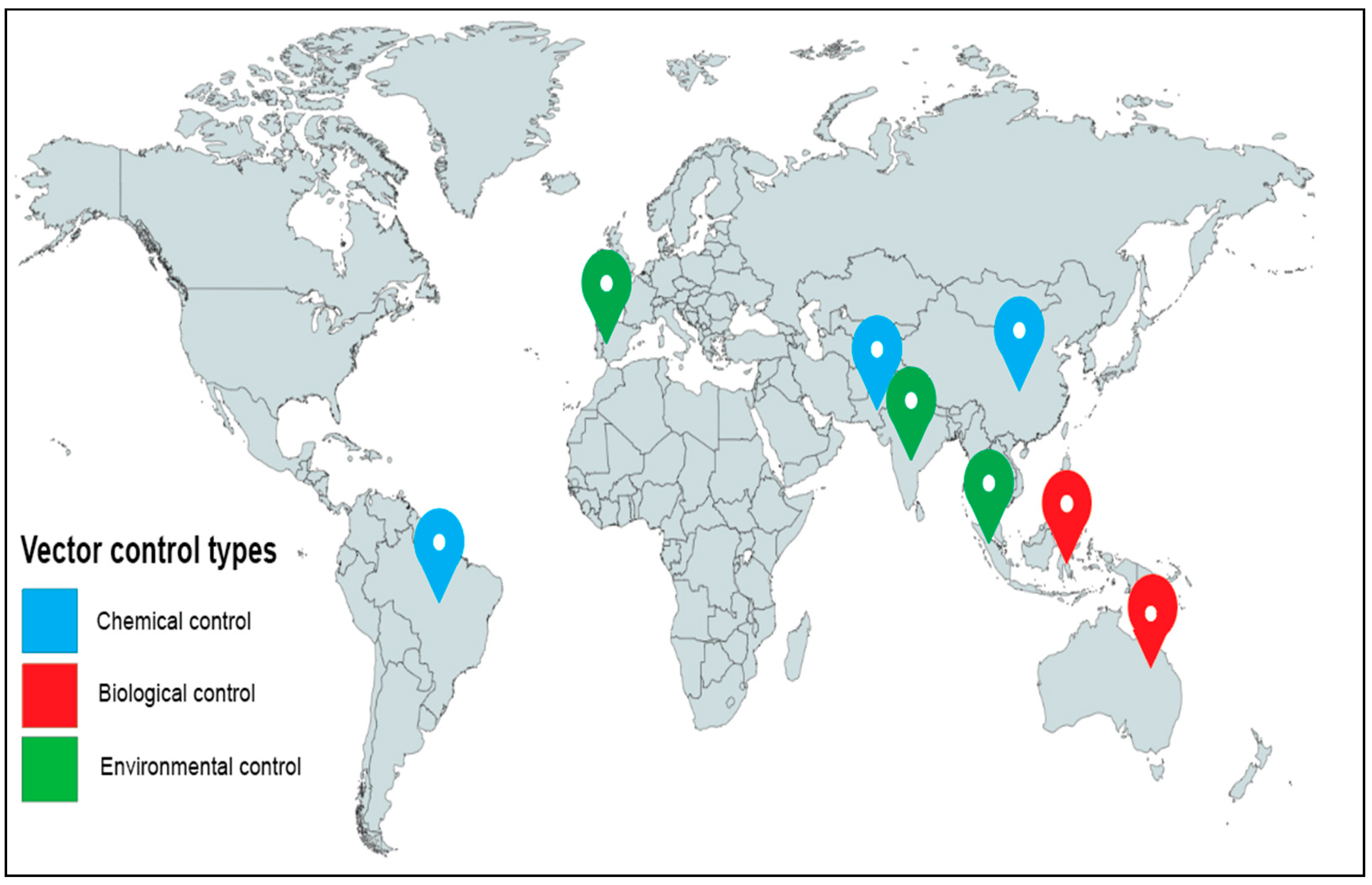

| S/No. | Reference | Year | Aims and Objectives of Study | Modelling Methods | Settings | Vector Control Technique | Summary of Findings/Conclusion |
|---|---|---|---|---|---|---|---|
| 1 | Abad-Franch et al. [46] | 2017 | Explored the mosquito-disseminated larvicide pyriproxyfen for vector control via arboviral blockage | DM | Brazil: Manacapuru | C | Following the mosquito-disseminated insecticides (pyriproxyfen), there were drastic decreases in the emergence and catch of adult and young Aedes mosquitoes, respectively. This reduction inhibited the transmission of Aedes-borne viruses, such as dengue, chikungunya, and Zika. |
| 2 | Agusto and Khan [37] | 2018 | Developed a deterministic dengue virus transmission model and parameterized it using 2017 dengue outbreak data in Pakistan. A sensitivity analysis was conducted, and optimal control theory was applied. | DM | Pakistan | C | There is a strong reciprocal relationship between vaccination and the use of insecticides. Nonetheless, the use of insecticides slightly increases when there is a decrease in vaccination levels as a result of an increase in cost. Application of the two time-dependent controls derived from the sensitivity analysis could decrease the total number of infected mosquitoes and humans. |
| 3 | Alphey et al. [38] | 2011 | Combined epidemiological models and mosquito population dynamics to investigate the effect of releasing RIDL (release of insects carrying a dominant lethal) on dengue virus transmission. | DM | n/a | B | Having derived a preliminary estimate of the potential cost-effectiveness of vector control, it was predicted that the genetic control technique could swiftly eliminate dengue disease from a human community at a very low expense. |
| 4 | Andraud et al. [39] | 2013 | Developed a simple periodic-forced vector-host model. This model was based on a previously formulated model that investigated the impact of vector control techniques during a dengue outbreak in Singapore in 2005. The model in this work considered the seasonal variations in vector density and estimated the parameters using dengue fever incidence data from August 2003 to the end of 2007. | DM | Singapore | E | After fitting the model outputs with the dengue incidence data, there was a good fit, which suggests that the impact of seasonality on dengue transmission dynamics was highly essential, even though the model did not consider the complex life cycle of the vector. Additionally, the seasonal fluctuations of the mosquito vector population occurred in phase with the variations in temperature. This signified a strong climatic effect on the vector abundance, thereby affecting the dengue virus transmission dynamics. |
| 5 | Barmak et al. [47] | 2014 | Presented a stochastic dynamical model for the transmission dynamics of dengue. This model accounted for the coevolution of human hosts and the spatial Aedes aegypti dynamics. | SM | n/a | C | For insecticide spraying techniques with different efficiencies, it was observed that the most efficient fumigation strategies could be effective during a dengue virus outbreak. Also, isolating infected humans with high compliance levels is an effective strategy; however, imposing restrictions on their movement is not likely to be effective. Therefore, combining fumigation and infected human isolation during a dengue outbreak would be a suitable strategy for mitigating the outbreaks. |
| 6 | Bliman et al. [32] | 2019 | Proposed a sex-structured model that captured the constant and periodic impulsive releases of sterile male Aedes mosquitoes in the hopes of eliminating wild-type mosquitos. This model serves as a foundation for vector control strategies. | DM | n/a | B | A mixed control strategy that requires the combination of open- and close-loop outputs that produce the best results regarding the total number of releases of sterile male mosquitoes to be effectively rolled out during the rollout program and the time required to achieve elimination. |
| 7 | Buonomo and Della Marca [48] | 2018 | Considered a mathematical model accounting for the use of insecticide-treated bed nets (ITN) by humans. The effect of seasonality, together with some varied rainfall and mean temperature scenarios, was investigated. The optimal control problem was used to mitigate the number of infected individuals, and a cost-effectiveness analysis was conducted to assess the most appropriate strategy for the elimination of dengue infection. | DM | n/a | C | The cost-effectiveness analysis showed that the benefits of the cost of intervention efforts were influenced by the shift in the periodic amplitude of the seasonal fluctuation. In general, of all the combination strategies for dengue disease control via its vectors considered, the most effective, averting the highest proportion of infections, is the use of ITN and insecticide spraying techniques. However, for areas with a low seasonality effect, only insecticide spraying campaigns should be conducted in the dengue control program as this is beneficial in terms of cost. |
| 8 | Cai et al. [40] | 2018 | Considered an interactive dynamical model of wild-type and sterile mosquitoes and accounted for the delay of the growth stage of the wild-type mosquito population. An analysis of the effect of the time delay of releasing sterile mosquitoes in two different rollouts was performed. | DM | n/a | B | At a constant release rate of sterile mosquitoes, the delay poses an insignificant effect on the system dynamics, and all the solutions of the system tend to an equilibrium point. At a release rate of sterile mosquitoes proportional to that of wild-type mosquitoes, the delay exhibits a significant effect on the system dynamics via some parameter ranges. For a small delay, the solutions tend to an equilibrium point. However, as the delay increases, the solutions of the system possess oscillatory behaviour by way of Hopf bifurcations. |
| 9 | Campo-Duarte et al. [41] | 2018 | A sex-structured population model was proposed describing the interaction between uninfected (male and female) and infected mosquitoes (via deliberate transinfection) with the wMelPop-Wolbachia strain in the same region. This model incorporated the natural introduction of the control or decision variable and introduced the optimal control approach to capture the dynamics of the wMelPop Wolbachia infection of the uninfected Aedes aegypti mosquito population. This was a targeted quest at estimating the number of Wolbachia-infected mosquitoes to be released in daily control action. | DM | n/a | B | The release policies derived from the model results, which are also consistent with Yeap et al. (2014), recommendations: (a) The release of Wolbachia-infected mosquitoes should be of considerable quantities; (b) releases of Wolbachia-infected mosquitoes should occur for a long time period; (c) the wMelPop Wolbachia strain invasion is only likely feasible in relatively isolated mosquito populations. Additionally, the method derived in this study can be advantageous to vector control interventions such that if the population density of wild-type mosquitoes is minimized at earlier stages by other control measures, such as SIT and insecticide spraying, the invasion of the wMelPop Wolbachia strain and replacement of wild mosquitoes can be swiftly attained at a low cost. |
| 10 | Chavez et al. [49] | 2017 | Presents a SIR model accounting for vector–host transmission dynamics and vice versa. The model incorporates pesticide control and seasonal variations of vector resurgence and disease transmission rates. Also, the effectiveness of the control strategy is investigated. | DM | n/a | C | Upon investigating the seasonal fluctuations, it was revealed that the timing of the applications of pesticides was highly influential in controlling dengue viral infection, i.e., in the required amount of pesticide to achieve tolerably moderate levels of infection. Also, time variations in the second pesticide application showed induced destabilization caused by a periodic-doubling bifurcation. Therefore, the solution within a year period loses stability, and a class of stable solutions within a two-year period occurs. Hence, the numerical investigations showed that avoiding the two-year periodic solution is best due to the drastic increase of dengue viral infections during the period. |
| 11 | Hancock et al. [50] | 2016 | Proposed a mathematical model to explain the transmission dynamics between Aedes aegypti mosquitoes and the intracellular bacterium, Wolbachia, which accounts for larval density-dependent fluctuation in fitness components of Wolbachia-infected and wild mosquitoes. This model was applied to study Wolbachia field releases and revealed how Wolbachia invasion end results could be highly dependent on the severity of the population density-dependent competition at the rollout locality. Following Wolbachia rollout programs, the period for establishing Wolbachia in the wild mosquito population can differ by over two years as this depends on the relative mosquito fitness of the field and laboratory conditions. | DM | n/a | B | The investigated models incorporating larval density-dependent demographical variation in mosquito traits are effective in elaborating Aedes aegypti mosquitos and Wolbachia dynamics in experimental mosquito populations. These models highlight the strong effects of mosquito density-dependence on Wolbachia dynamics in the field as well as help in controlling arboviral transmissions, such as Zika, dengue, and chikungunya, via the effective use of Wolbachia. |
| 12 | He et al. [51] | 2017 | Proposed multi-scale modelling incorporating the combination of a birth-pulse model with a genetically induced discrete model for the allelic frequencies. This model described the invasive spread of Wolbachia infection in mosquitoes resistant to CI. | DM | n/a | B | The results showed that the strategy for population eradication might not be actualised. However, a population replacement strategy may be feasibly realized with success to sensitive or resistant alleles. The failure or success of population replacement by Wolbachia may be dependent on the appropriate Wolbachia strain selected. Also, Wolbachia-induced parameters may cause catastrophic shifts in the stable states of the model system and may affect the rate of population replacement and density of wild mosquitoes. |
| 13 | Hughes and Britton [52] | 2013 | Developed a mathematical model used to describe the Human-mosquito dynamics in the presence of Wolbachia infection. The model further accounts for the introduction of Wolbachia-infected mosquitoes, which serves as a potential control measure for dengue transmission. | DM | n/a | B | The model results showed that the Wolbachia bacterium has the potential to control dengue transmissions in regions of moderate endemicity (that is, when the reproductive number, R0, is not too large). But if R0 is very high, Wolbachia can only have a slight effect on the population as it can only reduce but not eradicate the transmission of dengue. Moreover, if control strategies, such as mosquito population reduction, are adapted, combining the introduction of various strains of Wolbachia that completely inhibit dengue transmission may be worthwhile. |
| 14 | Li and Liu [53] | 2018 | Established a sex-structured model with birth pulse and investigated Wolbachia invasion dynamics and spread into the Aedes mosquito population. Additionally, it also studies the release strategies of Wolbachia-infected mosquitoes in wild mosquito populations. | DM | n/a | B | The modelling results showed that perfect maternal transmission drives a successful invasion of Wolbachia infection in mosquitoes. However, in the case of imperfect maternal transmission, either a partial replacement of the Wolbachia infection or Wolbachia extinction may occur. Further simulations revealed that the partial success of the Wolbachia replacement strategy is dependent on the number of initial Wolbachia-infected mosquitoes present. |
| 15 | Luz et al. [54] | 2011 | Developed a model describing the transmission dynamics of dengue that accounts for the evolution of insecticide resistance and immune responses in humans. In line with this, the dengue health burden of disability-adjusted life years was measured, and a cost-effectiveness analysis of insecticide control use was performed. Also, sensitivity and threshold analyses were performed to investigate the uncertainties of the parameters used in the results. | DM | n/a | C | Continual yearlong larval control can be ineffective at fuelling an increase in the burden of dengue epidemics as a result of the evolution of insecticide resistance and herd immunity loss. Additionally, six annual high-efficacy adult vector control applications have a cost-effectiveness ratio that may align with that of the WHO’s laydown standard. |
| 16 | Marini et al. [33] | 2019 | Developed a stochastic transmission model, which accounted for the geographical distribution of Aedes mosquitoes and human population and spatial transmission dynamics of dengue in Porto Alegre, Brazil. This model described the estimation of dengue cases that were avoided by ultra-low volume (ULV) insecticide spraying in the study region. | SM | Brazil: Porto Alegre | C | It was shown that a quarter of all the symptomatic cases were averted by insecticide spraying and low-income-induced Aedes aegypti mosquito death decreased intervention performance, as almost half of the mosquito population was killed by insecticide spraying. |
| 17 | Mishra et al. [55] | 2018 | Proposed a network model that described the host–vector dynamics in n patches to control dengue transmission. In this case, the control was based on sterile insect techniques (SIT). The required R0s were computed, and the existence and stability criteria for the steady states were analysed. Bifurcation effects were also investigated in relation to the disease-free and endemic equilibrium for an isolated patch. | NM | n/a | B | Following the analytical and numerical solutions, it was shown that dengue could be controlled in a network by adopting SIT in only one patch as it is required less to apply SIT to the whole network. This could be done by patch coupling. The applicable success of SIT relies on the coupling strength of the migration parameter and the recruitment rate of the sterile mosquito population. |
| 18 | Ndii et al. [42] | 2016 | Developed a mathematical model to investigate the effect of an endosymbiotic intracellular bacteria, Wolbachia, on the transmission dynamics and seasonality of dengue disease. The study focused on areas where dengue is not endemic but can spread as a result of human movement, especially with dengue imported cases. | DM | Australia: Cairns | B | The results of the study showed that Wolbachia decreased the total dengue case number by about 80%. Also, dengue outbreak times could be reduced by approximately 1.5 months annually in the presence of Wolbachia. The most significant effect was obtained when the seasonal force amplitude was low. Furthermore, the benefits of Wolbachia were dependent on the transmission rate. |
| 19 | Oki et al. [56] | 2011 | Formulated an SEIR model for dengue transmission capturing seasonal changes in mosquito lifespan and the optimal timing of insecticide fogging to mitigate the dengue disease burden in several wet season scenarios. Also, the assessment of insecticide fogging was simulated and studied at low and high levels of dengue endemicity over a 500-year time period producing an endemic state. | DM | n/a | C | The results showed that seasonal variation and the level of transmission intensity largely influenced the optimal timing of insecticide fogging and its impact. Insecticide fogging applications at optimal timing could control a substantial number of dengue virus cases. |
| 20 | O’Reilly et al. [43] | 2019 | Used the combination of multiple modelling methods for estimating the dengue disease burden to predict the dengue national case burden stratified by disease severity. Three different sources of data were used to map the spatial distribution of disease burden. Following a national release program of Wolbachia, the estimation of decreased dengue cases was performed using a collection of transmission models. | DM | Indonesia: Yogyakarta city | B | The results showed that about 7.8 million were estimated to have symptomatic cases of dengue in Indonesia in 2015. This estimated number of cases was related to about 3.23 thousand DALYs. The majority of the burden was due to underreporting as some asymptomatic or less severe dengue patients sought medical attention or had difficulty with disease diagnosis, respectively. The implementation of the national Wolbachia rollout program was estimated to significantly decrease dengue cases by 86.2% over the long term. |
| 21 | Pleydell and Bouyer [57] | 2019 | Modelled the dynamics of Aedes mosquito populations incorporating the SIT, boosted SIT with pupicide pyriproxifen (BSIT), and/or auto dissemination technique (ADT). Additionally, the rate of rolling out sterile male mosquitoes and competitiveness threshold were identified. | DM | n/a | B | Boosting decreased the thresholds in sterile male release rate and fuelled the mosquito’s destabilisation. There was no bifurcation in the ADT sub-model. Also, BSIT could avert over 95% of the overall rollout to mitigate dengue burden than SIT, suggesting that BSIT is effective in the control management of Aedes mosquitoes. |
| 22 | Qu et al. [44] | 2018 | Developed a two-sex mosquito model to describe the potential effectiveness of Wolbachia transmission for controlling mosquito-borne diseases. This model accounts for the Wolbachia transmission dynamics and incorporates the aquatic stage and various pregnant stages of adult female mosquitoes and heterosexual transmission. The R0 was computed. A threshold effect, which is driven by a backward bifurcation with three coexisting equilibria, is identified. The sensitivity analysis of the model parameters and the effectiveness of different migration strategies were investigated. | DM | n/a | B | It was shown that if R0 is less than one, the endemic equilibrium can still be stable via the backward bifurcation effect. Furthermore, there is a threshold condition for which a proportion of mosquitoes must exceed for Wolbachia establishment to occur in wild-type mosquitoes. In addition, the best way to establish Wolbachia infection in mosquitoes is to decrease the wild-type mosquito population either by insecticide spraying or mosquito traps and then introduce male and pregnant female mosquitoes infected with Wolbachia infections. |
| 23 | Robert et al. [58] | 2013 | A reduce and replace (RandR) strategic model, which numerically accounts for the release of insects (dengue vector Aedes aegypti mosquitoes) possessing the anti-pathogenic and female-killing trait, was proposed. In other words, this model described the strategic release of Aedes aegypti mosquito carrying RandR strain to suppress mosquito-borne diseases, such as dengue. | DM | n/a | B | Following the modelling results, it was shown that continuous release of RandR may temporarily reduce the density of the Aedes mosquito population, and this reduction may be long-lasting in the absence of fitness cost being related to the anti-pathogenic gene. Also, the swift RandR strain releases have a long-term reduction of vector densities compared to only female-killing rollout. Furthermore, the degree of reduction in overall mosquito densities depends on female inclusion in the rollout strategy, the release duration and release proportion. |
| 24 | Salami et al. [59] | 2020 | A deterministic model was adopted to portray the transmission dynamics of dengue in the Aedes aegypti mosquito population. This model accounts for the influence of seasonal fluctuating temperatures by integrating empirical and idealistic parameter tools. The epidemic dynamics of the seasonality influence were investigated following an imported case via the arrival of an infectious person. A sensitivity analysis was also performed on the interested quantities: peak time, epidemic peak size, and final epidemic size. | DM | Funchal, Madeira Island | E | The model results showed that the autumn and summer seasons could fuel dengue transmission, with the arrival date of an infectious person greatly affecting the time and peak size distribution of the dengue epidemic. Interestingly, late-summer infectious individual arrivals could generate large epidemics within a short time amplitude. It was also revealed that seasonality affects the epidemic dynamics. This suggests that large epidemics with a short time amplitude could be produced with starting warm temperatures and vice versa. The sensitivity analysis showed that the interested quantities were most sensitive to changes in the arrival date, seasonal temperature, mortality and transmission rates and mosquito population. |
| 25 | Senapati et al. [60] | 2019 | A general multi-patch dengue model was formulated to describe the Spatio-temporal transmission dynamics of dengue disease and the effectiveness of various adult mosquito controls (i.e., efficacy and environmental persistence) to reduce the dengue burden. This model was fitted to monthly data of dengue cases in five regions of Kolkata, India, for a period of two years (from 2014 to 2015). | SM | India: Kolkata | E | The results showed that control strategies with higher environmental persistence are more effective compared with the strategies with low environmental persistence. Also, the effectiveness of adult control strategies is greatly influenced by the spatial coupling (connectedness) between the regions. Amongst the three control strategies considered—ultra-low-volume (ULV) spray of insecticides; insecticide treatment of surfaces and materials; and use of lethal ovitraps—the most effective in reducing dengue cases is the treatment of surfaces and materials, while the least effective is ULV. |
| 26 | Strugarek et al. [34] | 2019 | Derived a minimalistic mathematical model incorporating the sterile insect technique (SIT) and incompatible insect technique (IIT) to eliminate the Aedes mosquito population. Unlike other previous models, the model considered in this study is bistable as it accommodates mosquito population elimination and survival. Different types of releases, which are constant, periodic, or impulsive releases, were considered as the necessary conditions for elimination were shown. Estimation of the parameters using an Aedes polynesiensis population study and both sufficient and minimal treatment times were performed, and both analytical and numerical results were analysed. | DM | n/a | B | The results showed that the mating competitiveness of the SIT control strategy needs to be close to one for effectiveness. If this is not the case, there may be limited efficacy if there are too few numbers of wild-type mosquitoes. Also, the mating parameter in the model is very important in the duration of controlling vectors via the SIT method, and it is suggested that entomologists focus more on the probability of mating between a male and a female mosquito with respect to the size of their habitat in their prospective experiments. |
| 27 | Tang et al. [61] | 2016 | Developed a mathematical model to imitate the impulsive vector control program and continuous treatment of patients and isolation in the Guangdong Province of China during the 2014 dengue outbreak. This vector program has occurred every week (specifically on Friday afternoons) since its inception. The accumulated dengue infection data were fitted using the parameterized model to perform a retrospective analysis. This analysis was used to estimate the basic and control R0 and the mosquito-killing ratios. | DM | China: Guangdong | C | The results showed the estimation of both basic and control R0 to be 1.7425 and 0.1709, respectively, suggesting a highly effective control of the dengue outbreak during the intervention program. It was also observed that when a Friday was skipped during the integrated program, this would not increase the control R0 to one; rather, it would increase the number of accumulated infections at the end of the disease outbreak. In all, a rapid and regular impulsive vector control implementation leads to an effective decrease in the control R0, which in turn significantly reduces new infections. |
| 28 | Thome et al. [62] | 2010 | Presented a mathematical model that captured the introduction of sterile male mosquitoes, besides the use of chemicals (insecticides), to biologically control the mosquito population. The optimal control strategy was used to search for the minimal effort required to decrease female mosquitoes that are productive by considering the cost of sterile male mosquito production, the cost of delivery to experimental sites, together with the social cost, and the cost of chemical application, such as insecticide. | DM | n/a | B | The model results showed that the social cost should be considered in controlling mosquito vectors as its exception when reducing the cost of other control strategies could result in unsuitable strategies. Furthermore, at the initial stage of the control strategy, high chemical insecticide application was required and then gradually decreased with time. Unless the social cost was multiplied by a hundred, the sterile male mosquito release should follow a bell-like curve with an increase and decrease at both ends together with a moderately flat middle. |
| 29 | Wijaya et al. [63] | 2014 | Presented an optimal control model, which described the dynamics of mosquito reduction management using chemicals, such as temephos, and conducted fumigation in dengue-endemic regions where mosquitoes are prevalent. The basic R0 was computed, and equilibrium stabilities were analysed. | DM | n/a | C | The results showed that if R0 is less than 1, the disease-free equilibrium (DFE) existed and was locally asymptotically stable, while the coexistence equilibrium (CE) did not exist. On the other hand, if R0 was greater than 1, the DFE was unstable, but the CE existed and was globally asymptotically stable in a positive region. Also, the best mosquito control strategies obtained from the optimal control analysis were obtained if the number of mosquitoes was small at the initial stages of control and additionally combined the use of temephos and fumigating activities. |
| 30 | Winskill et al. [64] | 2014 | Designed a compartmental model that accounted for the release dynamics of adult and pupal mosquitoes carrying RIDL. This model was used to fit an experimental data, which described the large-scale pupal mark release/recapture phenomena to determine pupal release dynamics. The simulation of pulsed releases of adult, pupae, or the combination of both was shown. Various release mechanisms of mosquito-carrying RIDL to sustain a long-lasting decrease in the wild-type mosquito population are investigated. | DM | n/a | B | For regular recurring releases, model simulations showed that releasing only adult-carrying RIDL mosquitoes performs better compared with the other releases: pupae only and combined adult-pupae releases, and vice versa for less recurring releases. The relative efficacy of releasing pupae is affected by the pupal emergence rate from the release apparatus. For a sustained, long-lasting reduction of wild mosquitoes in the presence of low recurrence, the combined adult–pupae mosquito release is more effective than the pupae-only or adult-only releases. |
| 31 | Zhang and Lui [35] | 2020 | Developed a mathematical model to investigate the Wolbachia transmission dynamics in Aedes-aegypti mosquitoes as a means of suppressing the spread of dengue. This model considered only female mosquitoes as they give infectious bites or obtain protein via bites to maturate their eggs. Equal numbers of male and female mosquitoes were assumed. Sensitivity and optimal control analysis were performed on model parameters. | DM | n/a | B | The model analysis revealed that without release, the model is bistable. This indicates that only one interior steady state is stable whenever it exists. Optimal control theory showed that halting a release after a continuous release for two years would allow the Wolbachia-only equilibrium to be locally asymptotically stable with time, suggesting the invasion of Wolbachia in all the mosquitoes and then resulting in the prevention of the spread of dengue viral infection. |
| 32 | Zhang et al. [65] | 2015 | Proposed a model that described the spread and invasion of Wolbachia infections accounting for the effects of CI and fitness effects. This model explored whether augmentation could inhibit the transmission of dengue in the field and also considered the question of why some rollout strategies were unsuccessful and what caused this failure in establishing population replacement. | DM | n/a | B | The stability analysis showed that some phenomena may have contributed to the failure of the Wolbachia invasion in wild mosquitoes. Such attractors include backward bifurcation and augmentation mechanisms, such as frequency, quantity, and timing. In all, the modelling result revealed that the successful establishment of Wolbachia infection via replacing the wild mosquitoes with Wolbachia-infected mosquitoes would depend on the type of Wolbachia strains selected for deployment and appropriate augmentation techniques. |
| S/No. | Author | Year | Aims and Objectives/Abstract | Intervention Comparators | Outcome Measures Defined | Model Structure and Flowchart | Modelling Methods | Parameters Specified | Assumptions Explicit and Justified | Quality of Data and Uncertainty and/or Sensitivity Analyses | Model Validation | Presentation of Results | Interpretation and Discussion of Results | Conflicts of or Competing Interest Declared | Final Point | Final Score (%) | Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abad-Franch et al. [46] | 2017 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 21 | 87.50 | Very High |
| 2 | Agusto and Khan [37] | 2018 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 21 | 87.50 | Very High |
| 3 | Alphey et al. [38] | 2011 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 22 | 91.67 | Very High |
| 4 | Andraud et al. [39] | 2013 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 19 | 79.17 | High |
| 5 | Barmak et al. [47] | 2014 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 20 | 83.33 | Very High |
| 6 | Bliman et al. [32] | 2019 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 2 | 18 | 75.00 | High |
| 7 | Buonomo and Della Marca [48] | 2018 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 0 | 18 | 75.00 | High |
| 8 | Cai et al. [40] | 2018 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 0 | 16 | 66.67 | High |
| 9 | Campo-Duarte et al. [41] | 2018 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 20 | 83.33 | Very High |
| 10 | Chavez et al. [49] | 2017 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 0 | 17 | 70.83 | High |
| 11 | Hancock et al. [50] | 2016 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 21 | 87.50 | Very High |
| 12 | He et al. [51] | 2017 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 2 | 18 | 75.00 | High |
| 13 | Hughes and Britton [52] | 2013 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 0 | 19 | 79.17 | High |
| 14 | Li and Liu [53] | 2018 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 0 | 18 | 75.00 | High |
| 15 | Luz et al. [54] | 2011 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 22 | 91.67 | Very High |
| 16 | Marini et al. [33] | 2019 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 22 | 91.67 | Very High |
| 17 | Mishra et al. [55] | 2018 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 0 | 17 | 70.83 | High |
| 18 | Ndii et al. [42] | 2016 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 20 | 83.33 | Very High |
| 19 | Oki et al. [56] | 2011 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 2 | 19 | 79.17 | High |
| 20 | O’Reilly et al. [43] | 2019 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 22 | 91.67 | Very High |
| 21 | Pleydell and Bouyer [57] | 2019 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 19 | 79.17 | High |
| 22 | Qu et al. [44] | 2018 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 20 | 83.33 | Very High |
| 23 | Robert et al. [58] | 2013 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 2 | 19 | 79.17 | High |
| 24 | Salami et al. [59] | 2020 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 21 | 87.50 | High |
| 25 | Senapati et al. [60] | 2019 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 18 | 75.00 | High |
| 26 | Strugarek et al. [34] | 2019 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 0 | 16 | 66.67 | High |
| 27 | Tang et al. [61] | 2016 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 18 | 75.00 | High |
| 28 | Thome et al. [62] | 2010 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 0 | 17 | 70.83 | High |
| 29 | Wijaya et al. [63] | 2014 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 0 | 18 | 75.00 | High |
| 30 | Winskill et al. [64] | 2014 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 20 | 83.33 | Very High |
| 31 | Zhang and Lui [35] | 2020 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 21 | 87.50 | Very High |
| 32 | Zhang et al. [65] | 2015 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 0 | 18 | 75.00 | High |
| Study Quality | Very High | High | Medium | Low | Total | |
|---|---|---|---|---|---|---|
| Vector Control Types | ||||||
| Chemical | 5 (50%) | 5 (50%) | 0 | 0 | 10 | |
| Biological | 8 (42%) | 11 (58%) | 0 | 0 | 19 | |
| Environmental | 0 (0%) | 3 (100%) | 0 | 0 | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogunlade, S.T.; Meehan, M.T.; Adekunle, A.I.; McBryde, E.S. A Systematic Review of Mathematical Models of Dengue Transmission and Vector Control: 2010–2020. Viruses 2023, 15, 254. https://doi.org/10.3390/v15010254
Ogunlade ST, Meehan MT, Adekunle AI, McBryde ES. A Systematic Review of Mathematical Models of Dengue Transmission and Vector Control: 2010–2020. Viruses. 2023; 15(1):254. https://doi.org/10.3390/v15010254
Chicago/Turabian StyleOgunlade, Samson T., Michael T. Meehan, Adeshina I. Adekunle, and Emma S. McBryde. 2023. "A Systematic Review of Mathematical Models of Dengue Transmission and Vector Control: 2010–2020" Viruses 15, no. 1: 254. https://doi.org/10.3390/v15010254
APA StyleOgunlade, S. T., Meehan, M. T., Adekunle, A. I., & McBryde, E. S. (2023). A Systematic Review of Mathematical Models of Dengue Transmission and Vector Control: 2010–2020. Viruses, 15(1), 254. https://doi.org/10.3390/v15010254







