Abstract
The real-world benefits of direct-acting antiviral (DAA)-induced sustained virologic response (SVR) on the de novo occurrence and progression of esophageal varices (EV) remain unclear in patients with hepatitis C virus (HCV)-related liver cirrhosis (LC). This is a retrospective cohort study evaluating all patients with Child-Pugh class A HCV-related LC during 2013 to 2020 in the Chang Gung Medical System. A total of 215 patients fit the inclusion criteria and were enrolled. Of them, 132 (61.4%) patients achieved DAA induced-SVR and 83 (38.6%) did not receive anti-viral treatment. During a median follow-up of 18.4 (interquartile range, 10.1–30.9) months, the 2-year incidence of de novo EV occurrence was 8 (7.0%) in the SVR group and 7 (12.7%) in the treatment-naïve group. Compared to the treatment-naïve group, the SVR group was associated with a significantly lower incidence of EV occurrence (adjusted hazard ratio [aHR]: 0.47, p = 0.030) and a significantly lower incidence of EV progression (aHR: 0.55, p = 0.033). The risk of EV progression was strongly correlated with the presence of baseline EV (p < 0.001). To the best of our knowledge, this is the first study to demonstrate that DAA-induced SVR is associated with decreased risk of de novo EV occurrence and progression in the real world.
1. Introduction
The natural history of patients with hepatitis C virus (HCV)-related compensated liver cirrhosis is now well characterized [1]. About 24% to 80% of patients with liver cirrhosis present with varices [2]. The development of hemorrhagic esophageal varices (EV)—a consequence of portal hypertension—is a major cause of cirrhosis-related morbidity and mortality [3]. Despite major improvements in management, mortality remains as high as 15% after the first episode of EV bleeding [4]. In a prospective study, the rate of incidence of EV was found to be 5% by year 1 and 28% by year 3, and the rate of EV progression was 12% at year 1 and 31% at year 3 for patients with cirrhosis and small EV [5]. The patients with small varices upon enrolment had a higher two-year risk of bleeding from EV than those without varices (12% vs. 2%) [5].
Predicting the development, bleeding, and mortality of EV in patients with cirrhosis is a key concern in clinical practice. It is well established that sustained virologic response (SVR) after interferon (IFN)-based anti-HCV treatment can prevent de novo EV development in patients with HCV-induced cirrhosis [5]. Since 2017, IFN-free direct-acting antiviral (DAA) drugs with a rate of SVR superior to that obtained from IFN-based treatment have been reimbursed by National Health Insurance (NHI) in Taiwan for treating HCV [6]. DAAs showed high efficacy against HCV infection. Pan-genotypic regimens, such as glecaprevir/pibrentasvir (GLE/PIB) and sofosbuvir/velpatasvir (SOF/VEL), showed particular promise during previous clinical studies focused on Taiwanese patients [7,8]. DAA-induced SVR was associated with improvements in liver stiffness and portal hypertension, which could be translate clinically into reductions in hepatic decompensation [9,10,11]. However, the effect of DAA-induced SVR on de novo EV development and progression was not clear. Although a previous study demonstrated that DAAs reduced the risk of bleeding from EV [12], this study included patients with alcoholism, hepatitis B-related cirrhosis, and cryptogenic cirrhosis. To our knowledge, no study has yet assessed the incidence of de novo occurrence and progression of EV following DAA-induced SVR.
2. Materials and Methods
2.1. Case Enrollment and Data Organization
The present retrospective cohort study, from January 2013 to December 2020, consists of a subgroup analysis based on electronic medical records obtained from the Chang Gung Research Database (CGRD), which is a de-identified and anonymous database [13,14]. The information comes from the various hospitals of the Chang Gung Medical System, the largest hospital system in Taiwan. The patient selection criteria are as follows: (i) presence of anti-HCV antibody and detectable HCV RNA; (ii) cirrhosis diagnosed for the first time during routine surveillance on transient elastography (TE) [15], acoustic radiation force impulse (ARFI) [16], ultrasound [17,18], and histology; and (iii) Child-Pugh class A. The exclusion criteria are as follows: (i) concurrent hepatitis B or human immunodeficiency virus infection; (ii) alcoholism; (iii) previous episodes of decompensation, defined as ascites, spontaneous bacterial peritonitis, encephalopathy, gastroesophageal varices, and hepatorenal syndrome; (iv) previous or active hepatocellular carcinoma (HCC), or cholangiocarcinoma (CCC); (v) previous local treatment for EV, such as EV ligation or endoscopic injection sclerotherapy (EIS); (vi) incomplete model for end-stage liver disease (MELD) score; and (vii) patients without SVR in the DAA group.
A total of 5606 patients with HCV-related liver cirrhosis were enrolled. We excluded patients with confounding factors, including 567 patients with HBV co-infection, 50 patients with HIV co-infection, 447 patients with alcoholism, 1542 patients with a history of HCC/CCC, and 196 patients who underwent previous EVL/EIS treatment. We also excluded 2483 patients without a record of esophago-gastro-duodenoscopy (EGD) prior to inclusion, 54 patients with enlarged EV, 521 patients without follow-up data to evaluate de novo occurrence and progression of EV, 84 patients without a complete MELD score, and 11 patients without SVR in the DAA group (Figure 1).
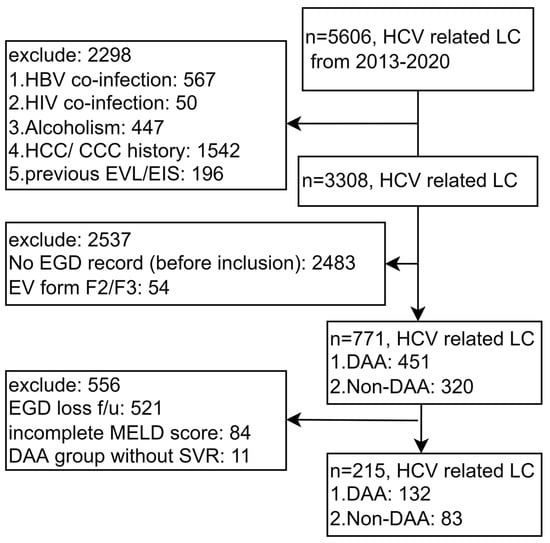
Figure 1.
Flowchart of the study. Abbreviations: EGD, esophago-gastro-duodenoscopy; HCV, hepatitis C virus; LC, liver cirrhosis; DAA, direct-acting antivirals; HBV, hepatitis B virus; HIV, human immunodeficiency virus; EVL, esophageal varices ligation, EIS, endoscopic injection sclerotherapy; HCC, hepatocellular carcinoma; CCC, cholangiocarcinoma; f/u follow up; MELD score, Model for End-Stage Liver Disease score; SVR, sustained virologic response.
2.2. EV and Endoscopy Criteria
The present study only considered patients who had undergone endoscopy prior to the time of enrollment (within 6 months) and were found to be EV-free or display a F1 degree of severity (grade 1 EV). Patients with EV severity of F2 or worse were excluded. Endoscopic procedures for EV assessment were performed by skilled endoscopists, and EV size was determined via medium insufflation and classified according to the North Italian Endoscopic Club score [19].
2.3. EV Occurrence/Progression
Retrospective endoscopic observation for EV was logged according to the following definitions. EV occurrence was defined as the development of EV in previously EV-free (F0) patients. EV progression was defined as the occurrence of F1~F3 EV in previously F0 patients, the occurrence of F2~F3 EV in previously F1 patients, or the onset of portal hypertension (PHT)-related bleeding episodes.
2.4. SVR in the DAA Group
Designation of patients in the DAA group to receive anti-HCV treatment was determined at the discretion of the treating physician on the basis of the labels approved by the Taiwan Food and Drug Administration and in compliance with the standard of care recommended by international guidelines for HCV infection [20]. SVR is defined as undetectable HCV RNA 12 weeks after the completion of DAA therapy [20].
2.5. Statistical Analysis
We considered inverse probability of treatment weighting (IPTW) with propensity score to estimate treatment efficacy in this study [21]. The IPTW-ATE (Average Treatment Effect) weighting method was used to account for variables such as age, sex, diabetes, dyslipidemia, arterial hypertension, obesity, non-carvedilol beta blocker usage, HCV genotype 1b, thrombocytopenia, MELD score, and/or initial EV form. The standardized mean difference (SMD) index was considered to evaluate whether these variables were sufficiently accounted for. When the absolute value of SMD is less than 0.1, it is accepted that there is no difference between the distributions of treatment modalities.
The incidence and progression of varices were visualized as Kaplan-Meier plots. Baseline demographic, clinical, and biochemical parameters, as well as ultrasonographic and endoscopic findings, were analyzed as possible predictors of study endpoints. Multivariate analyses were conducted using a Cox proportional hazards regression model. Results are presented as the adjusted hazard ratio (aHR) with the accompanying 95% confidence interval (CI) after adjustment for potential confounding factors. p-values are two-sided with p < 0.05 taken as statistically significant. All analyses were performed using SAS version 9.4 (SAS Inc., Cary, NC, USA).
3. Results
3.1. Baseline Case Characteristics
As demonstrated in Table 1, patients treated with DAAs (n = 132, 61.4%) and those treated without DAAs (n = 83, 38.6%) were of similar mean age (63.2 vs. 63.5 years, SMD = −0.057) and sex (49.3% vs. 46.5% male, SMD = −0.026) after IPTW. Comorbidities such as DM (43.9% vs. 43.3%, SMD = 0.013), dyslipidemia (32.2% vs. 29.1%, SMD = 0.067), arterial hypertension (58.7% vs. 57.0%, SMD = 0.035), obesity (6.0% vs. 5.7%, SMD = 0.009), history of beta blocker usage (6.1% vs. 6.9%, SMD = 0.033), HCV genotype 1b distribution (47.7% vs. 49.2%, SMD = −0.030), thrombocytopenia (49.3% vs. 49.3%, SMD = −0.016), MELD score (7.3 vs. 7.0, SMD < 0.001), and baseline EV F0 distribution (78.0% vs. 78.8%, SMD = −0.002) were also similarly distributed after IPTW. The median follow-up period was 18.4 months (interquartile range 10.1–30.9 months).

Table 1.
Baseline characteristics of patients.
3.2. Association between DAA and EV Occurrence/Progression
As shown in Figure 2a, the DAA group showed a lower rate of EV occurrence compared to that of the non-DAA group, with incidences of 6.0% vs. 13.3% at year 1, and 11.6% vs. 17.5% at year 2. Multivariable Cox proportional hazards model analysis after IPTW revealed that DAA usage (aHR 0.47, 95% CI 0.24–0.93, p = 0.030), MELD score (aHR 1.08, 95% CI 1.02–1.14 for 1 unit increase, p = 0.006), and female gender (aHR 22.5, 95% CI 5.58–91.1, p < 0.001) were significantly associated with the occurrence of EV (Table 2). Neither HCV genotype 1b nor thrombocytopenia was associated with the occurrence of EV.
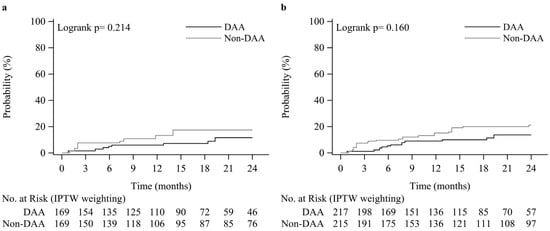
Figure 2.
Kaplan-Meier plots of the EV occurrence rate (a) and EV progression rate (b).

Table 2.
Multivariable Cox proportional hazards model analysis of factors associated with EV occurrence in HCV patients with liver cirrhosis.
As shown in Figure 2b, the DAA group showed a lower rate of EV progression compared to that of the non-DAA group, with incidences of 9.0% vs. 13.7% at year 1, and 15.1 % vs. 21.1% at year 2. Multivariable Cox proportional hazards model analysis after IPTW revealed that DAA usage (aHR 0.55, 95% CI 0.32–0.95, p = 0.033), baseline EV of grade F1 (vs. F0) (aHR 3.12, 95% CI 1.72–5.67, p < 0.001), female gender (aHR 6.53, 95% CI 2.98–14.3, p < 0.001), arterial hypertension (aHR 2.48, 95% CI 1.31–4.71, p = 0.006), and diabetes (aHR 2.23, 95% CI 1.21–4.13, p = 0.010) were significantly associated with EV progression (Table 3). Neither MELD score, HCV genotype 1b, nor thrombocytopenia was associated with the progression of EV.

Table 3.
Multivariable Cox proportional hazards model analysis of factors associated with EV progression in HCV patients with liver cirrhosis.
3.3. Progression of EV as a Function of DAA and Baseline EV Form
The cumulative probabilities of EV progression according to endoscopic findings at inclusion and the achievement of viral suppression (DAA induced SVR) are shown in Figure 3. The cumulative progression of EV at 0.5, 1, and 2 years was 5.0%, 6.0%, and 11.6%, respectively, among patients with no EV at inclusion and to whom DAA was administered, and 7.7%, 13.3%, and 17.5%, respectively, among patients with no EV at inclusion and to whom DAA was not administered. Similarly, cumulative progression at these time points was 7.0%, 21.6%, and 21.6%, respectively, among patients with F1 EV at inclusion and to whom DAA was administered, and 16.7%, 21.4%, and 33.2%, respectively, among patients with F1 EV at inclusion and to whom DAA was not administered. DAA-induced SVR and the absence of EV at inclusion were associated with a potential reduced risk of EV progression in patients with HCV-related liver cirrhosis (p = 0.050).
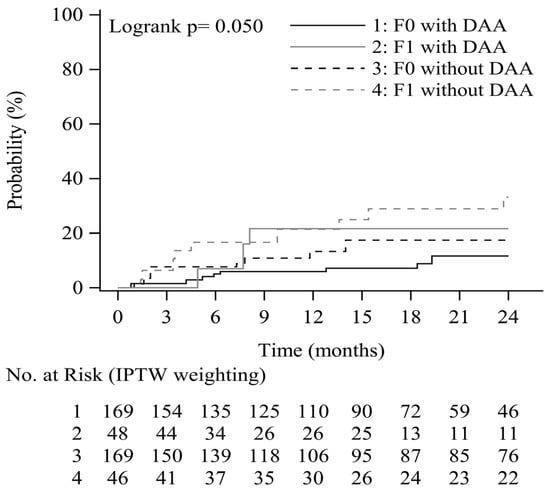
Figure 3.
EV progression rate by EV form and DAA treatment.
4. Discussion
This real-world multicenter retrospective study is based on a cohort of patients with HCV-related early cirrhosis who had received DAAs treatment or not. According to methods for comparative effectiveness, we used our observational data to emulate a hypothetical randomized trial by comparing DAA-exposed versus DAA-unexposed patients [22]. Its results show that patients treated with DAAs had significantly lower de novo EV occurrence and progression than those who did not receive DAAs. To the best of our knowledge, the benefit of DAAs on EV was demonstrated for the first time in our IPTW-ATE with propensity score analyses adjusted for demographic, liver function, and comorbidity-related characteristics.
DAAs are accepted as the standard of HCV care by many clinicians [23]. Therefore, it is impracticable to compare the patients who did versus those did not receive DAAs in a randomized controlled trial (RCT) design. As a result, the DAA and non-DAA groups may be compared using the IPTW-ATE method to correct for potential confounding.
The natural development and progression of EV are affected by many clinical factors such as the initial variceal status, EV ligation history, alcoholism, HBV or HIV coinfection, and hepatocellular carcinoma. We herein excluded these confounding factors to estimate causality due to treatment in this observational study. Other factors that may be involved in the development of EV, such as HCV genotype 1b [5], MELD score [5], and thrombocytopenia [24], were also pre-balanced.
In patients with pre-treatment cirrhosis, HCV eradication with SVR may stop progression or even induce regression of the fibrosis spontaneously, leading to improved portal hypertension and lower risk of variceal bleeding [25]. Savino et al. reported that HCV genotype 1b is an independent predictor of EV occurrence [5]. However, the higher occurrence of EV may be due to the lower IFN-induced SVR rate in patients with HCV genotype 1b [26], in which nonstructural 5A gene quasispecies mutations matters. In this study, HCV genotype is not a predictor owing to pangenotypic DAA with high potency.
The baseline MELD score was also an independent predictor of EV, which was noted by Bruno et al. [5] Similarly, in our study, the MELD score was significantly correlated with de novo EV occurrence (aHR 1.08; 95% CI 1.02–1.14 for 1 unit increase, p = 0.006), but not with EV progression (p = 0.299).
Sanyal et al. reported that the risk of having varices increases with decreasing platelet counts [24]. In our study, thrombocytopenia (platelet counts < 150,000/μL) has no significant correlation with de novo EV occurrence, nor with EV progression. This result is comparable with Qamar et al., who reported that “Platelet count is not a predictor of the presence or development of gastroesophageal varices in cirrhosis” [27].
DAA-induced SVR reduced the risk of EV bleeding in HCV-related liver cirrhosis patients in one clinical study [12]. Our study builds upon this work by considering key initial EV status data. We focus on de novo development of EV in only ethnic Asian patients to exclude confounding factors. In the previous study, data were obtained from the national Veterans Affairs (VA) healthcare system, which is composed of predominantly male (98.1%) patients. In the VA healthcare system, 50.3% of subjects consumed alcohol, and the ethnic makeup was 98% white, black, and Hispanic. Despite differences between the VA healthcare and our database, both studies infer that DAA reduces the risk of EV occurrence, progression, and bleeding. This has led to the hypothesis that DAA halts progression of HCV-related liver cirrhosis.
Besides SVR, metabolic syndrome also affects the natural history of PHT in cirrhosis [28]. In the previous IFN-based treatment for HCV, the patients with insulin resistance (type 2 DM) may be at higher risk for worse outcomes including a reduced SVR rate and early progression to fibrosis and cirrhosis [29,30]. In this study, DM (aHR 2.23, p = 0.010) and arterial hypertension (aHR 2.48, p = 0.006) have significant correlation with EV progression, but not EV occurrence. Morbidities of other metabolic syndromes such as obesity and dyslipidemia had no significant correlation with EV occurrence or progression.
Female gender correlated significantly with de novo EV occurrence and progression (p < 0.001) in this study. Khan et al. reported that women display a significantly different EV bleeding rate after particular treatment [31]. The possible mechanism may be due to hormones and pregnancy. Estrogen-increased venous capacitance and progesterone-weakened blood vessel walls were reported [32]. Pregnancy doubles blood volume in order to supply the baby. The increase in blood volume applies stress to the vessels and may cause varices [33].
According to the 2016 practice guidance about portal hypertensive bleeding in cirrhosis by the American Association for the Study of Liver Diseases (AASLD) [34], there is no evidence to support recommending non-selective beta blockers (NSBBs) in patients without varices. However, the primary prophylaxis of variceal hemorrhage is indicated for patients with small varices with red wale signs or decompensation. In clinical practice, early NSBBs to prevent EV development seems common particularly when hepatic venous pressure gradient (HVPG) information is not available. Despite that, this kind of practice may only be beneficial while administering carvedilol [35]. Other NSBBs, except for carvedilol, show a significantly higher incidence of adverse events. Nevertheless, these drugs offer no significance against EV development, upper gastrointestinal bleeding, or death [36]. In our study, non-carvedilol beta blockers showed potential higher EV progression (aHR 2.18, p = 0.051), which may be because patients with high-risk varices (e.g., red wale markings and enlarged EV) tend to also receive NSBBs. Conversely, NSBBs can not only be used to prevent variceal bleeding, but also to treat arterial hypertension, angina, tremor, and migraine; therefore, they are not considered specific enough. Hence, the use of NSBBs may be a potential confounder.
There are some limitations of this study. First, this is a study from Taiwan, where genotypes 4 and 5 are rare. Second, clinical data were not complete in this retrospective study and a majority of patients were therefore excluded. According to the flow chart (Figure 1), most of the patients were excluded because they did not undergo initial EGD or their EV condition was already very serious, which accounted for 2537 patients. In hope of proving that the included group was representative to the whole cohort, the Supplement Table S1 compared the differences between the “excluded” and “included” groups by the basic variables listed in “Table 1”. The numbers were divided into the included (n = 215) and excluded groups (n = 3093) from the total of 3308 in the flow chart (Figure 1). The result revealed that the only difference between the two groups was age. Other factors, such as comorbidity factors (DM, dyslipidemia, arterial HTN, obesity), platelets, MELD score, Fib4 score and DAA using were not significantly different (p > 0.05). Third, each modality for evaluating liver cirrhosis has particular shortcomings.
5. Conclusions
Despite early cirrhosis, SVR after DAAs significantly reduced the de novo occurrence and progression of EV during the short-term 24-month period study. In clinical practice, frequent EGD observation may be avoided in SVR patients. This study provides further evidence supporting the real-world benefits of early DAA treatment in HCV-positive patients with cirrhosis of the liver.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15010252/s1, Table S1: Comparison of included and excluded participants.
Author Contributions
Conception and design: Y.-Y.H. and T.-S.C.; methodology: Y.-Y.H., M.-H.L., Y.-H.L. and M.-S.L.; acquisition of data: Y.-Y.H., W.-M.C., K.-C.C., T.-S.C., C.-H.H., Y.-H.Y., S.-Y.T., K.-L.W., Y.-J.D., J.-H.H., Y.-T.H., C.-H.S., C.-S.W., M.-H.L. and C.-K.L.; analysis and interpretation of data (statistical analysis, biostatistics): Y.-Y.H. and M.-H.L.; writing, review, and revision of the manuscript: Y.-Y.H. and T.-S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by research grant from Chiayi Chang Gung Memorial Hospital, Taiwan (No. CGRPG6L0061).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Chang Gung Memorial Hospital (IRB 202100471B0, 13 April 2021).
Informed Consent Statement
The retrospective study used the data from Chang Gung Research Database (CGRD), which is a de-identified and anonymous database. The use of data during this study complied with the Personal Data Protection Law and IRB regulations. Our institutional review board reviewed and expedited this study by way of case study and/or clinical treatment/diagnosis. We ensure the security, privacy, and confidentiality of patient data, which was only used within the scope of the present study.
Data Availability Statement
The data that support the findings of this study are available on request from the first author with the permission of the IRB of Chang Gung Memorial Hospital, ChiaYi branch. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We thank the Health Information and Epidemiology Laboratory of Chiayi Chang Gung Memorial Hospital for their comments and assistance in the data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bruno, S.; Zuin, M.; Crosignani, A.; Rossi, S.; Zadra, F.; Roffi, L.; Borzio, M.; Redaelli, A.; Chiesa, A.; Silini, E.M.; et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: A long-term prospective study. Am. J. Gastroenterol. 2009, 104, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.; Fauerholdt, L.; Schlichting, P.; Juhl, E.; Poulsen, H.; Tygstrup, N. Aspects of the natural history of gastrointestinal bleeding in cirrhosis and the effect of prednisone. Gastroenterology 1981, 81, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Bosch, J. Management of varices and variceal hemorrhage in cirrhosis. N. Engl. J. Med. 2010, 362, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, N.; Pauwels, A.; Serfaty, L.; Fourdan, O.; Levy, V.G.; Poupon, R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology 2004, 40, 652–659. [Google Scholar] [CrossRef]
- Bruno, S.; Crosignani, A.; Facciotto, C.; Rossi, S.; Roffi, L.; Redaelli, A.; de Franchis, R.; Almasio, P.L.; Maisonneuve, P. Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology 2010, 51, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.H.; Pwu, R.F.; Chen, S.C. Achieving hepatitis C elimination in Taiwan-Overcoming barriers by setting feasible strategies. J. Formos Med. Assoc. 2018, 117, 1044–1045. [Google Scholar] [CrossRef]
- Huang, Y.T.; Hsieh, Y.Y.; Chen, W.M.; Tung, S.Y.; Wei, K.L.; Shen, C.H.; Chang, K.C.; Lu, C.K.; Yen, C.W.; Lu, S.N.; et al. Sofosbuvir/velpatasvir is an effective treatment for patients with hepatitis C and advanced fibrosis or cirrhosis in a real-world setting in Taiwan. BMC Gastroenterol. 2021, 21, 259. [Google Scholar] [CrossRef]
- Chang, K.C.; Tung, S.Y.; Wei, K.L.; Shen, C.H.; Hsieh, Y.Y.; Chen, W.M.; Chen, Y.H.; Chen, C.H.; Yen, C.W.; Xu, H.W.; et al. Real-world efficacy and safety of pangenotypic direct-acting antivirals against hepatitis C virus infection in Taiwan. Sci. Rep. 2021, 11, 13543. [Google Scholar] [CrossRef]
- Lens, S.; Alvarado-Tapias, E.; Marino, Z.; Londono, M.C.; Enrique, L.L.; Martinez, J.; Fortea, J.I.; Ibanez, L.; Ariza, X.; Baiges, A.; et al. Effects of All-Oral Anti-Viral Therapy on HVPG and Systemic Hemodynamics in Patients with Hepatitis C Virus-Associated Cirrhosis. Gastroenterology 2017, 153, 1273–1283. [Google Scholar] [CrossRef]
- Lens, S.; Baiges, A.; Alvarado-Tapias, E.; Enrique, L.L.; Martinez, J.; Fortea, J.I.; Ibanez-Samaniego, L.; Marino, Z.; Rodriguez-Tajes, S.; Gallego, A.; et al. Clinical outcome and hemodynamic changes following HCV eradication with oral antiviral therapy in patients with clinically significant portal hypertension. J. Hepatol. 2020, 73, 1415–1424. [Google Scholar] [CrossRef]
- Mandorfer, M.; Kozbial, K.; Schwabl, P.; Chromy, D.; Semmler, G.; Stattermayer, A.F.; Pinter, M.; Hernandez-Gea, V.; Fritzer-Szekeres, M.; Steindl-Munda, P.; et al. Changes in Hepatic Venous Pressure Gradient Predict Hepatic Decompensation in Patients Who Achieved Sustained Virologic Response to Interferon-Free Therapy. Hepatology 2020, 71, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.M.; Green, P.K.; Rockey, D.C.; Berry, K.; Ioannou, G.N. Hepatitis C eradication with direct-acting anti-virals reduces the risk of variceal bleeding. Aliment. Pharmacol. Ther. 2020, 51, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Lin, M.H.; Lee, C.P.; Yang, Y.H.; Chen, W.C.; Chang, G.H.; Tsai, Y.T.; Chen, P.C.; Tsai, Y.H. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed. J. 2017, 40, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.C.; Lai, E.C. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Changchien, C.S.; Hung, C.H.; Eng, H.L.; Tung, W.C.; Kee, K.M.; Chen, C.H.; Hu, T.H.; Lee, C.M.; Lu, S.N. FibroScan and ultrasonography in the prediction of hepatic fibrosis in patients with chronic viral hepatitis. J. Gastroenterol. 2009, 44, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Rust, M.; Nierhoff, J.; Lupsor, M.; Sporea, I.; Fierbinteanu-Braticevici, C.; Strobel, D.; Takahashi, H.; Yoneda, M.; Suda, T.; Zeuzem, S.; et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: A pooled meta-analysis. J. Viral Hepat. 2012, 19, e212–e219. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.Y.; Sheen, I.S.; Chiu, C.T.; Lin, S.M.; Kuo, Y.C.; Liaw, Y.F. Ultrasonographic changes of early liver cirrhosis in chronic hepatitis B: A longitudinal study. J. Clin. Ultrasound 1993, 21, 303–308. [Google Scholar] [CrossRef]
- Moon, K.M.; Kim, G.; Baik, S.K.; Choi, E.; Kim, M.Y.; Kim, H.A.; Cho, M.Y.; Shin, S.Y.; Kim, J.M.; Park, H.J.; et al. Ultrasonographic scoring system score versus liver stiffness measurement in prediction of cirrhosis. Clin. Mol. Hepatol. 2013, 19, 389–398. [Google Scholar] [CrossRef]
- North Italian Endoscopic Club for the, S.; Treatment of Esophageal, V. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N. Engl. J. Med. 1988, 319, 983–989. [Google Scholar] [CrossRef]
- Ghany, M.G.; Morgan, T.R.; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 2020, 71, 686–721. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef] [PubMed]
- Hernan, M.A.; Robins, J.M. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am. J. Epidemiol. 2016, 183, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Backus, L.I.; Belperio, P.S.; Shahoumian, T.A.; Mole, L.A. Impact of Sustained Virologic Response with Direct-Acting Antiviral Treatment on Mortality in Patients with Advanced Liver Disease. Hepatology 2019, 69, 487–497. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Fontana, R.J.; Di Bisceglie, A.M.; Everhart, J.E.; Doherty, M.C.; Everson, G.T.; Donovan, J.A.; Malet, P.F.; Mehta, S.; Sheikh, M.Y.; et al. The prevalence and risk factors associated with esophageal varices in subjects with hepatitis C and advanced fibrosis. Gastrointest. Endosc. 2006, 64, 855–864. [Google Scholar] [CrossRef]
- Rockey, D.C. Fibrosis reversal after hepatitis C virus elimination. Curr. Opin. Gastroenterol. 2019, 35, 137–144. [Google Scholar] [CrossRef]
- Pawlotsky, J.M.; Germanidis, G.; Neumann, A.U.; Pellerin, M.; Frainais, P.O.; Dhumeaux, D. Interferon resistance of hepatitis C virus genotype 1b: Relationship to nonstructural 5A gene quasispecies mutations. J. Virol. 1998, 72, 2795–2805. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.A.; Grace, N.D.; Groszmann, R.J.; Garcia-Tsao, G.; Bosch, J.; Burroughs, A.K.; Maurer, R.; Planas, R.; Escorsell, A.; Garcia-Pagan, J.C.; et al. Platelet count is not a predictor of the presence or development of gastroesophageal varices in cirrhosis. Hepatology 2008, 47, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Garcia-Tsao, G.; Bosch, J.; Grace, N.D.; Burroughs, A.K.; Morillas, R.; Escorsell, A.; Garcia-Pagan, J.C.; Patch, D.; Matloff, D.S.; et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology 2011, 54, 555–561. [Google Scholar] [CrossRef]
- Hammerstad, S.S.; Grock, S.F.; Lee, H.J.; Hasham, A.; Sundaram, N.; Tomer, Y. Diabetes and Hepatitis C: A Two-Way Association. Front. Endocrinol. 2015, 6, 134. [Google Scholar] [CrossRef]
- Romero-Gomez, M.; Del Mar Viloria, M.; Andrade, R.J.; Salmeron, J.; Diago, M.; Fernandez-Rodriguez, C.M.; Corpas, R.; Cruz, M.; Grande, L.; Vazquez, L.; et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology 2005, 128, 636–641. [Google Scholar] [CrossRef]
- Khan, M.S.; Majeed, A.; Ghauri, F. Comparison of carvedilol and esophageal variceal band ligation for prevention of variceal bleed among cirrhotic patients. Age Years 2017, 52, 54.07–54.54. [Google Scholar]
- Kristiansson, P.; Wang, J.X. Reproductive hormones and blood pressure during pregnancy. Hum. Reprod. 2001, 16, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Beebe-Dimmer, J.L.; Pfeifer, J.R.; Engle, J.S.; Schottenfeld, D. The epidemiology of chronic venous insufficiency and varicose veins. Ann. Epidemiol. 2005, 15, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017, 65, 310–335. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Kedarisetty, C.K.; Vashishtha, C.; Bhadoria, A.S.; Jindal, A.; Kumar, G.; Choudhary, A.; Shasthry, S.M.; Maiwall, R.; Kumar, M.; et al. Carvedilol delays the progression of small oesophageal varices in patients with cirrhosis: A randomised placebo-controlled trial. Gut 2017, 66, 1838–1843. [Google Scholar] [CrossRef]
- Qi, X.S.; Bao, Y.X.; Bai, M.; Xu, W.D.; Dai, J.N.; Guo, X.Z. Nonselective beta-blockers in cirrhotic patients with no or small varices: A meta-analysis. World J. Gastroenterol. 2015, 21, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).