Early Permissiveness of Central Nervous System Cells to Measles Virus Infection Is Determined by Hyperfusogenicity and Interferon Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Cells
2.3. Recombinant Virus Production and Analysis
2.4. Organotypic Cerebellar Culture Preparation and Treatment Post-Infection
2.5. Real-Time Quantitative PCR
2.6. Immunofluorescent Staining
2.7. Flow Cytometry
2.8. Statistical Analysis
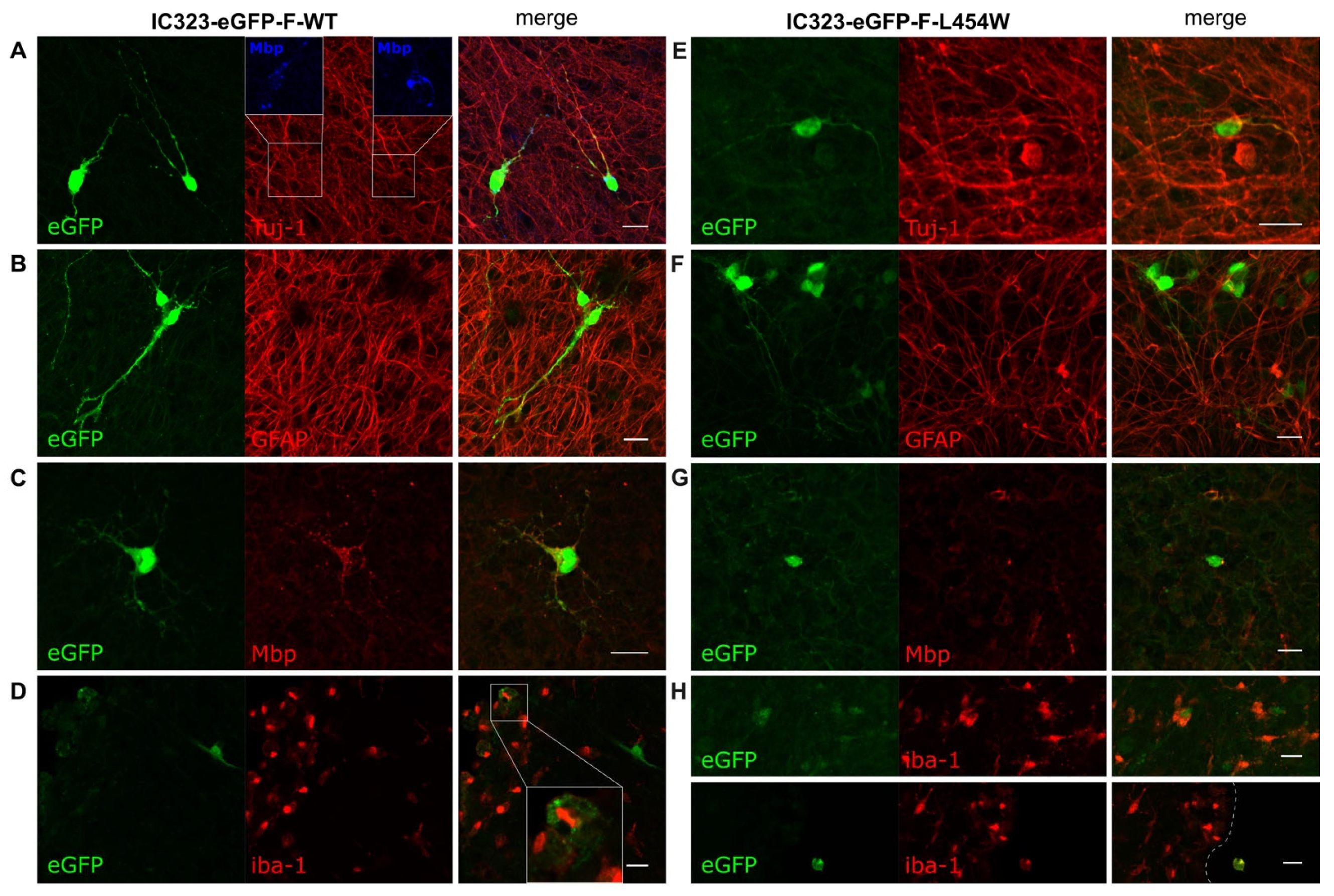
3. Results
3.1. Invasion of Hamster Cerebellum by MeV with wt F or F HRC Variant
3.2. Confocal Microscopy Analysis of MeV Early Neural Tropism
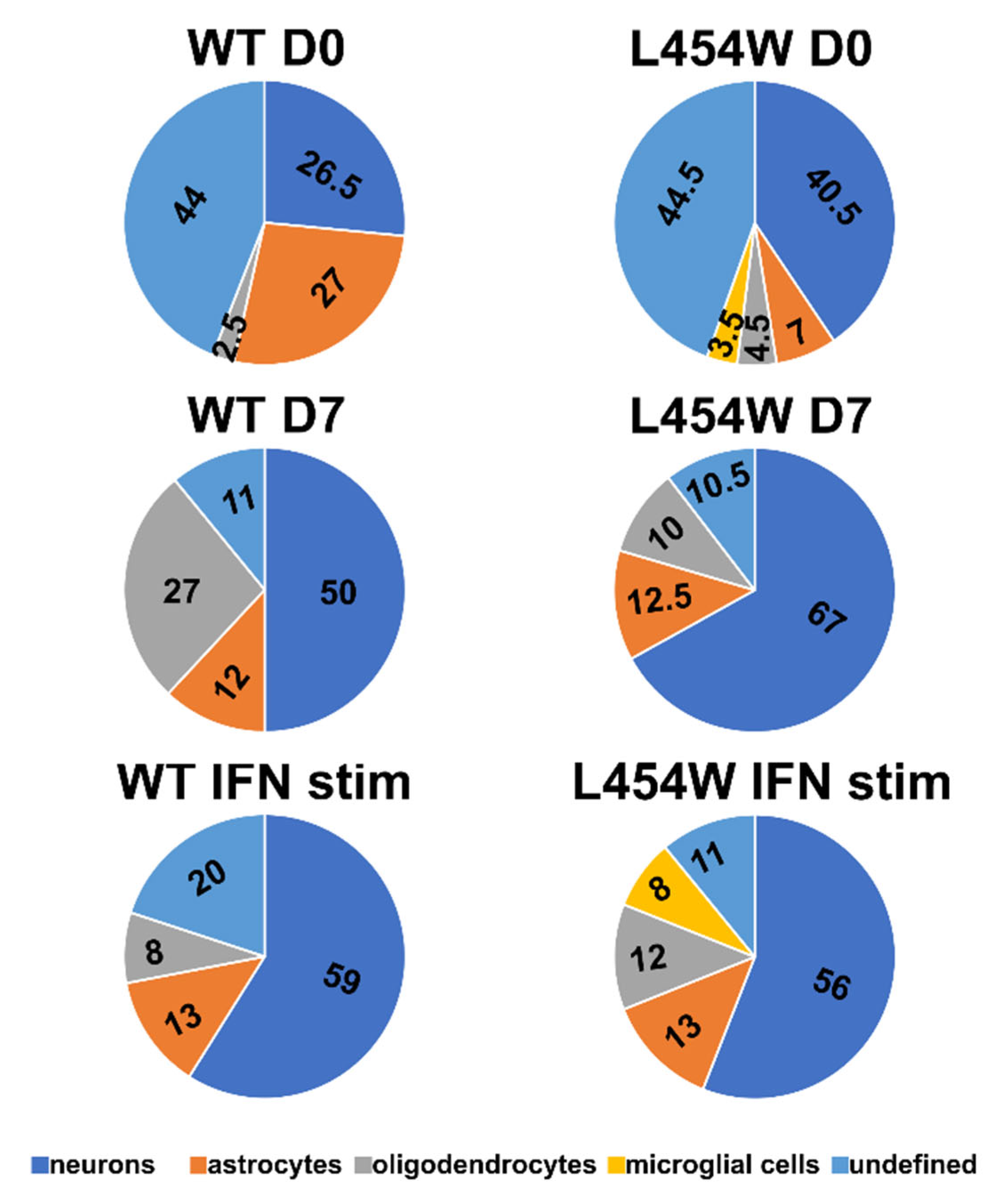
3.3. Early Neural Tropism of MeV Hyperfusogenic Variant Is Skewed toward Neurons
3.4. Astrogliosis Modifies Early Tropism of MeV
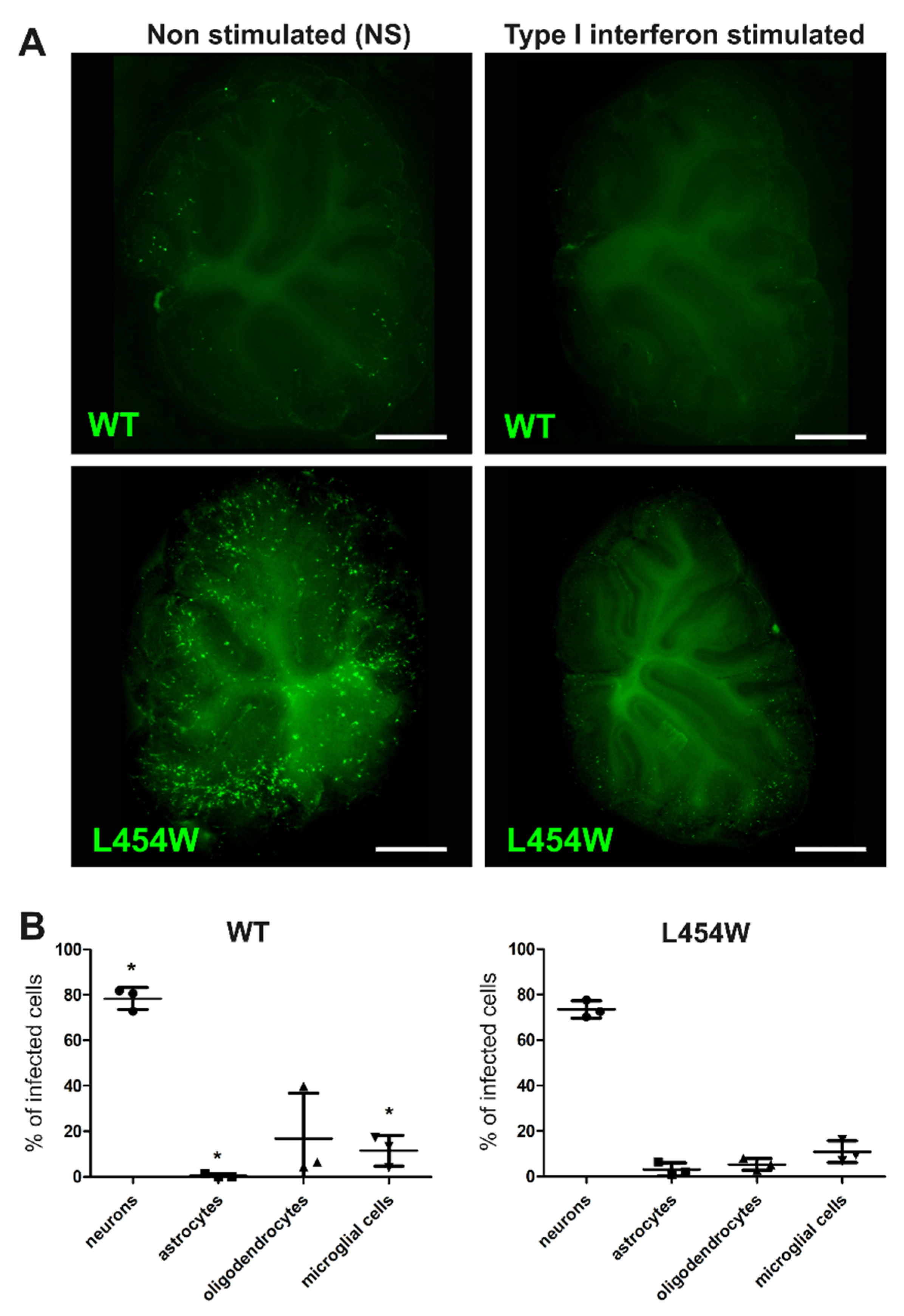
3.5. IFN-I Pretreatment Alters the Permissiveness of Neural Cells to MeV Infection
3.6. MeV Does Not Infect All Neurons
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, M.K.; Goodson, J.L.; Alexander, J.P.; Kretsinger, K.; Sodha, S.V.; Steulet, C.; Gacic-Dobo, M.; Rota, P.A.; McFarland, J.; Menning, L.; et al. Progress Toward Regional Measles Elimination—Worldwide, 2000–2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Ferren, M.; Horvat, B.; Mathieu, C. Measles Encephalitis: Towards New Therapeutics. Viruses 2019, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Noyce, R.S.; Bondre, D.G.; Ha, M.N.; Lin, L.-T.; Sisson, G.; Tsao, M.-S.; Richardson, C.D. Tumor Cell Marker PVRL4 (Nectin 4) Is an Epithelial Cell Receptor for Measles Virus. PLoS Pathog. 2011, 7, e1002240. [Google Scholar] [CrossRef] [PubMed]
- Mühlebach, M.D.; Mateo, M.; Sinn, P.L.; Prüfer, S.; Uhlig, K.M.; Leonard, V.H.J.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens Junction Protein Nectin-4 Is the Epithelial Receptor for Measles Virus. Nature 2011, 480, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Tatsuo, H.; Ono, N.; Tanaka, K.; Yanagi, Y. SLAM (CDw150) Is a Cellular Receptor for Measles Virus. Nature 2000, 406, 893–897. [Google Scholar] [CrossRef]
- Mathieu, C.; Ferren, M.; Jurgens, E.; Dumont, C.; Rybkina, K.; Harder, O.; Stelitano, D.; Madeddu, S.; Sanna, G.; Schwartz, D.; et al. Measles Virus Bearing Measles Inclusion Body Encephalitis-Derived Fusion Protein Is Pathogenic after Infection via the Respiratory Route. J. Virol. 2019, 93, e01862-18. [Google Scholar] [CrossRef]
- Mathieu, C.; Huey, D.; Jurgens, E.; Welsch, J.C.; DeVito, I.; Talekar, A.; Horvat, B.; Niewiesk, S.; Moscona, A.; Porotto, M. Prevention of Measles Virus Infection by Intranasal Delivery of Fusion Inhibitor Peptides. J. Virol. 2015, 89, 1143–1155. [Google Scholar] [CrossRef]
- Barrero, P.R.; Grippo, J.; Viegas, M.; Mistchenko, A.S. Wild-Type Measles Virus in Brain Tissue of Children with Subacute Sclerosing Panencephalitis, Argentina. Emerg. Infect. Dis. 2003, 9, 1333–1336. [Google Scholar] [CrossRef]
- Patterson, J.B.; Cornu, T.I.; Redwine, J.; Dales, S.; Lewicki, H.; Holz, A.; Thomas, D.; Billeter, M.A.; Oldstone, M.B. Evidence That the Hypermutated M Protein of a Subacute Sclerosing Panencephalitis Measles Virus Actively Contributes to the Chronic Progressive CNS Disease. Virology 2001, 291, 215–225. [Google Scholar] [CrossRef]
- Angius, F.; Smuts, H.; Rybkina, K.; Stelitano, D.; Eley, B.; Wilmshurst, J.; Ferren, M.; Lalande, A.; Mathieu, C.; Moscona, A.; et al. Analysis of a Subacute Sclerosing Panencephalitis Genotype B3 Virus from the 2009-2010 South African Measles Epidemic Shows That Hyperfusogenic F Proteins Contribute to Measles Virus Infection in the Brain. J. Virol. 2019, 93, e01700-18. [Google Scholar] [CrossRef]
- Jurgens, E.M.; Mathieu, C.; Palermo, L.M.; Hardie, D.; Horvat, B.; Moscona, A.; Porotto, M. Measles Fusion Machinery Is Dysregulated in Neuropathogenic Variants. MBio 2015, 6, e02528-14. [Google Scholar] [CrossRef]
- Mathieu, C.; Bovier, F.T.; Ferren, M.; Lieberman, N.A.P.; Predella, C.; Lalande, A.; Peddu, V.; Lin, M.J.; Addetia, A.; Patel, A.; et al. Molecular Features of the Measles Virus Viral Fusion Complex That Favor Infection and Spread in the Brain. MBio 2021, 12, e00799-21. [Google Scholar] [CrossRef]
- Mathieu, C.; Figueira, T.N.; Decker, A.R.; Ferren, M.; Bovier, T.F.; Jurgens, E.M.; Marcink, T.C.; Moscona, A.; Porotto, M. Measles Fusion Complexes from Central Nervous System Clinical Isolates: Decreased Interaction between Hemagglutinin and Fusion Proteins. bioRxiv 2021. [Google Scholar] [CrossRef]
- Welsch, J.C.; Charvet, B.; Dussurgey, S.; Allatif, O.; Aurine, N.; Horvat, B.; Gerlier, D.; Mathieu, C. Type I Interferon Receptor Signaling Drives Selective Permissiveness of Astrocytes and Microglia to Measles Virus during Brain Infection. J. Virol. 2019, 93, e00618-19. [Google Scholar] [CrossRef]
- Ferren, M.; Horvat, B.; Gerlier, D.; Mathieu, C. Type I interferon and selective permissiveness of central nervous system to measles virus infection. Med. Sci. 2021, 37, 22–25. [Google Scholar] [CrossRef]
- Watanabe, S.; Shirogane, Y.; Suzuki, S.O.; Ikegame, S.; Koga, R.; Yanagi, Y. Mutant Fusion Proteins with Enhanced Fusion Activity Promote Measles Virus Spread in Human Neuronal Cells and Brains of Suckling Hamsters. J. Virol. 2013, 87, 2648–2659. [Google Scholar] [CrossRef]
- Welsch, J.C.; Lionnet, C.; Terzian, C.; Horvat, B.; Gerlier, D.; Mathieu, C. Organotypic Brain Cultures: A Framework for Studying CNS Infection by Neurotropic Viruses and Screening Antiviral Drugs. Bio. Protoc. 2017, 7, e2605. [Google Scholar] [CrossRef]
- Ferren, M.; Favède, V.; Decimo, D.; Iampietro, M.; Lieberman, N.A.P.; Weickert, J.-L.; Pelissier, R.; Mazelier, M.; Terrier, O.; Moscona, A.; et al. Hamster Organotypic Modeling of SARS-CoV-2 Lung and Brainstem Infection. Nat. Commun. 2021, 12, 5809. [Google Scholar] [CrossRef]
- Allen, I.V.; McQuaid, S.; McMahon, J.; Kirk, J.; McConnell, R. The Significance of Measles Virus Antigen and Genome Distribution in the CNS in SSPE for Mechanisms of Viral Spread and Demyelination. J. Neuropathol. Exp. Neurol. 1996, 55, 471–480. [Google Scholar] [CrossRef]
- McQuaid, S.; Cosby, S.L. An Immunohistochemical Study of the Distribution of the Measles Virus Receptors, CD46 and SLAM, in Normal Human Tissues and Subacute Sclerosing Panencephalitis. Lab. Investig. A J. Tech. Methods Pathol. 2002, 82, 403–409. [Google Scholar] [CrossRef]
- Radecke, F.; Billeter, M.A. Appendix: Measles Virus Antigenome and Protein Consensus Sequences. Curr. Top. Microbiol. Immunol. 1995, 191, 181–192. [Google Scholar]
- Radecke, F.; Spielhofer, P.; Schneider, H.; Kaelin, K.; Huber, M.; Dötsch, C.; Christiansen, G.; Billeter, M.A. Rescue of Measles Viruses from Cloned DNA. EMBO J. 1995, 14, 5773–5784. [Google Scholar] [CrossRef]
- Hashimoto, K.; Ono, N.; Tatsuo, H.; Minagawa, H.; Takeda, M.; Takeuchi, K.; Yanagi, Y. SLAM (CD150)-Independent Measles Virus Entry as Revealed by Recombinant Virus Expressing Green Fluorescent Protein. J. Virol. 2002, 76, 6743–6749. [Google Scholar] [CrossRef] [PubMed]
- Welsch, J.C.; Talekar, A.; Mathieu, C.; Pessi, A.; Moscona, A.; Horvat, B.; Porotto, M. Fatal Measles Virus Infection Prevented by Brain-Penetrant Fusion Inhibitors. J. Virol. 2013, 87, 13785–13794. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Preibisch, S.; Saalfeld, S.; Tomancak, P. Globally Optimal Stitching of Tiled 3D Microscopic Image Acquisitions. Bioinformatics 2009, 25, 1463–1465. [Google Scholar] [CrossRef]
- Hardie, D.R.; Albertyn, C.; Heckmann, J.M.; Smuts, H.E. Molecular Characterisation of Virus in the Brains of Patients with Measles Inclusion Body Encephalitis (MIBE). Virol. J. 2013, 10, 283. [Google Scholar] [CrossRef]
- Holm, C.K.; Jensen, S.B.; Jakobsen, M.R.; Cheshenko, N.; Horan, K.A.; Moeller, H.B.; Gonzalez-Dosal, R.; Rasmussen, S.B.; Christensen, M.H.; Yarovinsky, T.O.; et al. Virus-Cell Fusion as a Trigger of Innate Immunity Dependent on the Adaptor STING. Nat. Immunol. 2012, 13, 737–743. [Google Scholar] [CrossRef]
- Herschke, F.; Plumet, S.; Duhen, T.; Azocar, O.; Druelle, J.; Laine, D.; Wild, T.F.; Rabourdin-Combe, C.; Gerlier, D.; Valentin, H. Cell-Cell Fusion Induced by Measles Virus Amplifies the Type I Interferon Response. J. Virol. 2007, 81, 12859–12871. [Google Scholar] [CrossRef]
- Delpeut, S.; Noyce, R.S.; Siu, R.W.C.; Richardson, C.D. Host Factors and Measles Virus Replication. Curr. Opin. Virol. 2012, 2, 773–783. [Google Scholar] [CrossRef]
- Schmitz, K.S.; Lange, M.V.; Gommers, L.; Handrejk, K.; Porter, D.P.; Alabi, C.A.; Moscona, A.; Porotto, M.; de Vries, R.D.; de Swart, R.L. Repurposing an In Vitro Measles Virus Dissemination Assay for Screening of Antiviral Compounds. Viruses 2022, 14, 1186. [Google Scholar] [CrossRef]
- Hosoya, M.; Mori, S.; Tomoda, A.; Mori, K.; Sawaishi, Y.; Kimura, H.; Shigeta, S.; Suzuki, H. Pharmacokinetics and Effects of Ribavirin Following Intraventricular Administration for Treatment of Subacute Sclerosing Panencephalitis. Antimicrob. Agents Chemother. 2004, 48, 4631–4635. [Google Scholar] [CrossRef]
- Tomoda, A.; Nomura, K.; Shiraishi, S.; Hamada, A.; Ohmura, T.; Hosoya, M.; Miike, T.; Sawaishi, Y.; Kimura, H.; Takashima, H.; et al. Trial of Intraventricular Ribavirin Therapy for Subacute Sclerosing Panencephalitis in Japan. Brain Dev. 2003, 25, 514–517. [Google Scholar] [CrossRef]
- Kwak, M.; Yeh, H.-R.; Yum, M.-S.; Kim, H.-J.; You, S.J.; Ko, T.-S. A Long-Term Subacute Sclerosing Panencephalitis Survivor Treated with Intraventricular Interferon-Alpha for 13 Years. Korean J. Pediatr. 2019, 62, 108–112. [Google Scholar] [CrossRef]




| Antibody | Dilution Used | Reference |
|---|---|---|
| Mouse monoclonal anti-Myelin Basic Protein (clone MBP101); Abcam, France | 1/200 | Cat# ab62631 |
| Rabbit polyclonal anti-Glial Fibrillary Acidic Protein (GFAP); Agilent Technologies, Dako, France | 1/500 | Cat# Z0334 |
| Rabbit anti-beta III Tubulin (Tuj-1); Abcam, France | 1/500 | Cat# ab18207 |
| Goat polyclonal anti-Olig2; R&D system, France | 1/200 | Cat# AF2418 |
| Rabbit polyclonal anti-iba-1; Wako, USA | 1/200 | Cat# 016-20001 |
| Goat polyclonal anti-calbindin D-28K (CB28K); Invitrogen, France | 1/500 | Cat# PA5-46936 |
| Alexa FluorTM 555 Donkey anti-mouse IgG (H+L); ThermoFisher Scientific, France | 1/500 | Cat# A31570 |
| Alexa FluorTM 555 Donkey anti-rabbit IgG (H+L); ThermoFisher Scientific, France | 1/500 | Cat# A31572 |
| Alexa FluorTM 647 Donkey anti-mouse IgG (H+L); ThermoFisher Scientific, France | 1/500 | Cat# A31571 |
| Alexa FluorTM 647 Donkey anti-goat IgG (H+L); ThermoFisher Scientific, France | 1/500 | Cat# A21447 |
| Cell Population | Antibody | Fluorochrome | Reference | Dilution |
|---|---|---|---|---|
| Neurons | Monoclonal Anti-GABA A Receptor | PE/Atto 594 | Merk/sigma; SAB5202234-100UG | 1/100 |
| Astrocytes | Anti GLAST (ACSA-1), anti-human/mouse/rat | PE | Miltenyi; 130-118-344 | 1/10 |
| Oligodendrocytes | Clone mGalC Anti-Galactocerebroside | Alexa fluor 647 | Merk/sigma; MAB342-AF647 | 1/100 |
| Microglial cells | CD68 Rat-Anti-Mouse | BV421 | BD; 566388 | 1/50 |
| IC323-eGFP-F-wt | IC323-eGFP-F-L454W | |
|---|---|---|
| Tubulin-positive neurons (Tuj-1) | ++ | ++++ |
| Astrocytes (GFAP) | ++ | + |
| Oligodendrocytes (Mbp) | + | + |
| Microglial cells (iba-1) | − | + |
| Oligodendrocytes-engaged cells (Olig2) | ++ | +++ |
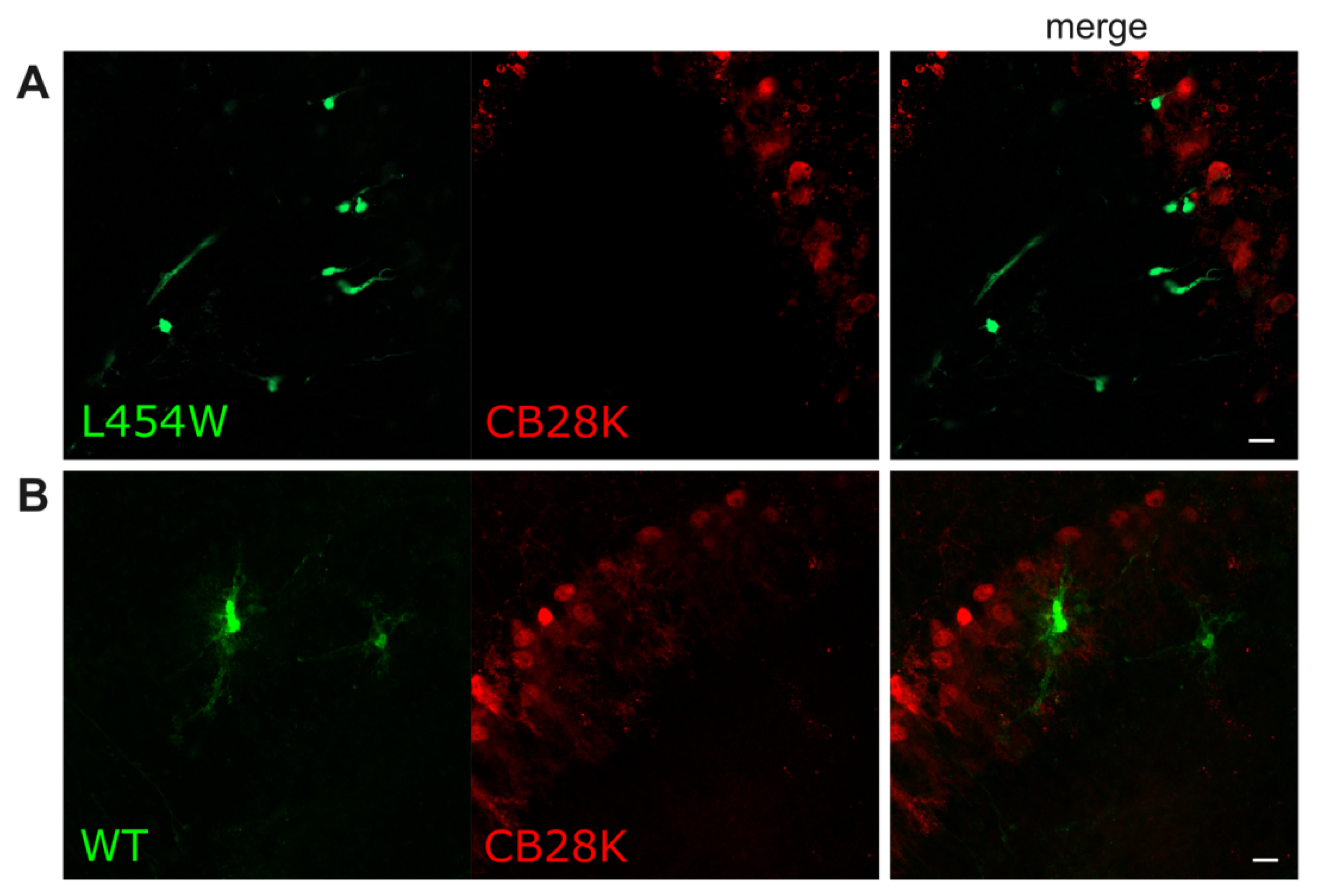
| CB28K-positive neurons de (Purkinje and Golgi cells) | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferren, M.; Lalande, A.; Iampietro, M.; Canus, L.; Decimo, D.; Gerlier, D.; Porotto, M.; Mathieu, C. Early Permissiveness of Central Nervous System Cells to Measles Virus Infection Is Determined by Hyperfusogenicity and Interferon Pressure. Viruses 2023, 15, 229. https://doi.org/10.3390/v15010229
Ferren M, Lalande A, Iampietro M, Canus L, Decimo D, Gerlier D, Porotto M, Mathieu C. Early Permissiveness of Central Nervous System Cells to Measles Virus Infection Is Determined by Hyperfusogenicity and Interferon Pressure. Viruses. 2023; 15(1):229. https://doi.org/10.3390/v15010229
Chicago/Turabian StyleFerren, Marion, Alexandre Lalande, Mathieu Iampietro, Lola Canus, Didier Decimo, Denis Gerlier, Matteo Porotto, and Cyrille Mathieu. 2023. "Early Permissiveness of Central Nervous System Cells to Measles Virus Infection Is Determined by Hyperfusogenicity and Interferon Pressure" Viruses 15, no. 1: 229. https://doi.org/10.3390/v15010229
APA StyleFerren, M., Lalande, A., Iampietro, M., Canus, L., Decimo, D., Gerlier, D., Porotto, M., & Mathieu, C. (2023). Early Permissiveness of Central Nervous System Cells to Measles Virus Infection Is Determined by Hyperfusogenicity and Interferon Pressure. Viruses, 15(1), 229. https://doi.org/10.3390/v15010229







