Development and Preliminary Application of a Droplet Digital PCR Assay for Quantifying the Oncolytic Herpes Simplex Virus Type 1 in the Clinical-Grade Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Cells
2.2. Primers, Probes, and Plasmid
2.3. Genome Extraction
2.4. Droplet Digital PCR
2.5. Production of oHSV Samples
2.6. Virus Titration
2.7. Statistical Analysis
3. Results
3.1. The Establishment of a Digital Droplet PCR (ddPCR) for Quantifying the Genomic Copy Number of oHSV-1 Samples
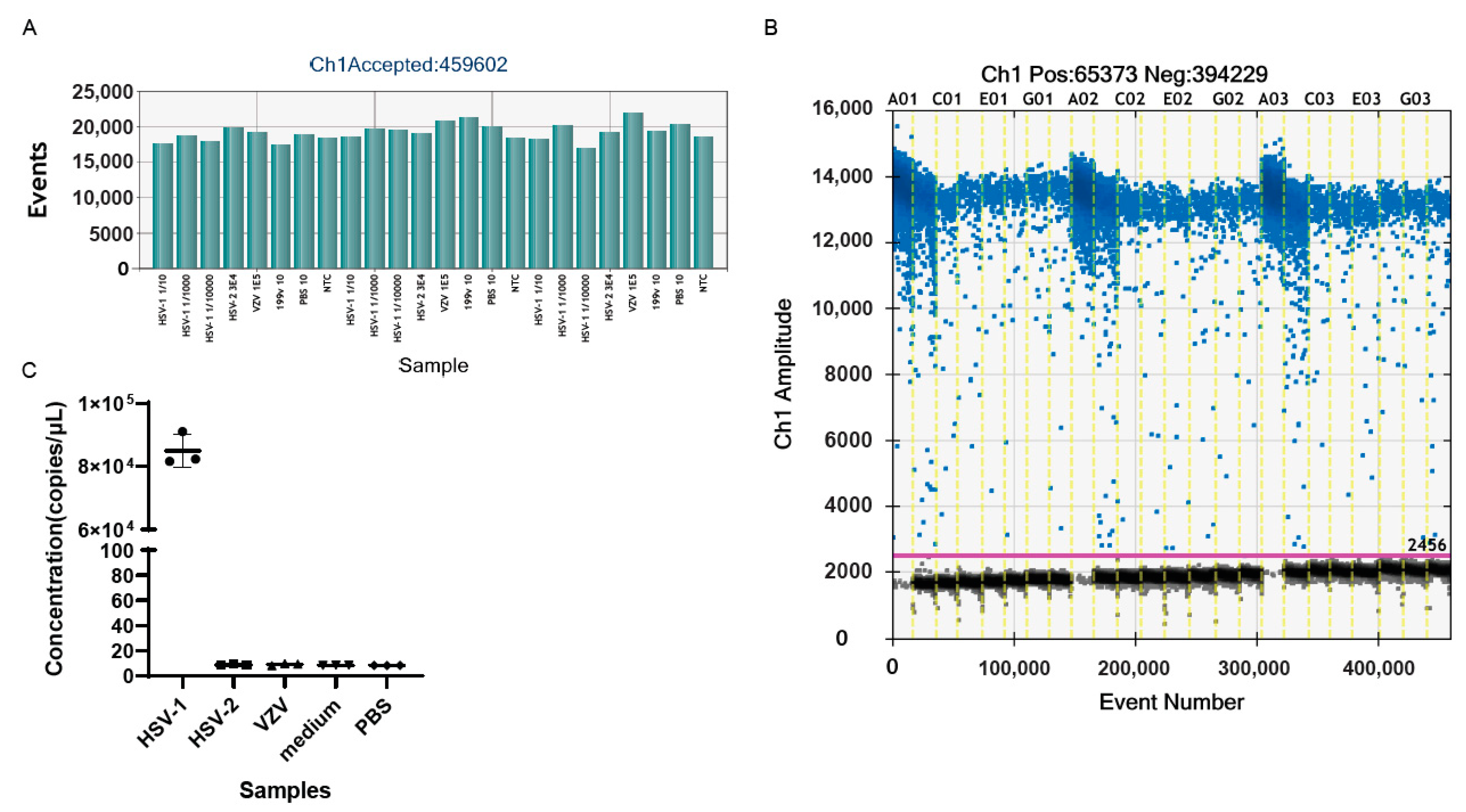
3.2. Specificity of the ddPCR Assay for Detecting oHSV-1
3.3. The Plasmid DNA and Limit of Detection of the ddPCR Assay
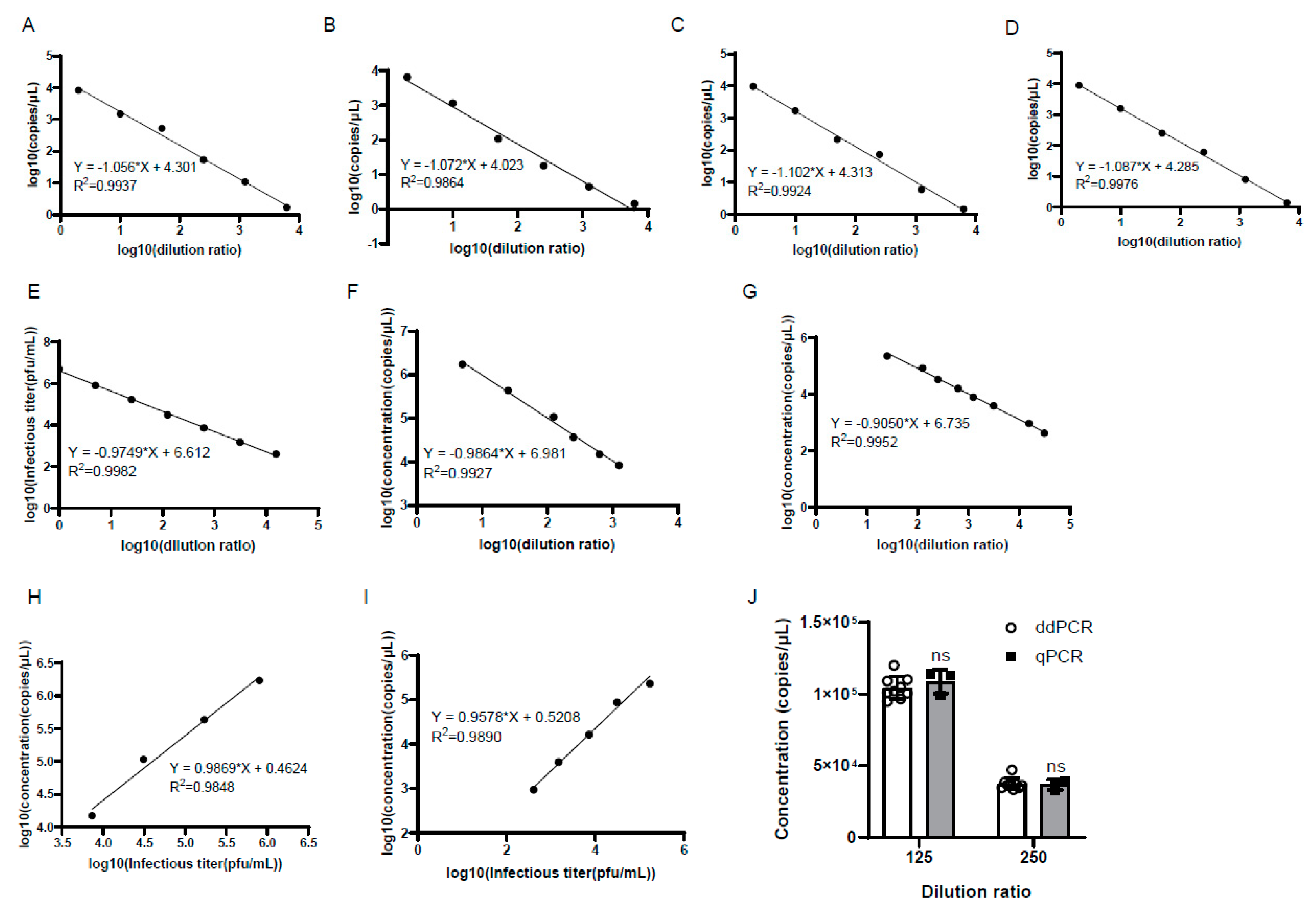
3.4. The Accuracy and Reproducibility of Genomic Copy Numbers of Samples Using ddPCR
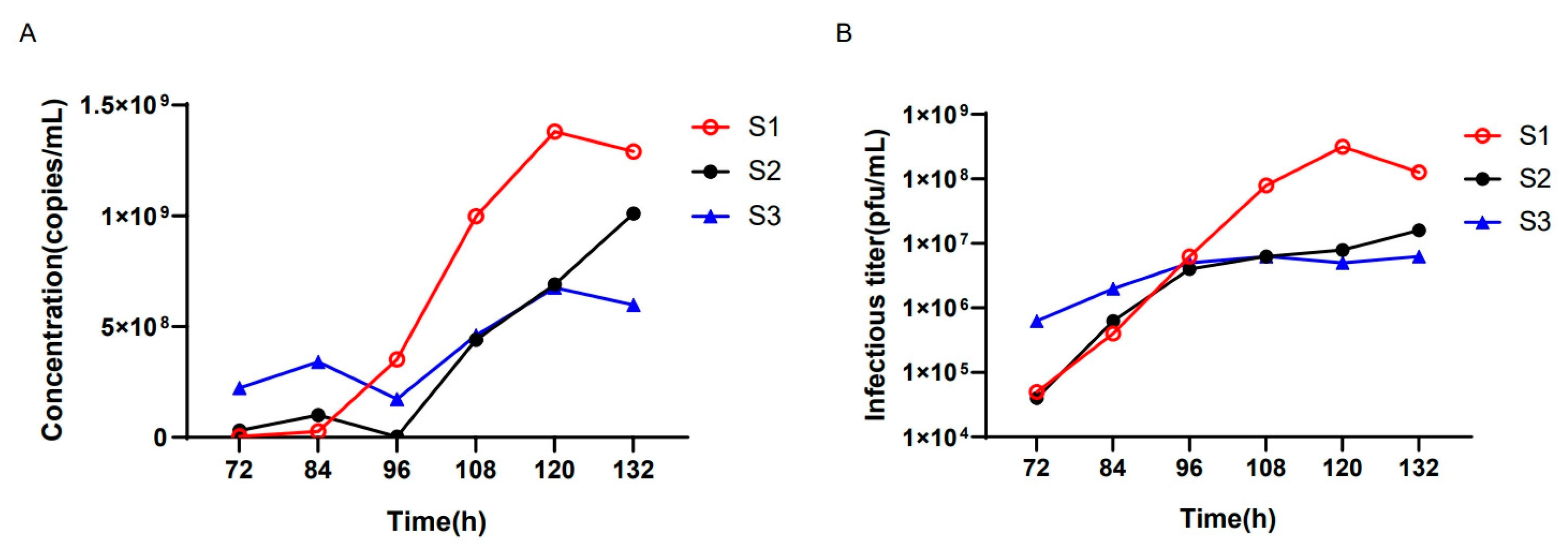
3.5. The Application of the ddPCR Assay during the oHSV Production
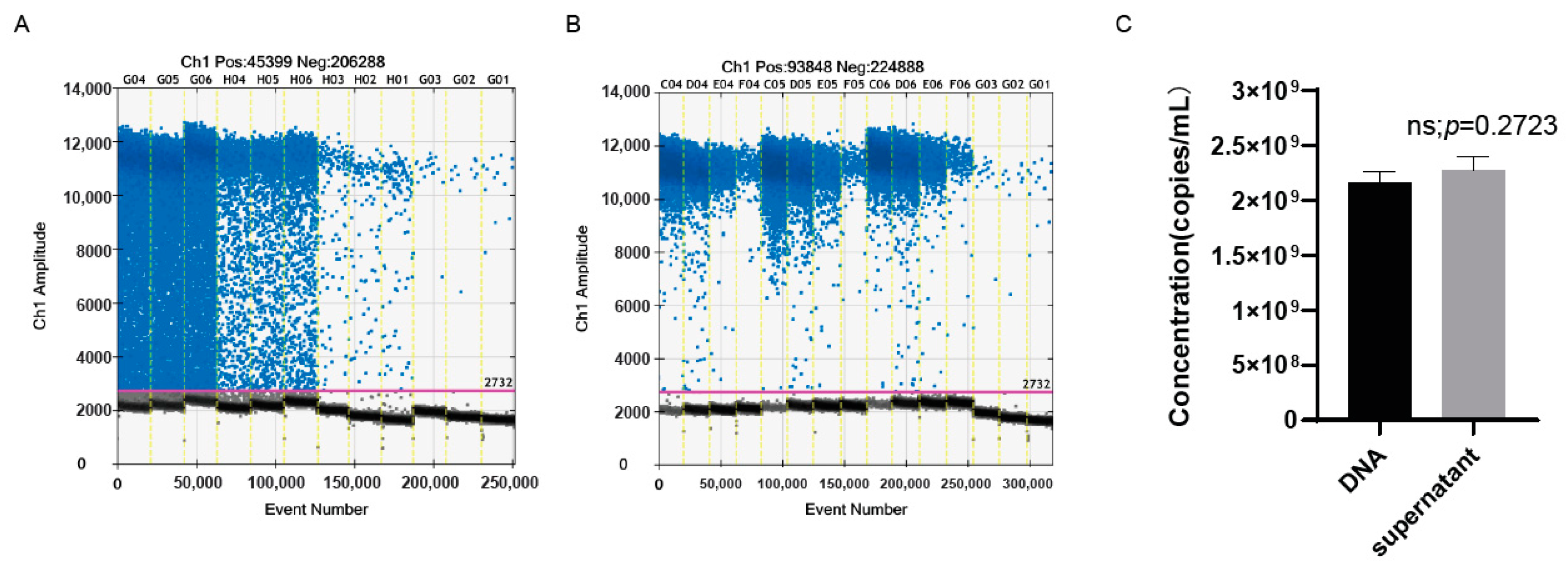
3.6. The ddPCR Assay Could Accurately Detect the Genomic copy number of oHSV-1 without Viral DNA Extraction
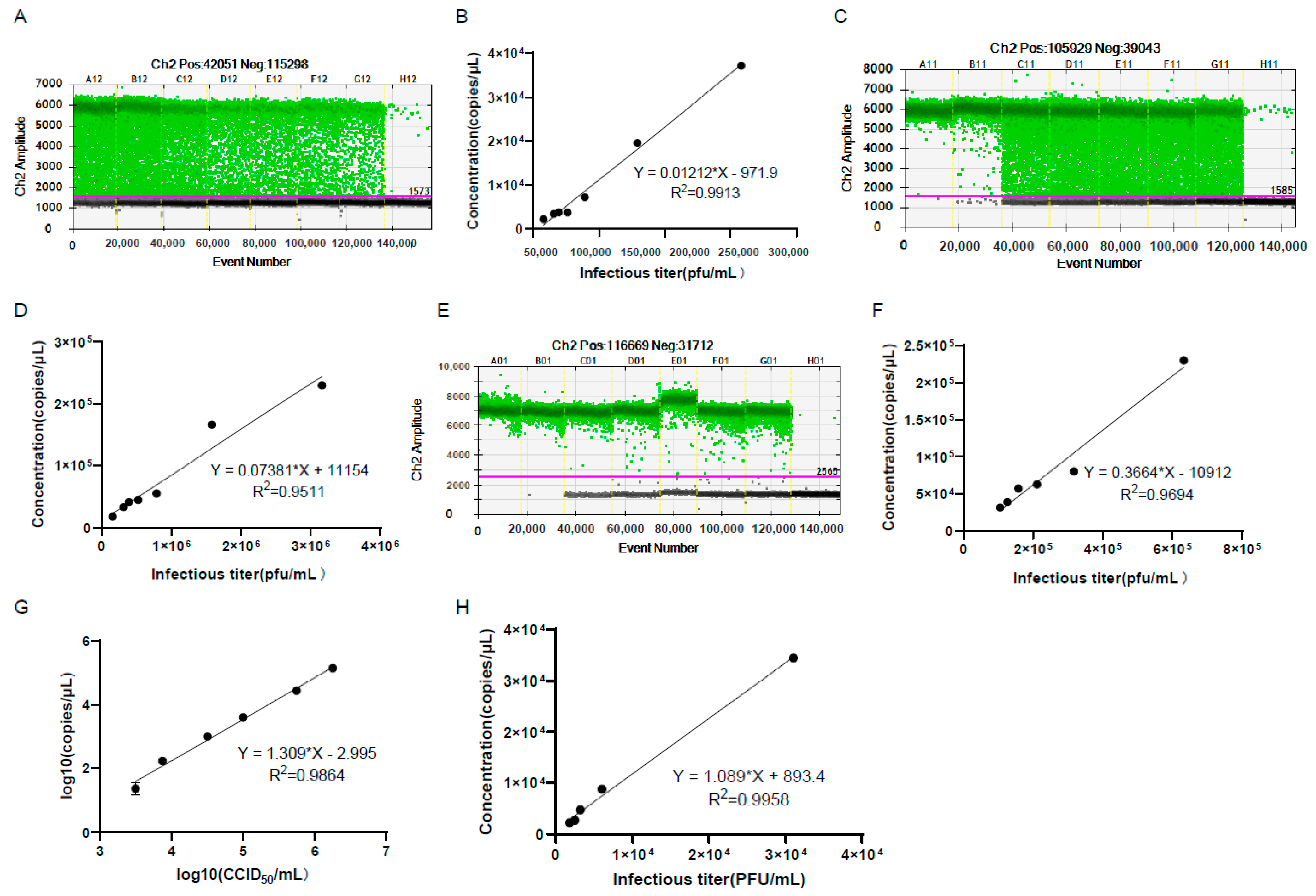
3.7. The Genomic Copy Numbers of the Harvested and Purified Virus Showed Good Linear Relationships with Viral Infectious Titer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herpes Simplex Virus. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 1 September 2022).
- Raman, S.S.; Hecht, J.R.; Chan, E. Talimogene laherparepvec: Review of its mechanism of action and clinical efficacy and safety. Immunotherapy 2019, 11, 705–723. [Google Scholar] [CrossRef]
- LeGoff, J.; Pere, H.; Belec, L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol. J. 2014, 11, 83. [Google Scholar] [CrossRef]
- Karlen, Y.; McNair, A.; Perseguers, S.; Mazza, C.; Mermod, N. Statistical significance of quantitative PCR. BMC Bioinform. 2007, 8, 131. [Google Scholar] [CrossRef]
- Mendoza, E.J.; Manguiat, K.; Wood, H.; Drebot, M. Two Detailed Plaque Assay Protocols for the Quantification of Infectious SARS-CoV-2. Curr. Protoc. Microbiol. 2020, 57, ecpmc105. [Google Scholar] [CrossRef]
- Howes, D.W. Overlap and the errors of plaque counting. I. The overlap biases of observed counts and their correction. Epidemiol. Infect. 1969, 67, 317–334. [Google Scholar] [CrossRef]
- Grigorov, B.; Rabilloud, J.; Lawrence, P.; Gerlier, D. Rapid Titration of Measles and Other Viruses: Optimization with Determination of Replication Cycle Length. PLoS ONE 2011, 6, e24135. [Google Scholar] [CrossRef]
- Watzinger, F.; Suda, M.; Preuner, S.; Baumgartinger, R.; Ebner, K.; Baskova, L.; Niesters, H.G.M.; Lawitschka, A.; Lion, T. Real-time quantitative PCR assays for detection and monitoring of pathogenic human viruses in immunosuppressed pediatric patients. J. Clin. Microbiol. 2004, 42, 5189–5198. [Google Scholar] [CrossRef]
- Pandori, M.W.; Lei, J.; Wong, E.H.; Klausner, J.; Liska, S. Real-Time PCR for detection of herpes simplex virus without nucleic acid extraction. BMC Infect. Dis. 2006, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, S.S.; Chandak, N.H.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F.; Kashyap, R.S. Determination of viral load by quantitative real-time PCR in herpes simplex encephalitis patients. Intervirology 2014, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shaha, M.; Roy, B.; Islam, M. Detection of herpes simplex virus 2: A SYBR-Green-based real-time PCR assay. F1000Research 2021, 10, 655. [Google Scholar] [PubMed]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR inhibitors—Occurrence, properties and removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.L.; Sauzade, M.; Brouzes, E. dPCR: A Technology Review. Sensors 2018, 18, 1271. [Google Scholar] [CrossRef] [PubMed]
- Nyaruaba, R.; Mwaliko, C.; Kering, K.K.; Wei, H. Droplet digital PCR applications in the tuberculosis world. Tuberculosis 2019, 117, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiao, L.; Lin, H.; Chen, S.; Yang, M.; An, W.; Wang, Y.; Yang, Z.; Yao, X.; Tang, Z. Development and application of a droplet digital polymerase chain reaction (ddPCR) for detection and investigation of African swine fever virus. Can. J. Vet. Res. 2018, 82, 70–74. [Google Scholar]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Miotke, L.; Lau, B.T.; Rumma, R.T.; Ji, H.P. High sensitivity detection and quantitation of DNA copy number and single nucleotide variants with single color droplet digital PCR. Anal. Chem. 2014, 86, 2618–2624. [Google Scholar] [CrossRef]
- Beck, J.; Bierau, S.; Balzer, S.; Andag, R.; Kanzow, P.; Schmitz, J.; Gaedcke, J.; Moerer, O.; Slotta, J.E.; Walson, P.; et al. Digital droplet PCR for rapid quantification of donor DNA in the circulation of transplant recipients as a potential universal biomarker of graft injury. Clin. Chem. 2013, 59, 1732–1741. [Google Scholar] [CrossRef]
- Tang, H.; Cai, Q.; Li, H.; Hu, P. Comparison of droplet digital PCR to real-time PCR for quantification of hepatitis B virus DNA. Biosci. Biotechnol. Biochem. 2016, 80, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- McDermott, G.P.; Do, D.; Litterst, C.M.; Maar, D.; Hindson, C.M.; Steenblock, E.R.; Legler, T.C.; Jouvenot, Y.; Marrs, S.H.; Bemis, A.; et al. Multiplexed target detection using DNA-binding dye chemistry in droplet digital PCR. Anal. Chem. 2013, 85, 11619–11627. [Google Scholar] [CrossRef]
- Rački, N.; Dreo, T.; Gutierrez-Aguirre, I.; Blejec, A.; Ravnikar, M. Reverse transcriptase droplet digital PCR shows high resilience to PCR inhibitors from plant, soil and water samples. Plant Methods 2014, 10, 42. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.; et al. The need for transparency and good practices in the qPCR literature. Nat. Methods 2013, 10, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Morella, N.M.; Yang, S.C.; Hernandez, C.A.; Koskella, B. Rapid quantification of bacteriophages and their bacterial hosts in vitro and in vivo using droplet digital PCR. J. Virol. Methods 2018, 259, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hernandez, F.; Garcia-Heredia, I.; Gomez, M.L.; Maestre-Carballa, L.; Martínez, J.M.; Martinez-Garcia, M. Droplet Digital PCR for Estimating Absolute Abundances of Widespread Pelagibacter Viruses. Front. Microbiol. 2019, 10, 1226. [Google Scholar] [CrossRef]
- Azizi, A.; Aidoo, F.; Gisonni-Lex, L.; McNeil, B. Determination of HSV-1 UL5 and UL29 gene copy numbers in an HSV complementing Vero cell line. J. Biotechnol. 2013, 168, 382–387. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, P.; Lu, L.; Zhong, H.; Xu, M.; Jia, R.; Su, L.; Cao, L.; Sun, Y.; Guo, M.; et al. Development of a multiplex droplet digital PCR assay for detection of enterovirus, parechovirus, herpes simplex virus 1 and 2 simultaneously for diagnosis of viral CNS infections. Virol. J. 2022, 19, 70. [Google Scholar] [CrossRef]
- Xiu, L.; Zhang, C.; Li, Y.; Wang, F.; Peng, J. Simultaneous detection of eleven sexually transmitted agents using multiplexed PCR coupled with MALDI-TOF analysis. Infect. Drug Resist. 2019, 12, 2671–2682. [Google Scholar] [CrossRef] [PubMed]
- Shehata, H.R.; Li, J.; Chen, S.; Redda, H.; Cheng, S.; Tabujara, N.; Li, H.; Warriner, K.; Hanner, R. Droplet digital polymerase chain reaction (ddPCR) assays integrated with an internal control for quantification of bovine, porcine, chicken and turkey species in food and feed. PLoS ONE 2017, 12, e0182872. [Google Scholar] [CrossRef]
- Huggett, J.F. The Digital MIQE Guidelines Update: Minimum Information for Publication of Quantitative Digital PCR Experiments for 2020. Clin. Chem. 2020, 66, 1012–1029. [Google Scholar]
- Tozaki, T.; Ohnuma, A.; Iwai, S.; Kikuchi, M.; Ishige, T.; Kakoi, H.; Hirota, K.; Kusano, K.; Nagata, S. Robustness of Digital PCR and Real-Time PCR in Transgene Detection for Gene-Doping Control. Anal. Chem. 2021, 93, 7133–7139. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Paweletz, C.P.; Kuang, Y.; Mach, S.L.; O’Connell, A.; Messineo, M.M.; Luke, J.J.; Butaney, M.; Kirschmeier, P.; Jackman, D.M.; et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin. Cancer Res. 2014, 20, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Persaud, D.; Gay, H.; Ziemniak, C.; Chen, Y.H.; Piatak, M.; Chun, T.-W.; Strain, M.; Richman, D.; Luzuriaga, K. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N. Engl. J. Med. 2013, 369, 1828–1835. [Google Scholar] [CrossRef]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.C.; Miura, J.T.; Naqvi, S.M.H.; Kim, Y.; Holstein, A.; Lee, D.; Sarnaik, A.A.; Zager, J.S. Talimogene Laherparepvec (TVEC) for the Treatment of Advanced Melanoma: A Single-Institution Experience. Ann. Surg. Oncol. 2018, 25, 3960–3965. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xie, D.; Yang, L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Vähä-Koskela, M.J.; Heikkilä, J.E.; Hinkkanen, A.E. Oncolytic viruses in cancer therapy. Cancer Lett. 2007, 254, 178–216. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, S.; Fong, Y.; Warner, S. Oncolytic Virotherapy for Cancer: Clinical Experience. Biomedicines 2021, 9, 419. [Google Scholar] [CrossRef]
- Haines, B.B.; Denslow, A.; Grzesik, P.; Lee, J.S.; Farkaly, T.; Hewett, J.; Wambua, D.; Kong, L.; Behera, P.; Jacques, J.; et al. ONCR-177, an Oncolytic HSV-1 Designed to Potently Activate Systemic Antitumor Immunity. Cancer Immunol. Res. 2021, 9, 291–308. [Google Scholar] [CrossRef]
- Yan, R.; Zhou, X.; Chen, X.; Liu, X.; Tang, Y.; Ma, J.; Wang, L.; Liu, Z.; Zhan, B.; Chen, H.; et al. Enhancement of oncolytic activity of oHSV expressing IL-12 and anti PD-1 antibody by concurrent administration of exosomes carrying CTLA-4 miRNA. Immunotherapy 2019, 5, 92. [Google Scholar] [CrossRef]
- Chouljenko, D.; Ding, J.; Lee, I.-F.; Murad, Y.; Bu, X.; Liu, G.; Delwar, Z.; Sun, Y.; Yu, S.; Samudio, I.; et al. Induction of Durable Antitumor Response by a Novel Oncolytic Herpesvirus Expressing Multiple Immunomodulatory Transgenes. Biomedicines 2020, 8, 484. [Google Scholar] [CrossRef]
- Ungerechts, G.; Bossow, S.; Leuchs, B.; Holm, P.S.; Rommelaere, J.; Coffey, M.; Coffin, R.; Bell, J.; Nettelbeck, D.M. Moving oncolytic viruses into the clinic: Clinical-grade production, purification, and characterization of diverse oncolytic viruses. Mol. Ther. Methods Clin. Dev. 2016, 3, 16018. [Google Scholar] [CrossRef]


| Name | Sequence |
|---|---|
| gH-F1 | GGCTGCGTGTCAAAGGCTA |
| gH-R1 | GTGTTTTCGAGCGACGTGC |
| gH-P1 | 5′6-FAM-ACGGCCTTGTTGCTATTTCCAAA-3′BHQ1 |
| gH-F2 | CGGCGATTTGCTGCTGT |
| gH-R2 | ACCCGGGATATCGAGTCCAA |
| gH-P2 | 5′HEX-CACCCAAAACCAGCGCGACCT-3′BHQ1 |
| gH plasmid DNA | CGGGCTGCGTGTCAAAGGCTAGCAAATGAATGACGGTTCCGTTTGGAAATAGCAACAAGGCCGTGGACGGCACGTCGCTCGAAAACACGCTCGGGGCGCCCTCCGTCGGCCCGGCGGCGATTTGCTGCTGTGTGTTGTCCGTATCCACCAGCAACACAGACATGACCTCCCCGGCTGGGGTGTAGCGCATAAACACGGCCCCCACGAGCCCCAGGTCGCGCTGGTTTTGGGTGCGCACCAGCCGCTTGGACTCGATATCCCGGGTGGAGCCTTCGCATGTCGCGGTGAGGTAGGTTAGGAACAGTGGGCGTCGGA |
| ICP8-F | TCGCCACGAACACGCTACT |
| ICP8-R | CCCGCTCCTTATTTTTGACC |
| ICP8-P | 5′6-FAM-CCCGCCCTCGGAGATAATGC-3′BHQ1 |
| Primer Pair | Dilution Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 16 | 32 | 64 | 128 | 256 | 512 | 1024 | 2048 | 4096 | |
| gH-1 | 55,400 | 25,980 | 10,440 | 4220 | 1932 | 798 | 348 | 152 | 88 |
| 57,220 | 25,340 | 13,080 | 4500 | 1982 | 788 | 324 | 138 | 72 | |
| gH-2 | 60,560 | 27,560 | 10,640 | 4660 | 1834 | 850 | 360 | 130 | 54 |
| 62,860 | 29,660 | 11,900 | 4940 | 1994 | 756 | 286 | 118 | 58 | |
| ICP8 | 71,000 | 31,540 | 13,100 | 4760 | 1868 | 1040 | 304 | 134 | 66 |
| 58,560 | 24,040 | 11,240 | 4760 | 1880 | 814 | 284 | 150 | 62 | |
| Average | 60,933.33 | 27,353.33 | 11,733.33 | 4640.00 | 1915.00 | 841.00 | 317.67 | 137.00 | 66.67 |
| RSD | 8.35 | 9.42 | 9.09 | 4.94 | 3.09 | 11.10 | 9.16 | 8.50 | 16.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Deng, L.; Liang, H.; Du, Y.; Gao, W.; Tian, N.; Bi, Y.; Li, J.; Ma, T.; Zhang, Y.; et al. Development and Preliminary Application of a Droplet Digital PCR Assay for Quantifying the Oncolytic Herpes Simplex Virus Type 1 in the Clinical-Grade Production. Viruses 2023, 15, 178. https://doi.org/10.3390/v15010178
Guo M, Deng L, Liang H, Du Y, Gao W, Tian N, Bi Y, Li J, Ma T, Zhang Y, et al. Development and Preliminary Application of a Droplet Digital PCR Assay for Quantifying the Oncolytic Herpes Simplex Virus Type 1 in the Clinical-Grade Production. Viruses. 2023; 15(1):178. https://doi.org/10.3390/v15010178
Chicago/Turabian StyleGuo, Miaomiao, Li Deng, Hongyang Liang, Yuyao Du, Wenrui Gao, Na Tian, Ying Bi, Jinghua Li, Tiancong Ma, Yuntao Zhang, and et al. 2023. "Development and Preliminary Application of a Droplet Digital PCR Assay for Quantifying the Oncolytic Herpes Simplex Virus Type 1 in the Clinical-Grade Production" Viruses 15, no. 1: 178. https://doi.org/10.3390/v15010178
APA StyleGuo, M., Deng, L., Liang, H., Du, Y., Gao, W., Tian, N., Bi, Y., Li, J., Ma, T., Zhang, Y., & Wang, H. (2023). Development and Preliminary Application of a Droplet Digital PCR Assay for Quantifying the Oncolytic Herpes Simplex Virus Type 1 in the Clinical-Grade Production. Viruses, 15(1), 178. https://doi.org/10.3390/v15010178






