Measles Outbreak Response Activity in Japan, and a Discussion for a Possible Strategy of Outbreak Response Using Cycle Threshold Values of Real-Time Reverse Transcription PCR for Measles Virus in Measles Elimination Settings
Abstract
1. Introduction
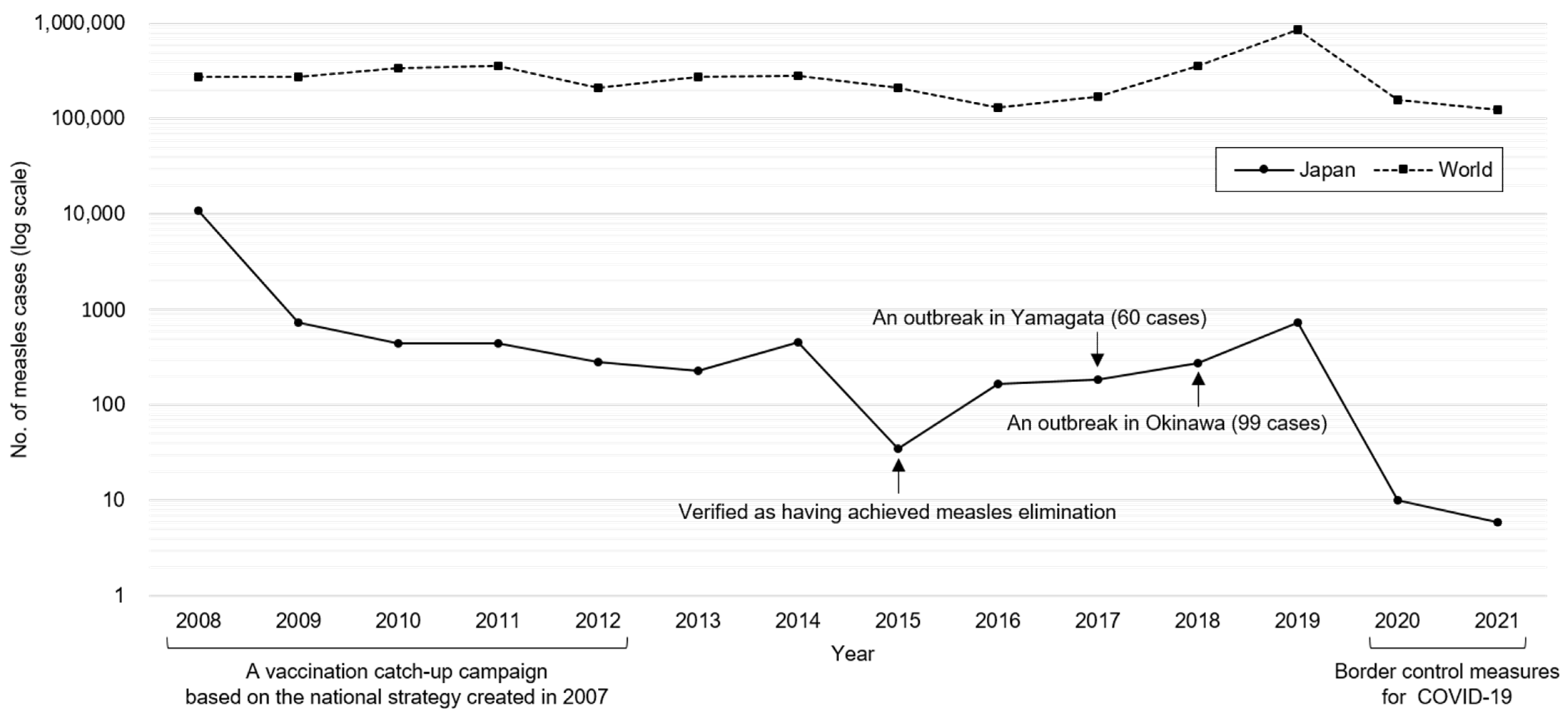
2. History of Measles Elimination in Japan
3. Outbreak Response Using Ct Values of Real-Time RT-PCR
3.1. Requirements for Accurate Real-Time RT-PCR Results
3.2. Outbreak Response Using Ct Values
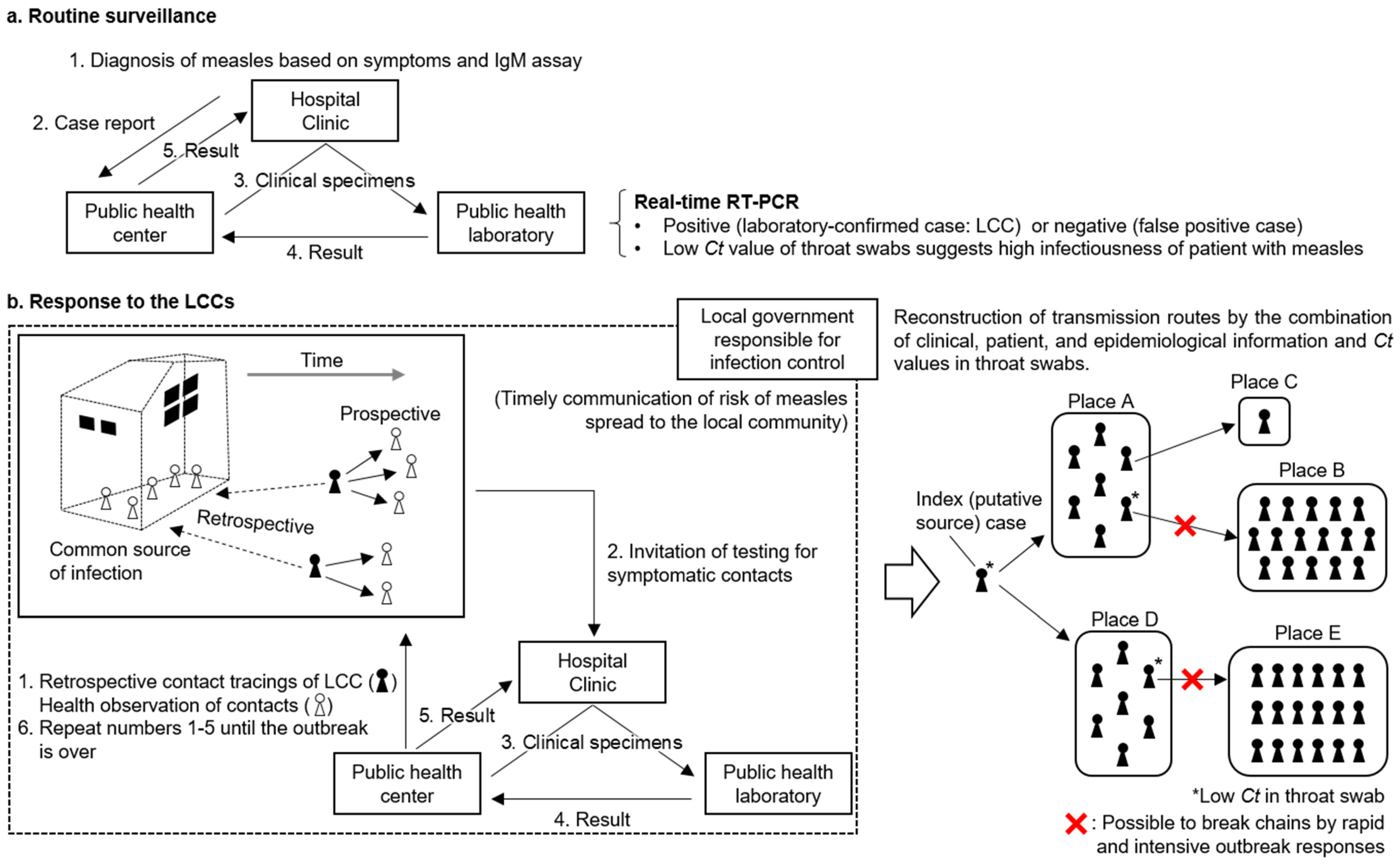
3.2.1. Routine Surveillance
3.2.2. Response to Laboratory Confirmed Cases
3.3. Other Potential Laboratory Diagnostic Methods
3.4. Limitations
| Ct Value in Throat Swab | |||
|---|---|---|---|
| Low 1 | High or Not Detected | ||
| IgG avidity 2 | Low | Naïve | Improbable |
| High | Debatable | Breakthrough | |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hübschen, J.M.; Gouandjika-Vasilache, I.; Dina, J. Measles. Lancet 2022, 399, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Seki, F.; Miyoshi, M.; Ikeda, T.; Nishijima, H.; Saikusa, M.; Itamochi, M.; Minagawa, H.; Kurata, T.; Ootomo, R.; Kajiwara, J.; et al. Nationwide molecular epidemiology of measles virus in Japan between 2008 and 2017. Front. Microbiol. 2019, 10, 1470. [Google Scholar] [CrossRef] [PubMed]
- WHO. Provisional Monthly Measles and Rubella Data. Available online: http://who-wiise-frontend-prod-cdn.azureedge.net/listing.html?topic=measles-rubella&location= (accessed on 23 October 2022).
- WHO. Measles—Number of Reported Cases. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/measles---number-of-reported-cases (accessed on 23 October 2022).
- Oshitani, H. Cluster-based approach to coronavirus disease 2019 (COVID-19) response in Japan, from February to April 2020. Jpn. J. Infect. Dis. 2020, 73, 491–493. [Google Scholar] [CrossRef]
- Mercader, S.; Garcia, P.; Bellini, W.J. Measles virus IgG avidity assay for use in classification of measles vaccine failure in measles elimination settings. Clin. Vaccine Immunol. 2012, 19, 1810–1817. [Google Scholar] [CrossRef]
- Jacobson, R.M.; St Sauver, J.L.; Finney Rutten, L.J. Vaccine hesitancy. Mayo Clin. Proc. 2015, 90, 1562–1568. [Google Scholar] [CrossRef]
- WHO. Measles Outbreak Guide. Available online: https://apps.who.int/iris/handle/10665/360891 (accessed on 23 October 2022).
- Komabayashi, K.; Seto, J.; Tanaka, S.; Suzuki, Y.; Ikeda, T.; Onuki, N.; Yamada, K.; Ahiko, T.; Ishikawa, H.; Mizuta, K. The largest measles outbreak, including 38 modified measles and 22 typical measles cases in its elimination era in Yamagata, Japan, 2017. Jpn. J. Infect. Dis. 2018, 71, 413–418. [Google Scholar] [CrossRef]
- Seto, J.; Ikeda, T.; Tanaka, S.; Komabayashi, K.; Matoba, Y.; Suzuki, Y.; Takeuchi, S.; Yamauchi, T.; Mizuta, K. Detection of modified measles and super-spreader using a real-time reverse transcription PCR in the largest measles outbreak, Yamagata, Japan, 2017 in its elimination era. Epidemiol. Infect. 2018, 146, 1707–1713. [Google Scholar] [CrossRef]
- Sundell, N.; Dotevall, L.; Sansone, M.; Andersson, M.; Lindh, M.; Wahlberg, T.; Tyrberg, T.; Westin, J.; Liljeqvist, J.; Bergström, T.; et al. Measles outbreak in Gothenburg urban area, Sweden, 2017 to 2018: Low viral load in breakthrough infections. Euro Surveill. 2019, 24, 1900114. [Google Scholar] [CrossRef]
- Kurata, T.; Yamamoto, S.P.; Nishimura, H.; Yumisashi, T.; Motomura, K.; Kinoshita, M. A measles outbreak in Kansai International Airport, Japan, 2016: Analysis of the quantitative difference and infectivity of measles virus between patients who are immunologically naive versus those with secondary vaccine failure. J. Med. Virol. 2020, 6, 3446–3454. [Google Scholar] [CrossRef]
- James, A.; Pitchford, J.W.; Plank, M.J. An event-based model of superspreading in epidemics. Proc. Biol. Sci. 2007, 274, 741–747. [Google Scholar] [CrossRef]
- Bech, V. Measles epidemics in Greenland. Am. J. Dis. Child 1962, 103, 252–253. [Google Scholar] [CrossRef] [PubMed]
- Rota, J.S.; Hickman, C.J.; Sowers, S.B.; Rota, P.A.; Mercader, S.; Bellini, W.J. Two case studies of modified measles in vaccinated physicians exposed to primary measles cases: High risk of infection but low risk of transmission. J. Infect. Dis. 2011, 204, S559–S563. [Google Scholar] [CrossRef] [PubMed]
- Hübschen, J.M.; Bork, S.M.; Brown, K.E.; Mankertz, A.; Santibanez, S.; Ben Mamou, M.; Mulders, M.N.; Muller, C.P. Challenges of measles and rubella laboratory diagnostic in the era of elimination. Clin. Microbiol. Infect. 2017, 23, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Hummel, K.B.; Lowe, L.; Bellini, W.J.; Rota, P.A. Development of quantitative gene-specific real-time RT-PCR assays for the detection of measles virus in clinical specimens. J. Virol. Methods 2006, 132, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, Y. Measles. Nihon Shippeishi (History of Disease in Japan). Tohodo Shoten. 1912, Volume 1, pp. 161–199. Available online: https://dl.ndl.go.jp/info:ndljp/pid/833367 (accessed on 18 October 2022). (In Japanese).
- Suzuki, A. Measles and the spatio-temporal structure of modern Japan. Econ. Hist. Rev. 2009, 62, 828–856. [Google Scholar] [CrossRef]
- National Institute of Infectious Diseases. Deaths from Measles by Age. Available online: http://idsc.nih.go.jp/iasr/22/261/dj261a.html (accessed on 23 October 2022). (In Japanese)
- eStat: Portal Site of Official Statistics of Japan. Available online: https://www.e-stat.go.jp (accessed on 23 October 2022). (In Japanese).
- Nakatani, H.; Sano, T.; Iuchi, T. Development of vaccination policy in Japan: Current issues and policy directions. Jpn. J. Infect. Dis. 2002, 55, 101–111. [Google Scholar] [PubMed]
- WHO. Measles Vaccination Coverage. Available online: https://immunizationdata.who.int/pages/coverage/MCV.html?CODE=JPN&ANTIGEN=&YEAR= (accessed on 23 October 2022).
- Sunagawa, T.; Shimada, T.; Ueno-Yamamoto, K.; Yamashita, K.; Tanaka-Taya, K.; Tada, Y.; Yasui, Y.; Matsui, T.; Taniguchi, K.; Kobayashi, J.; et al. Progress toward measles elimination–Japan, 1999–2008. Morb. Mortal. Wkly. Rep. 2008, 57, 1049–1052. [Google Scholar]
- Hunt, E.; Lurie, P.; Lute, J.; Moll, M.; Stafford, H.; Bart, J.; Gray, A.; Urdaneta, V.; Ostroff, S.; Blostein, J.; et al. Multistate measles outbreak associated with an international youth sporting event-Pennsylvania, Michigan, and Texas, August-September 2007. Morb. Mortal. Wkly. Rep. 2008, 57, 169–173. [Google Scholar]
- Redd, S.B.; Kutty, P.K.; Parker, A.A.; LeBaron, C.W.; Barskey, A.E.; Seward, J.F.; Rota, J.S.; Rota, P.A.; Lowe, L.; Bellini, W.J. Measles-United States, January 1-April 25, 2008. Morb. Mortal. Wkly. Rep. 2008, 57, 494–498. [Google Scholar]
- National Institute of Infectious Diseases. Number of Cases with National Epidemiological Surveillance of Infectious Diseases (NESID). Available online: https://www.niid.go.jp/niid/ja/ydata/10410-report-ja2020-30.html (accessed on 23 October 2022). (In Japanese)
- Mizumoto, K.; Kobayashi, T.; Chowell, G. Transmission potential of modified measles during an outbreak, Japan, March–May 2018. Euro. Surveill. 2018, 23, 1800239. [Google Scholar] [CrossRef]
- WHO. Manual for the Laboratory-Based Surveillance of Measles, Rubella, and Congenital Rubella Syndrome. Chapter 3. Clinical Specimens for the Laboratory Confirmation and Molecular Epidemiology of Measles, Rubella, and CRS. Available online: https://www.who.int/docs/default-source/immunization/vpd_surveillance/lab_networks/measles_rubella/manual/chapter-3.pdf (accessed on 22 November 2022).
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef] [PubMed]
- WHO. Measles and Rubella Strategic Framework 2021–2030. Available online: https://www.who.int/publications/i/item/measles-and-rubella-strategic-framework-2021-2030 (accessed on 23 October 2022).
- National Institute of Infectious Diseases. Infectious Agents Surveillance Report: Measles surveillance in Japan. Available online: https://www.niid.go.jp/niid/ja/typhi-m/iasr-reference/2569-related-articles/related-articles-511/11518-511r07.html (accessed on 23 October 2022). (In Japanese)
- Bailey, A.; Sapra, A. MMR Vaccine; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554450/ (accessed on 23 October 2022).
- Seto, J.; Aoki, Y.; Tanaka, S.; Komabayashi, K.; Ikeda, T.; Mizuta, K. A seroepidemiologic study of a measles outbreak, Yamagata Prefecture, Japan, 2017: The estimation of spreaders using serological assays in a measles elimination setting. J. Infect. Chemother. 2022, 28, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.D.; Heath, J.; Collins, J.; Greene, T.; Antipa, L.; Rota, P.; Bellini, W.; McChesney, M. Experimental measles. II. Infection and immunity in the rhesus macaque. Virology 1997, 233, 85–92. [Google Scholar] [CrossRef] [PubMed]

| Category | Subcategory | Content |
|---|---|---|
| Sample quality | Specimen collection | Collect throat (or nasopharyngeal) swabs using a synthetic fiber swab. Serum is unsuitable for the breakthrough cases. |
| Multiple specimens | Collect multiple specimens (e.g., throat swab, whole-blood, and urine). PBMCs, extracted from whole-blood, and throat swabs show a high positive rate among the breakthrough cases. | |
| Collection timing | Collect specimens at the time of initial symptoms. Ideally collect specimens within 7 days of rash onset. | |
| Transport conditions | Require cold chain and rapid transportation using a viral transport media to minimize the fragmentation of viral RNA. | |
| Multiple tests | Collect specimens again when initial testing is negative, but measles is strongly suspected. | |
| Inspection accuracy | Accuracy assurance | Prepare standard operating procedure for specimen storage, specimen pretreatment, RNA extraction, real-time RT-PCR, and result determination. Implement the internal quality control and the external quality assessment. Implement regular equipment maintenance. |
| Negative control | Confirm negative result in negative control in every test to confirm absence of laboratory cross-contamination. | |
| Positive control | Confirm the detection of measles virus standard RNA with low copy numbers (e.g., 50 copies) in every test to ensure the inspection accuracy. | |
| Calibration curve | Ideally obtain a calibration curve using serially diluted standard RNA samples in every test to calculate genome copies of MeV. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seto, J.; Aoki, Y.; Komabayashi, K.; Yamada, K.; Ishikawa, H.; Ichikawa, T.; Ahiko, T.; Mizuta, K. Measles Outbreak Response Activity in Japan, and a Discussion for a Possible Strategy of Outbreak Response Using Cycle Threshold Values of Real-Time Reverse Transcription PCR for Measles Virus in Measles Elimination Settings. Viruses 2023, 15, 171. https://doi.org/10.3390/v15010171
Seto J, Aoki Y, Komabayashi K, Yamada K, Ishikawa H, Ichikawa T, Ahiko T, Mizuta K. Measles Outbreak Response Activity in Japan, and a Discussion for a Possible Strategy of Outbreak Response Using Cycle Threshold Values of Real-Time Reverse Transcription PCR for Measles Virus in Measles Elimination Settings. Viruses. 2023; 15(1):171. https://doi.org/10.3390/v15010171
Chicago/Turabian StyleSeto, Junji, Yoko Aoki, Kenichi Komabayashi, Keiko Yamada, Hitoshi Ishikawa, Tomoo Ichikawa, Tadayuki Ahiko, and Katsumi Mizuta. 2023. "Measles Outbreak Response Activity in Japan, and a Discussion for a Possible Strategy of Outbreak Response Using Cycle Threshold Values of Real-Time Reverse Transcription PCR for Measles Virus in Measles Elimination Settings" Viruses 15, no. 1: 171. https://doi.org/10.3390/v15010171
APA StyleSeto, J., Aoki, Y., Komabayashi, K., Yamada, K., Ishikawa, H., Ichikawa, T., Ahiko, T., & Mizuta, K. (2023). Measles Outbreak Response Activity in Japan, and a Discussion for a Possible Strategy of Outbreak Response Using Cycle Threshold Values of Real-Time Reverse Transcription PCR for Measles Virus in Measles Elimination Settings. Viruses, 15(1), 171. https://doi.org/10.3390/v15010171






