Tixagevimab/Cilgavimab in SARS-CoV-2 Prophylaxis and Therapy: A Comprehensive Review of Clinical Experience
Abstract
:1. Introduction
2. Methods
3. Tixagevimab/Cilgavimab
4. Prophylaxis
4.1. Clinical Trial Data
4.2. Real-World Evidence on Pre-Exposure Prophylaxis
5. Therapy
5.1. Clinical Trial Data
5.2. RWE on Therapy
6. Expert Opinion and Future Directions
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Cevik, M.; Kuppalli, K.; Kindrachuk, J.; Peiris, M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ 2020, 371, m3862. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.P.Y.; Cheung, M.C.; Perera, R.; Ng, K.C.; Bui, C.H.T.; Ho, J.C.W.; Ng, M.M.T.; Kuok, D.I.T.; Shih, K.C.; Tsao, S.W.; et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: An analysis in ex-vivo and in-vitro cultures. Lancet Respir. Med. 2020, 8, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef] [PubMed]
- Backer, J.A.; Eggink, D.; Andeweg, S.P.; Veldhuijzen, I.K.; van Maarseveen, N.; Vermaas, K.; Vlaemynck, B.; Schepers, R.; van den Hof, S.; Reusken, C.B.; et al. Shorter serial intervals in SARS-CoV-2 cases with Omicron BA.1 variant compared with Delta variant, the Netherlands, 13 to 26 December 2021. Eurosurveillance 2022, 27, 2200042. [Google Scholar] [CrossRef]
- Embi, P.J.; Levy, M.E.; Naleway, A.L.; Patel, P.; Gaglani, M.; Natarajan, K.; Dascomb, K.; Ong, T.C.; Klein, N.P.; Liao, I.C.; et al. Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults-Nine States, January-September 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1553–1559. [Google Scholar] [CrossRef]
- Fisher, A.M.; Schlauch, D.; Mulloy, M.; Dao, A.; Reyad, A.I.; Correll, M.; Fromell, G.J.; Pittman, J.; Bingaman, A.W.; Sankarapandian, B.; et al. Outcomes of COVID-19 in hospitalized solid organ transplant recipients compared to a matched cohort of non-transplant patients at a national healthcare system in the United States. Clin. Transplant. 2021, 35, e14216. [Google Scholar] [CrossRef]
- National Institutes of Health COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 10 December 2022).
- Takashita, E.; Yamayoshi, S.; Simon, V.; van Bakel, H.; Sordillo, E.M.; Pekosz, A.; Fukushi, S.; Suzuki, T.; Maeda, K.; Halfmann, P.; et al. Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants. N. Engl. J. Med. 2022, 387, 468–470. [Google Scholar] [CrossRef]
- Liu, C.; Ginn, H.M.; Dejnirattisai, W.; Supasa, P.; Wang, B.; Tuekprakhon, A.; Nutalai, R.; Zhou, D.; Mentzer, A.J.; Zhao, Y.; et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell 2021, 184, 4220–4236.E13. [Google Scholar] [CrossRef]
- Planas, D.; Bruel, T.; Staropoli, I.; Guivel-Benhassine, F.; Porrot, F.; Maes, P.; Grzelak, L.; Prot, M.; Mougari, S.; Planchais, C.; et al. Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies. BioRxiv 2022. [Google Scholar] [CrossRef]
- Cox, M.; Peacock, T.P.; Harvey, W.T.; Hughes, J.; Wright, D.W.; Consortium, C.-G.U.; Willett, B.J.; Thomson, E.; Gupta, R.K.; Peacock, S.J.; et al. SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nat. Rev. Microbiol. 2022, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tuekprakhon, A.; Nutalai, R.; Dijokaite-Guraliuc, A.; Zhou, D.; Ginn, H.M.; Selvaraj, M.; Liu, C.; Mentzer, A.J.; Supasa, P.; Duyvesteyn, H.M.E.; et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. BioRxiv 2022, 1–3. [Google Scholar] [CrossRef]
- Rockett, R.; Basile, K.; Maddocks, S.; Fong, W.; Agius, J.E.; Johnson-Mackinnon, J.; Arnott, A.; Chandra, S.; Gall, M.; Draper, J.; et al. Resistance Mutations in SARS-CoV-2 Delta Variant after Sotrovimab Use. N. Engl. J. Med. 2022, 386, 1477–1479. [Google Scholar] [CrossRef]
- Gliga, S.; Luebke, N.; Killer, A.; Gruell, H.; Walker, A.; Dilthey, A.T.; Lohr, C.; Flasshove, C.; Orth, H.M.; Feldt, T.; et al. Rapid selection of sotrovimab escape variants in SARS-CoV-2 Omicron infected immunocompromised patients. Clin. Infect. Dis. 2022, ciac802. [Google Scholar] [CrossRef]
- Birnie, E.; Biemond, J.J.; Appelman, B.; de Bree, G.J.; Jonges, M.; Welkers, M.R.A.; Wiersinga, W.J. Development of Resistance-Associated Mutations After Sotrovimab Administration in High-risk Individuals Infected with the SARS-CoV-2 Omicron Variant. JAMA 2022, 328, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Jary, A.; Marot, S.; Faycal, A.; Leon, S.; Sayon, S.; Zafilaza, K.; Ghidaoui, E.; Quoc, S.N.; Nemlaghi, S.; Choquet, S.; et al. Spike Gene Evolution and Immune Escape Mutations in Patients with Mild or Moderate Forms of COVID-19 and Treated with Monoclonal Antibodies Therapies. Viruses 2022, 14, 226. [Google Scholar] [CrossRef]
- Pommeret, F.; Colomba, J.; Bigenwald, C.; Laparra, A.; Bockel, S.; Bayle, A.; Michot, J.M.; Hueso, T.; Albiges, L.; Tiberghien, P.; et al. Bamlanivimab + etesevimab therapy induces SARS-CoV-2 immune escape mutations and secondary clinical deterioration in COVID-19 patients with B-cell malignancies. Ann. Oncol. 2021, 32, 1445–1447. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Tzou, P.L.; Kosakovsky Pond, S.L.; Ioannidis, J.P.A.; Shafer, R.W. Susceptibility of SARS-CoV-2 Omicron Variants to Therapeutic Monoclonal Antibodies: Systematic Review and Meta-analysis. Microbiol. Spectr. 2022, 10, e0092622. [Google Scholar] [CrossRef]
- Bruel, T.; Stefic, K.; Nguyen, Y.; Toniutti, D.; Staropoli, I.; Porrot, F.; Guivel-Benhassine, F.; Bolland, W.H.; Planas, D.; Hadjadj, J.; et al. Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4, and BA.5 in patients receiving monoclonal antibodies. Cell Rep. Med. 2022, 3, 100850. [Google Scholar] [CrossRef]
- FDA. Fact Sheet for Healthcare Providers: Emergency Use Authorization for Evusheld™; FDA: Silver Spring, MD, USA, 2022.
- Bruel, T.; Hadjadj, J.; Maes, P.; Planas, D.; Seve, A.; Staropoli, I.; Guivel-Benhassine, F.; Porrot, F.; Bolland, W.H.; Nguyen, Y.; et al. Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat. Med. 2022, 28, 1297–1302. [Google Scholar] [CrossRef]
- Stuver, R.; Shah, G.L.; Korde, N.S.; Roeker, L.E.; Mato, A.R.; Batlevi, C.L.; Chung, D.J.; Doddi, S.; Falchi, L.; Gyurkocza, B.; et al. Activity of AZD7442 (tixagevimab-cilgavimab) against Omicron SARS-CoV-2 in patients with hematologic malignancies. Cancer Cell 2022, 40, 590–591. [Google Scholar] [CrossRef]
- COVID-19 Updates: Increased dosage of tixagevimab/cilgavimab (Evusheld). Med. Lett. Drugs Ther. 2022, 64, 48.
- Infectous Diseases Society of America IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#Recommendations20-21:NeutralizingAntibodiesforPre-ExposureandPost-ExposureProphylaxis (accessed on 15 December 2022).
- Bellino, S. COVID-19 treatments approved in the European Union and clinical recommendations for the management of non-hospitalized and hospitalized patients. Ann. Med. 2022, 54, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- White, H.; McDonald, S.J.; Barber, B.; Davis, J.; Burr, L.; Nair, P.; Mukherjee, S.; Tendal, B.; Elliott, J.; McGloughlin, S.; et al. Care for adults with COVID-19: Living guidelines from the National COVID-19 Clinical Evidence Taskforce. Med. J. Aust. 2022, 217, 368–378. [Google Scholar] [CrossRef]
- Bartoletti, M.; Azap, O.; Barac, A.; Bussini, L.; Ergonul, O.; Krause, R.; Martin-Quiros, A.; Pano-Pardo, J.R.; Power, N.; Sibani, M.; et al. European society of clinical microbiology and infectious diseases guidelines for coronavirus disease 2019: An update on treatment of patients with mild/moderate disease. Clin. Microbiol. Infect. 2022, 28, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schafer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Sockolosky, J.T.; Szoka, F.C. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv. Drug Deliv. Rev. 2015, 91, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Loo, Y.M.; McTamney, P.M.; Arends, R.H.; Abram, M.E.; Aksyuk, A.A.; Diallo, S.; Flores, D.J.; Kelly, E.J.; Ren, K.; Roque, R.; et al. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Sci. Transl. Med. 2022, 14, eabl8124. [Google Scholar] [CrossRef]
- Robbie, G.J.; Criste, R.; Dall’acqua, W.F.; Jensen, K.; Patel, N.K.; Losonsky, G.A.; Griffin, M.P. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob. Agents Chemother. 2013, 57, 6147–6153. [Google Scholar] [CrossRef] [Green Version]
- Oganesyan, V.; Gao, C.; Shirinian, L.; Wu, H.; Dall’Acqua, W.F. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr. D Biol. Crystallogr. 2008, 64, 700–704. [Google Scholar] [CrossRef] [Green Version]
- Mallory, R.M.; Ali, S.O.; Takas, T.; Kankam, M.; Dubovsky, F.; Tseng, L. A phase 1 study to evaluate the safety and pharmacokinetics of MEDI8852, an anti-influenza A monoclonal antibody, in healthy adult volunteers. Biologicals 2017, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J.; et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of COVID-19. N. Engl. J. Med. 2022, 386, 2188–2200. [Google Scholar] [CrossRef] [PubMed]
- Kleiboeker, H.L.; Jorgenson, M.R.; Smith, J.A. Myalgia in liver transplant recipients after receiving tixagevimab/cilgavimab for pre-exposure prophylaxis of COVID-19: A case series. Transpl. Infect. Dis. 2022, 24, e13932. [Google Scholar] [CrossRef]
- Birabaharan, M.; Hill, E.; Begur, M.; Kaelber, D.C.; Martin, T.C.; Mehta, S.R. Cardiovascular outcomes after tixagevimab and cilgavimab use for pre-exposure prophylaxis against COVID-19: A population-based propensity-matched cohort study. Clin. Infect. Dis. 2022, ciac894. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, J.; Murthy, S.; Afra, K. Cardiac and vascular serious adverse events following tixagevimab-cilgavimab. Lancet Respir. Med. 2022, 11, E5–E6. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Loo, Y.M.C.Y.; Ren, K.; Rosenthal, K.; Flores, D.; Aksyuk, A.; Wilkins, D.; Stanley, A.M.; Roque, R.; Diallo, S.; Campbell, D.; et al. The SARS-CoV-2 Monoclonal Antibody Combination AZD7442 (Tixagevimab/Cilgavimab) Does Not Interfere with COVID-19 Vaccine-induced Immunogenicity. In Proceedings of the 31st ECCMID, Lisbon, Portugal, 23–26 April 2022. [Google Scholar]
- NCT04625972 Phase III Double-Blind, Placebo-Controlled Study of AZD7442 for Post-Exposure Prophylaxis of COVID-19 in Adults (STORM CHASER). Available online: https://clinicaltrials.gov/ct2/show/NCT04625972 (accessed on 10 December 2022).
- Young-Xu, Y.; Epstein, L.; Marconi, V.C.; Davey, V.; Zwain, G.; Smith, J.; Korves, C.; Cunningham, F.; Bonomo, R.; Ginde, A.A. Tixagevimab/Cilgavimab for Prevention of COVID-19 during the Omicron Surge: Retrospective Analysis of National VA Electronic Data. medRxiv 2022. [Google Scholar] [CrossRef]
- Al Jurdi, A.; Morena, L.; Cote, M.; Bethea, E.; Azzi, J.; Riella, L.V. Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave. Am. J. Transplant. 2022, 22, 3130–3136. [Google Scholar] [CrossRef]
- Calabrese, C.; Kirchner, E.; Villa-Forte, A.; Hajj-Ali, R.A.; Moss, B.P.; Fernandez, J.P.; Calabrese, L. Early experience with tixagevimab/cilgavimab pre-exposure prophylaxis in patients with immune-mediated inflammatory disease undergoing B cell depleting therapy and those with inborn errors of humoral immunity. RMD Open 2022, 8, e002557. [Google Scholar] [CrossRef]
- Karaba, A.H.; Kim, J.D.; Chiang, T.P.; Alejo, J.L.; Abedon, A.T.; Mitchell, J.; Chang, A.; Eby, Y.; Johnston, T.S.; Aytenfisu, T.; et al. Omicron BA.1 and BA.2 Neutralizing Activity Following Pre-Exposure Prophylaxis with Tixagevimab plus Cilgavimab in Vaccinated Solid Organ Transplant Recipients. medRxiv 2022. [Google Scholar] [CrossRef]
- Kertes, J.; Shapiro Ben David, S.; Engel-Zohar, N.; Rosen, K.; Hemo, B.; Kantor, A.; Adler, L.; Shamir Stein, N.; Mizrahi Reuveni, M.; Shahar, A. Association Between AZD7442 (Tixagevimab-Cilgavimab) Administration and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, Hospitalization, and Mortality. Clin. Infect. Dis. 2022, ciac625. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.; Flahault, A.; Chavarot, N.; Melenotte, C.; Cheminant, M.; Deschamps, P.; Carlier, N.; Lafont, E.; Thomas, M.; Flamarion, E.; et al. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Clin. Microbiol. Infect. 2022, 28, 1654-e1. [Google Scholar] [CrossRef] [PubMed]
- Ordaya, E.E.; Beam, E.; Yao, J.D.; Razonable, R.R.; Vergidis, P. Characterization of Early-Onset Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Immunocompromised Patients Who Received Tixagevimab-Cilgavimab Prophylaxis. Open Forum Infect. Dis. 2022, 9, ofac283. [Google Scholar] [CrossRef]
- Conte, W.L.; Golzarri-Arroyo, L. Tixagevimab and Cilgavimab (Evusheld) boosts antibody levels to SARS-CoV-2 in patients with multiple sclerosis on b-cell depleters. Mult. Scler. Relat. Disord. 2022, 63, 103905. [Google Scholar] [CrossRef] [PubMed]
- Ocon, A.J.; Mustafa, S.S. Real-World Experience of Tixagevimab and Cilgavimab (Evusheld) in Rheumatologic Patients on Rituximab. J. Clin. Rheumatol. 2022. [Google Scholar] [CrossRef]
- Montgomery, H.; Hobbs, F.D.R.; Padilla, F.; Arbetter, D.; Templeton, A.; Seegobin, S.; Kim, K.; Campos, J.A.S.; Arends, R.H.; Brodek, B.H.; et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2022, 10, 985–996. [Google Scholar] [CrossRef]
- Kaminski, H.; Gigan, M.; Vermorel, A.; Charrier, M.; Guirle, L.; Jambon, F.; Lacapere, A.; Menard, C.; Moreau, K.; Neau-Cransac, M.; et al. COVID-19 morbidity decreases with tixagevimab-cilgavimab preexposure prophylaxis in kidney transplant recipient nonresponders or low-vaccine responders. Kidney Int. 2022, 102, 936–938. [Google Scholar] [CrossRef]
- Al-Obaidi, M.M.; Gungor, A.B.; Kurtin, S.E.; Mathias, A.E.; Tanriover, B.; Zangeneh, T.T. The Prevention of COVID-19 in High-Risk Patients Using Tixagevimab-Cilgavimab (Evusheld): Real-World Experience at a Large Academic Center. Am. J. Med. 2022, 136, 96–99. [Google Scholar] [CrossRef]
- Davis, J.A.; Granger, K.; Roubal, K.; Smith, D.; Gaffney, K.J.; McGann, M.; Cendagorta, A.M.; Thurlapati, A.; Herbst, A.; Hendrickson, L.; et al. Efficacy of tixagevimab-cilgavimab in preventing SARS-CoV-2 for patients with B-cell malignancies. Blood 2022. [Google Scholar] [CrossRef]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Stein, N.; Saliba, W. Effectiveness of Evusheld in Immunocompromised Patients: Propensity Score-Matched Analysis. Clin. Infect. Dis. 2022, ciac855. [Google Scholar] [CrossRef]
- Zerbit, J.; Detroit, M.; Meyer, A.; Decroocq, J.; Deau-Fischer, B.; Deschamps, P.; Birsen, R.; Mondesir, J.; Franchi, P.; Miekoutima, E.; et al. Patients with Hematological Malignancies Treated with T-Cell or B-Cell Immunotherapy Remain at High Risk of Severe Forms of COVID-19 in the Omicron Era. Viruses 2022, 14, 2377. [Google Scholar] [CrossRef]
- Chen, B.H.N. Binkin N, Law N, Horton LE, Yam N, Chen V, Abeles S, Real World Effectiveness of Tixagevimab/cilgavimab (Evusheld) in the 2 Omicron Era. medRxiv 2022. [Google Scholar] [CrossRef]
- Bertrand, D.; Laurent, C.; Lemee, V.; Lebourg, L.; Hanoy, M.; Le Roy, F.; Nezam, D.; Pruteanu, D.; Grange, S.; de Nattes, T.; et al. Efficacy of anti-SARS-CoV-2 monoclonal antibody prophylaxis and vaccination on the Omicron variant of COVID-19 in kidney transplant recipients. Kidney Int. 2022, 102, 440–442. [Google Scholar] [CrossRef]
- Benotmane, I.; Velay, A.; Thaunat, O.; Gautier, V.G.; Olagne, J.; Fafi-Kremer, S.; Caillard, S. Pre-exposure prophylaxis with Evusheld™ elicits limited neutralizing activity against the omicron variant in kidney transplant patients. medRxiv 2022. [Google Scholar] [CrossRef]
- Benotmane, I.; Velay, A.; Gautier-Vargas, G.; Olagne, J.; Obrecht, A.; Cognard, N.; Heibel, F.; Braun-Parvez, L.; Keller, N.; Martzloff, J.; et al. Breakthrough COVID-19 cases despite prophylaxis with 150 mg of tixagevimab and 150 mg of cilgavimab in kidney transplant recipients. Am. J. Transplant. 2022, 22, 2675–2681. [Google Scholar] [CrossRef]
- Jondreville, L.; D’Aveni, M.; Labussiere-Wallet, H.; Le Bourgeois, A.; Villate, A.; Berceanu, A.; Bezsera, S.M.; Thiebaut, A.; Boissard-Simonet, M.; Legrand, M.; et al. Pre-exposure prophylaxis with tixagevimab/cilgavimab (AZD7442) prevents severe SARS-CoV-2 infection in recipients of allogeneic hematopoietic stem cell transplantation during the Omicron wave: A multicentric retrospective study of SFGM-TC. J. Hematol. Oncol. 2022, 15, 169. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.; Salto-Alejandre, S.; Barker, L.; Langlee, J.; Freed, K.; Carter, D.; Bannon, J.; Goddard, D.; Mostafa, H.; Werbel, W.; et al. COVID-19 Outcomes in Solid Organ Transplant Recipients Who Received Tixagevimab-cilgavimab Prophylaxis and/or Bebtelovimab Treatment in a Nurse-driven Monoclonal Antibody Program During the Omicron Surge. Transplantation 2022. [Google Scholar] [CrossRef]
- Aqeel, F.; Geetha, D. Tixagevimab and Cilgavimab (Evusheld) in Rituximab-treated Antineutrophil Cytoplasmic Antibody Vasculitis Patients. Kidney Int. Rep. 2022, 7, 2537–2538. [Google Scholar] [CrossRef]
- Woopen, C.; Konofalska, U.; Akgun, K.; Ziemssen, T. Case report: Variant-specific pre-exposure prophylaxis of SARS-CoV-2 infection in multiple sclerosis patients lacking vaccination responses. Front. Immunol. 2022, 13, 897748. [Google Scholar] [CrossRef]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Khoury, J.; Amar, M.; Stein, N.; Goldstein, L.H.; Saliba, W. Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients. Clin. Infect. Dis. 2022, ciac443. [Google Scholar] [CrossRef]
- Estiri, H.; Strasser, Z.H.; Murphy, S.N. Individualized prediction of COVID-19 adverse outcomes with MLHO. Sci. Rep. 2021, 11, 5322. [Google Scholar] [CrossRef]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw. Open 2021, 4, e210830. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Paredes, R.; Murray, T.A.; Engen, N.; Grandits, G.; Vekstein, A.; Ivey, N.; Mourad, A.; Sandkovsky, U.; Gottlieb, R.L.; et al. Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: A randomised, double-blind, phase 3 trial. Lancet Respir. Med. 2022, 10, 972–984. [Google Scholar]
- Benotmane IOJGautier Vargas, G.; Cognard, N.; Heibel, F.; Braun-Parvez, L.; Keller, N.; Martzloff, J.; Perrin, P.; Pszczolinski, R.; Moulin, B.; Fafi-Kremer, S. Tixagevimab-cilgavimab as an early treatment for COVID-19 in kidney transplant recipients. MedRxiv 2022. [Google Scholar] [CrossRef]
- Lafont, E.; Pere, H.; Lebeaux, D.; Cheminet, G.; Thervet, E.; Guillemain, R.; Flahault, A. Targeted SARS-CoV-2 treatment is associated with decreased mortality in immunocompromised patients with COVID-19. J. Antimicrob. Chemother. 2022, 77, 2688–2692. [Google Scholar] [CrossRef]
- Otiniano, A.; van de Wyngaert, Z.; Brissot, E.; Dulery, R.; Gozlan, J.; Daguenel, A.; Abi Aad, Y.; Ricard, L.; Stocker, N.; Banet, A.; et al. Tixagevimab/cilgavimab for Omicron SARS-CoV-2 infection in patients with haematologic diseases. Bone Marrow Transplant. 2022, 1–3. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Zhu, K.; Xu, C.; Wang, D.; Hou, M. The effect of tixagevimab-cilgavimab on clinical outcomes in patients with COVID-19: A systematic review with meta-analysis. J. Infect. 2022, 86, E15–E17. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: UK will not buy Evusheld owing to “insufficient data” on protection, government says. BMJ 2022, 378, o2021. [Google Scholar] [CrossRef]
- Hypersensitivity reactions with tixagevimab/cilgavimab (Evusheld). Med. Lett. Drugs Ther. 2022, 64, 112.
- Vellas, C.; Tremeaux, P.; Del Bello, A.; Latour, J.; Jeanne, N.; Ranger, N.; Danet, C.; Martin-Blondel, G.; Delobel, P.; Kamar, N.; et al. Resistance mutations in SARS-CoV-2 omicron variant in patients treated with sotrovimab. Clin. Microbiol. Infect. 2022, 28, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Vellas, C.; Kamar, N.; Izopet, J. Resistance mutations in SARS-CoV-2 omicron variant after tixagevimab-cilgavimab treatment. J. Infect. 2022, 85, e162–e163. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Casadevall, A. A Critical Analysis of the Use of Cilgavimab plus Tixagevimab Monoclonal Antibody Cocktail (Evusheld) for COVID-19 Prophylaxis and Treatment. Viruses 2022, 14, 1999. [Google Scholar] [CrossRef] [PubMed]
- Magawa, S.; Nii, M.; Maki, S.; Enomoto, N.; Takakura, S.; Maegawa, Y.; Osato, K.; Tanaka, H.; Kondo, E.; Ikeda, T. Evaluation of the tolerability of monoclonal antibody therapy for pregnant patients with COVID-19. J. Obstet. Gynaecol. Res. 2022, 48, 2325–2333. [Google Scholar] [CrossRef]
- Chang, M.H.; Cowman, K.; Guo, Y.; Bao, H.; Bernstein, P.S.; Gendlina, I.; Nori, P. A real-world assessment of tolerability and treatment outcomes of COVID-19 monoclonal antibodies administered in pregnancy. Am. J. Obstet. Gynecol. 2022, 226, 743–745. [Google Scholar] [CrossRef]
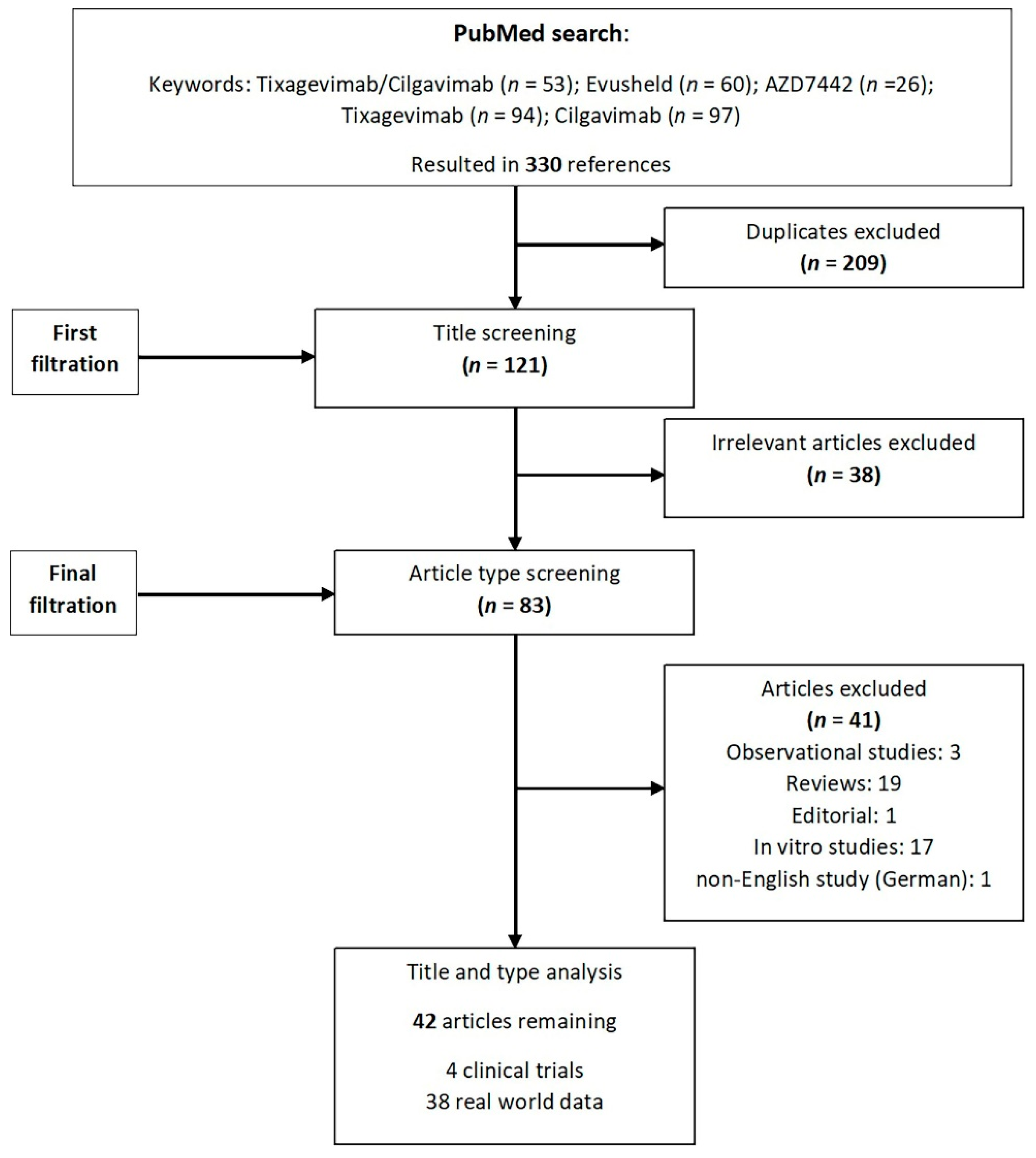
| Lineage | Tixagevimab/Cilgavimab | Sotrovimab (no Longer Recommended, According to NIH Living Guidance) | Casirivimab/ Imdevimab (no Longer Recommended, According to NIH Living Guidance) | Bebtelovimab (no Longer Recommended, According to NIH Living Guidance) | Bamlanivimab/ Etesevimab (no Longer Recommended, According to NIH Living Guidance) |
|---|---|---|---|---|---|
| Alpha | |||||
| Beta | |||||
| Gamma | |||||
| Delta | |||||
| Omicron | |||||
| BA.1 | |||||
| BA.1.1 | |||||
| BA.2 | |||||
| BA.2.12.1 | |||||
| BA.2.75.2 | |||||
| BA.4 | |||||
| BA.4.6 | |||||
| BA.5 | |||||
| BQ.1/BQ1.1 | |||||
| XBB (BA2.10.1 and BA.2.75 recombinant) |
| Reference | Country of Origin | Population Characteristics | Outcomes |
|---|---|---|---|
| Pre-exposure prophylaxis | |||
| Young-Xu, et al. Tixagevimab/Cilgavimab for Prevention of COVID-19 during the Omicron Surge: Retrospective Analysis of National VA Electronic Data. medRxiv 2022. [42] https://doi.org/10.1101/2022.05.28.22275716 (accessed on 10 December 2022) | USA |
| Composite endpoints: SARS-CoV-2 infection, COVID-19-related hospitalization, all-cause mortality Lower incidence of composite outcome 17/1733 (1.0%) vs 206/6354 (3.2%); HR 0.31; 95% CI, 0.18–0.53) lower SARS-CoV-2 infection (HR 0.34; 95% CI, 0.13–0.87) lower COVID-19 hospitalization (HR 0.13; 95% CI, 0.02–0.99) lower all-cause mortality (HR 0.36; 95% CI, 0.18–0.73) |
| Ordaya EE, et al. Characterization of Early-Onse Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Immunocompromised Patients Who Received Tixagevimab-Cilgavimab Prophylaxis Open Forum Infect Dis. 2022. [48] https://doi.org/10.1093/ofid/ofac283 (accessed on 10 December 2022) | USA |
| Endpoint: SARS-CoV-2 infection, COVID-19-related hospitalization, all-cause mortality 8/674 (1.2%) infected with COVID-19 2/8 required hospitalization No deaths |
| Kertes J et al. Association Between AZD7442 (Tixagevimab-Cilgavimab) Administration and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, Hospitalization, and Mortality, Clinical Infectious Diseases, 2022; ciac625, [46] https://doi.org/10.1093/cid/ciac625 (accessed on 10 December 2022) | Israel |
| Endpoint: SARS-CoV-2 infection, COVID-19-related hospitalization, all-cause mortality 29/825 (3.5%) and 308/4299 (7.2%) infected with COVID-19 1/825(0.1%) compared with 27/4299(0.6%) hospitalized 0/825 compared with 40/4299(0.9%) mortality rate The AZD7442 group was half as likely to become infected with SARS-CoV-2 (OR: 0.51; 95% CI: 0.30–0.84) 92% less likely to be hospitalized/die than those not administered AZD7442 (OR: 0.08; 95% CI: 0.01–0.54). |
| Stuver, R. et al. Activity of AZD7442 (tixagevimab-cilgavimab) against Omicron SARS-CoV-2 in patients with hematologic malignancies, Cancer Cell 2022, 40, 590–591. [23] https://doi.org/10.1016/j.ccell.2022.05.007 (accessed on 10 December 2022) | USA |
| Endpoint: anti-S IgG titers 47/47(100%) high titers neutralization of wild-type (WT) receptor-binding domain (RBD) 47/47 (100%) who received single dose 150 mg 5/5(100% who received additional 150 mg 5/5(100%) who received single dose of 300 mg neutralizing activity against Omicron-RBD (positive cut-off value = 30%) The median neutralization by subgroup of 47 was <30%; those who received 300 mg in total had a mean neutralization >30% (9/10 above 30%) differential neutralizing capacity against various Omicron sublineages; 300 mg dose of tixa/cilga for pre-exposure prophylaxis |
| Benotmane, I. et al. Pre-exposure prophylaxis with Evusheld™elicits limited neutralizing activity against the Omicron variant in kidney transplant patients. medRxiv 2022. [59] https://doi.org/10.1101/2022.03.21.22272669 (accessed on 10 December 2022) | France |
| Primary endpoint: Omicron BA.1 neutralization activity after 29 days (median) 9.5% (6/63) of those who received Evusheld 71% (10/14) of those positive during the fifth wave 2.6% (1/39) of those who received casirivimab-imdevimab Secondary endpoint: anti-RBD IgG titers generally low after Evusheld injection high interindividual variability the patients’ body mass index has an inverse correlation with anti-RBD IgG titers no neutralizing activity with anti-RBD titers <2500 BAU/mL after Evusheld |
| Bertrand, D. et al. Efficacy of anti–SARS-CoV-2 monoclonal antibody prophylaxis and vaccination on the Omicron variant of COVID-19 in kidney transplant recipients. Letter. Kidney Int. 2022. [58] https://doi.org/10.1016/j.kint.2022.05.007(accessed on 10 December 2022) | France |
| Endpoint: Incidence of Omicron SARS-CoV-2 infection, COVID-19-related hospitalization, all-cause mortality 113/860(13.1%) infected with COVID-19 21/860(2%) required hospitalization (8 in the ICU) 5/860 (0.6%) COVID-19-related deaths The occurrence of infection, symptomatic infection, hospitalization, intensive care unit hospitalization, and COVID-19 death were significantly increased in patients in group 3 Patients in groups 1 and 2 showed similar results |
| Kaminski, H. et al. COVID-19 morbidity decreases with tixagevimab–cilgavimab preexposure prophylaxis in kidney transplant recipient nonresponders or low-vaccine responders. Letter in press. Kidney Int. 2022. [52] https://doi.org/10.1016/j.kint.2022.07.008 (accessed on 10 December 2022) | France |
| Endpoint: Incidence of Omicron SARS-CoV-2 infection, COVID-19-related hospitalization, all-cause mortality 41/333 (12.3%) and 42/97 (43.3%) infected with COVID-19 4/333 (1.2%) and 11/97 (11.3%) required hospitalization (2 and 6 KTR respectively required in the ICU) 1/333 (0.3%) and 2/97 (2%) COVID-19-related deaths preexposure prophylaxis with tixagevimab–cilgavimab is effective onCOVID-19 infection caused by Omicron in KTRs |
| Al Jurdi et al. Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the Omicron wave. Online ahead of print. Am. J Transplant. 2022. [43] https://doi.org/10.1111/ajt.17128 (accessed on 10 December 2022) | USA |
| Endpoint: Incidence of breakthrough COVID-19 infection Breakthrough infection in 11 (5%) from group 1 and 32 (14%) from group 2 150–150 mg dose subgroup had a higher incidence of breakthrough infections compared to those who received the 300–300 mg dose |
| Karaba et al. Omicron BA.1 and BA.2 Neutralizing Activity following Pre-Exposure Prophylaxis with Tixagevimab plus Cilgavimab inVaccinated Solid Organ Transplant Recipients. medRxiv 2022. [45] https://doi.org/10.1101/2022.05.24.22275467 (accessed on 10 December 2022) | USA |
| Endpoints: Neutralization of SARS-CoV-2 variants after tixa/cilga (achieving ≥20% ACE2 inhibition) Omicron BA.1: from 5/61 (8%) to 10/61 (16%) (p-value:0.06) Omicron BA.2: from 4/61 (7%) to 44/61 (72%) (p-value < 0.001) The change in titer was similar for those receiving a single 300 + 300 mg dose, versus two 150 + 150 mg doses. |
| Conte, W.L. et al. Tixagevimab and Cilgavimab (Evusheld) boost antibody levels to SARS-CoV-2 in patients with multiple sclerosis on b-cell depleters. Mult Scler Relat Disord. 2022. [49] https://doi.org/10.1016/j.msard.2022.103905(accessed on 10 December 2022) | USA |
| Endpoints: Level of SARS-CoV-2 antibody response At baseline 12/18 were lower than 0.8 U/mL and 6/18 were above threshold Two weeks after tixa/cilga 100% had an antibody response above threshold (>250 U/mL; p-value < 0.001) |
| Ocon, A.J. et al. Real-World experience of Tixagevimab and Cilgavimab (Evusheld) in rheumatologic patients on Rituximab [50] https://doi.org/10.1097/rhu.0000000000001907 (accessed on 10 December 2022) | USA |
| Endpoint: Infection with SARS-CoV-2 after 100 ± 33 days 1/43 experienced symptomatic infection No serious adverse events occurred |
| Calabrese, C. et al. Early experience with tixagevimab/cilgavimab pre-exposure prophylaxis in patients with immune-mediated inflammatory disease undergoing B cell depleting therapy and those with inborn errors of humoral immunity. Letter and supplementary data. RMD Open. 2022;8(2):e002557 [44] http://dx.doi.org/10.1136/rmdopen-2022-002557 (accessed on 10 December 2022) | USA |
| Endpoints: SARS-CoV-2 infection, COVID-19-related hospitalization, mortality 12/412 (2.91%) developed breakthrough COVID-19 6 were from group 1 and 6 from group 2 Group 1 patients developedinfection a median of 19 days (13–84) Group 2 patients developed infection a median of 38.5 days (19–72) One patient required hospitalization and high-flow oxygen There were no deaths |
| Chen, B. et al. Real-World Effectiveness of Tixagevimab/cilgavimab (Evusheld) in the Omicron Era. Pre-print. medRxiv. 2022 [57] https://doi.org/10.1101/2022.09.16.22280034 (accessed on 10 December 2022) | USA |
| Endpoints: SARS-CoV-2 infection, COVID-19-related hospitalization, mortality SARS-CoV-2 infection: 121/1295 (9.3%) before and 102/1295 (7.9%) after receiving tixa/cilga Hospitalization: 36/121 (29.8%) (8/36 required ICU) and 6/102 (5.9%) No COVID-19-related deaths occurred |
| Nguyen, Y. et al. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Research note in press. Clin Microbiol Infect. 2022. [47] https://doi.org/10.1016/j.cmi.2022.07.015 (accessed on 10 December 2022) | France |
| Endpoints: SARS-CoV-2 infection, severity of illness, mortality after median 63 (49–73) days SARS-CoV-2 infection: 49/1112 (4.4%) ≥ 5 days after treatment Mild to moderate illness: 43/49 (88%) Moderate-to-severe illness:6/49 (12%) Deaths:2/49 (4%) |
| Benotmane, I. et al. Breakthrough COVID-19 cases despite prophylaxis with 150 mg of tixagevimab and 150 mg of cilgavimab in kidney transplant recipients. [60] https://doi.org/10.1111/ajt.17121 (accessed on 10 December 2022) | France |
| Endpoints: SARS-CoV-2 infection, COVID-19-related hospitalization, mortality SARS-CoV-2 infection: 39/419 (9.4%) Hospitalization:14/39 (35.9%) (3 patients were admitted to the ICU) Deaths: 2/39 (5.1%) Pre-exposure prophylaxis with Evusheld™ does not adequately protect KTRs against Omicron |
| Al-Obaidi, M.M., Gungor A.B., Kurtin S.E., Mathias A.E., Tanriover B., and Zangeneh, T.T. The Prevention of COVID-19 in High-Risk Patients Using Tixagevimab-Cilgavimab (Evusheld): Real-World Experience at a Large Academic Center. Am J Med. 2022. [53] https://doi.org/10.1016/j.amjmed.2022.08.019 (accessed on 10 December 2022) | USA |
| Endpoints: SARS-CoV-2 infection, COVID-19-related hospitalization, mortality SARS-CoV-2 infection: 6/98 (who had PCR test available) Hospitalization: 42/463 (9.1%) Deaths: 4/463(0.9%). no deaths were attributed to COVID-19 |
| Davis, J.A., Granger, K., Roubal, K., Smith, D., Gaffney, K.J., McGann, M. et al. Efficacy of tixagevimab-cilgavimab in preventing SARS-CoV-2 for patients with B-cell malignancies. Blood. 2022 [54] https://doi.org/10.1182/blood.2022018283 (accessed on 10 December 2022) | USA |
| Endpoints: incidence of COVID-19 breakthrough infections COVID-19-related hospitalization, mortality Breakthrough cases at median 91-day follow-up: 27/251 (10.7%) Hospitalization: 4/27 (15%) No deaths observed |
| Najjar-Debbiny, R., Gronich, N., Weber, G., Stein, N., Saliba, W. Effectiveness of Evusheld in Immunocompromised Patients: Propensity Score-Matched Analysis. Clin Infect. Dis. 2022 [55] https://doi.org/10.1093/cid/ciac855 (accessed on 10 December 2022) | Israel |
| Endpoints: SARS-CoV-2 infection, COVID-19-related hospitalization SARS-CoV-2 infection: 72/703 (10.2%) and 377/2812 (13.4%); HR 0.75 (95% CI, 0.58–0.96); p-value: 0.023 Hospitalization: 7/72 and 67/377; HR 0.41 (0.19–0.89); p-value: 0.025 |
| Zerbit, J. et al. Patients with Hematological Malignancies Treated with T-Cell or B-Cell Immunotherapy Remain at High Risk of Severe Forms of COVID-19 in the Omicron Era. Viruses 2022, 14, 2377. [56] https://doi.org/10.3390/v14112377 (accessed on 10 December 2022) | France |
| Endpoints: SARS-CoV-2 infection, COVID-19-related hospitalization, mortality SARS-CoV-2 infection: 57/338 (16.9%) Hospitalization: 13/57 (22.8%), of whom 11/13 (84.6%) required invasive mechanical ventilation 3 deaths were recorded |
| Jondreville L.; et al. Pre-exposure prophylaxis with tixagevimab/cilgavimab (AZD7442) prevents severe SARS-CoV-2 infection in recipients of allogeneic hematopoietic stem cell transplantation during the Omicron wave: a multicentric retrospective study of SFGM-TC. J Hematol Oncol. 2022 Nov 28;15(1):169 [61] https://doi.org/10.1186/s13045-022-01387-0 (accessed on 10 December 2022) | France |
| Endpoints: SARS-CoV-2 infection, COVID-19-related hospitalization, mortality 139/161 (86.3%) remained uninfected 22/161(13.7%) symptomatic SARS-CoV 2 infection 8/22 (36.4%) received an additional treatment No hospitalizations recorded No deaths recorded |
| Aqeel, F., and Geetha, D. (2022). Tixagevimab and Cilgavimab (Evusheld) in Rituximab-treated Antineutrophil Cytoplasmic Antibody Vasculitis Patients. Kidney International Reports, 7(11), 2537–2538. [63] https://doi.org/10.1016/j.ekir.2022.08.019 (accessed on 10 December 2022) | USA |
| Primary Endpoints: SARS-CoV-2 infection, COVID-19-related hospitalization, mortality The one patient who received the lower Evusheld dose was infected with SARS-CoV-2 122 days after receiving Evusheld 3/20 (15%) developed breakthrough COVID-19 disease No hospitalizations recorded No deaths recorded |
| Woopen, C., Konofalska, U., Akgün, K., and Ziemssen, T. (2022). Case report: Variant-specific pre-exposure prophylaxis of SARS-CoV-2 infection in multiple sclerosis patients lacking vaccination responses (Case Report). Frontiers in Immunology, 13. [64] https://doi.org/10.3389/fimmu.2022.897748 (accessed on 10 December 2022) | Germany |
| Endpoints: SARS-CoV-2 infection, COVID-19-related hospitalization, mortality 1/6 asymptomatic SARS-CoV-2 infection before Tixa/Cilga No hospitalizations recorded No deaths recorded |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinosoglou, K.; Rigopoulos, E.-A.; Kaiafa, G.; Daios, S.; Karlafti, E.; Ztriva, E.; Polychronopoulos, G.; Gogos, C.; Savopoulos, C. Tixagevimab/Cilgavimab in SARS-CoV-2 Prophylaxis and Therapy: A Comprehensive Review of Clinical Experience. Viruses 2023, 15, 118. https://doi.org/10.3390/v15010118
Akinosoglou K, Rigopoulos E-A, Kaiafa G, Daios S, Karlafti E, Ztriva E, Polychronopoulos G, Gogos C, Savopoulos C. Tixagevimab/Cilgavimab in SARS-CoV-2 Prophylaxis and Therapy: A Comprehensive Review of Clinical Experience. Viruses. 2023; 15(1):118. https://doi.org/10.3390/v15010118
Chicago/Turabian StyleAkinosoglou, Karolina, Emmanouil-Angelos Rigopoulos, Georgia Kaiafa, Stylianos Daios, Eleni Karlafti, Eleftheria Ztriva, Georgios Polychronopoulos, Charalambos Gogos, and Christos Savopoulos. 2023. "Tixagevimab/Cilgavimab in SARS-CoV-2 Prophylaxis and Therapy: A Comprehensive Review of Clinical Experience" Viruses 15, no. 1: 118. https://doi.org/10.3390/v15010118
APA StyleAkinosoglou, K., Rigopoulos, E.-A., Kaiafa, G., Daios, S., Karlafti, E., Ztriva, E., Polychronopoulos, G., Gogos, C., & Savopoulos, C. (2023). Tixagevimab/Cilgavimab in SARS-CoV-2 Prophylaxis and Therapy: A Comprehensive Review of Clinical Experience. Viruses, 15(1), 118. https://doi.org/10.3390/v15010118








