Early Treatment with Monoclonal Antibodies or Convalescent Plasma Reduces Mortality in Non-Vaccinated COVID-19 High-Risk Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Therapy Regiments
2.2.1. Monoclonal Antibody Therapy
2.2.2. Treatment with High-Titer Convalescent Plasma
2.2.3. Best-Supportive Care
2.3. Ethics
2.4. Statistics
3. Results
3.1. Study Overview
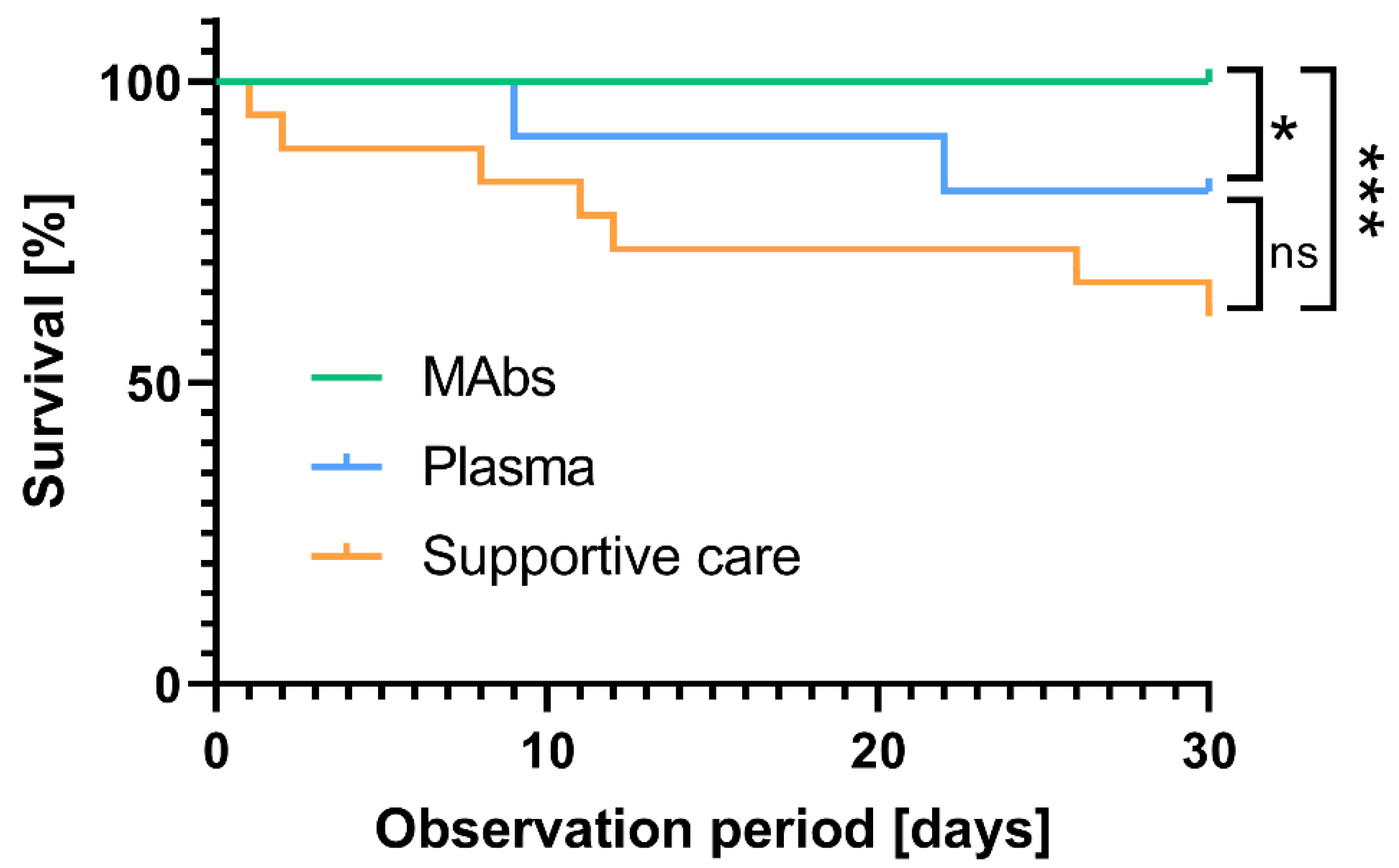
3.2. Antiviral Immunotherapy with Monoclonal Antibodies or Convalescent Plasma Reduced the 30-Day Mortality in High-Risk COVID-19 Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- van der Straten, K.; van Gils, M.J.; de Taeye, S.W.; de Bree, G.J. Optimization of Anti-SARS-CoV-2 Neutralizing Antibody Therapies: Roadmap to Improve Clinical Effectiveness and Implementation. Front. Med. Technol. 2022, 4, 867982. [Google Scholar] [CrossRef]
- Wunsch, K.; Anastasiou, O.E.; Alt, M.; Brochhagen, L.; Cherneha, M.; Thummler, L.; van Baal, L.; Madel, R.J.; Lindemann, M.; Taube, C.; et al. COVID-19 in Elderly, Immunocompromised or Diabetic Patients-From Immune Monitoring to Clinical Management in the Hospital. Viruses 2022, 14, 746. [Google Scholar] [CrossRef] [PubMed]
- Konik, M.; Lindemann, M.; Zettler, M.; Meller, L.; Dolff, S.; Rebmann, V.; Horn, P.A.; Dittmer, U.; Krawczyk, A.; Schipper, L.; et al. Long-Term SARS-CoV-2 Specific Immunity Is Affected by the Severity of Initial COVID-19 and Patient Age. J. Clin. Med. 2021, 10, 4606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhong, N.; Guan, Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N. Engl. J. Med. 2007, 357, 1450–1451. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, J.; Edwards, T.; de Lamballerie, X.; Semple, M.G.; Gallian, P.; Baize, S.; Horby, P.W.; Raoul, H.; Magassouba, N.; Antierens, A.; et al. Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. N. Engl. J. Med. 2016, 374, 33–42. [Google Scholar] [CrossRef]
- Eibl, M.M. History of immunoglobulin replacement. Immunol. Allergy Clin. N. Am. 2008, 28, 737–764. [Google Scholar] [CrossRef]
- Mair-Jenkins, J.; Saavedra-Campos, M.; Baillie, J.K.; Cleary, P.; Khaw, F.M.; Lim, W.S.; Makki, S.; Rooney, K.D.; Nguyen-Van-Tam, J.S.; Beck, C.R.; et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 2015, 211, 80–90. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, J.; Bao, L.; Shi, Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020, 20, 398–400. [Google Scholar] [CrossRef]
- Mungmunpuntipantip, R.; Wiwanitkit, V. COVID-19 convalescent plasma therapy and immunodeficiency. Transfus. Clin. Biol. 2021, 28, 306–307. [Google Scholar] [CrossRef]
- Rnjak, D.; Ravlic, S.; Sola, A.M.; Halassy, B.; Semnicki, J.; Superba, M.; Hecimovic, A.; Kurolt, I.C.; Kurtovic, T.; Macak Safranko, Z.; et al. COVID-19 convalescent plasma as long-term therapy in immunodeficient patients? Transfus. Clin. Biol. 2021, 28, 264–270. [Google Scholar] [CrossRef]
- Bormann, M.; van de Sand, L.; Witzke, O.; Krawczyk, A. Recent Antiviral Treatment and Vaccination Strategies Against SARS-CoV-2. Klin. Monbl. Augenheilkd. 2021, 238, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Thümmler, L.; Konik, M.; Lindemann, M.; Fisenkci, N.; Koldehoff, M.; Gäckler, A.; Horn, P.A.; Theodoropoulos, F.; Taube, C.; Zettler, M.; et al. Long-term cellular immune response in immunocompromised unvaccinated COVID-19 patients undergoing monoclonal antibody treatment. Front. Immunol. 2022, 13, 980698. [Google Scholar] [CrossRef] [PubMed]
- Paniskaki, K.; Konik, M.J.; Anft, M.; Meister, T.L.; Marheinecke, C.; Pfaender, S.; Jäger, J.; Krawczyk, A.; Zettler, M.; Dolff, S.; et al. Superior humoral immunity in vaccinated SARS-CoV-2 convalescence as compared to SARS-COV-2 infection or vaccination. Front. Immunol. 2022, 13, 1031254. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Wright, R.S.; Fairweather, D.; Senefeld, J.W.; Bruno, K.A.; Klassen, S.A.; Carter, R.E.; Klompas, A.M.; Wiggins, C.C.; Shepherd, J.R.; et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Investig. 2020, 130, 4791–4797. [Google Scholar] [CrossRef]
- Liu, S.T.H.; Lin, H.M.; Baine, I.; Wajnberg, A.; Gumprecht, J.P.; Rahman, F.; Rodriguez, D.; Tandon, P.; Bassily-Marcus, A.; Bander, J.; et al. Convalescent plasma treatment of severe COVID-19: A propensity score-matched control study. Nat. Med. 2020, 26, 1708–1713. [Google Scholar] [CrossRef]
- Perotti, C.; Baldanti, F.; Bruno, R.; Del Fante, C.; Seminari, E.; Casari, S.; Percivalle, E.; Glingani, C.; Musella, V.; Belliato, M.; et al. Mortality reduction in 46 severe COVID-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica 2020, 105, 2834–2840. [Google Scholar] [CrossRef]
- Group, R.C. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): A randomised controlled, open-label, platform trial. Lancet 2021, 397, 2049–2059. [Google Scholar] [CrossRef]
- Piechotta, V.; Iannizzi, C.; Chai, K.L.; Valk, S.J.; Kimber, C.; Dorando, E.; Monsef, I.; Wood, E.M.; Lamikanra, A.A.; Roberts, D.J.; et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2021, 5, CD013600. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.J.; Gebo, K.A.; Shoham, S.; Bloch, E.M.; Lau, B.; Shenoy, A.G.; Mosnaim, G.S.; Gniadek, T.J.; Fukuta, Y.; Patel, B.; et al. Early Outpatient Treatment for Covid-19 with Convalescent Plasma. N. Engl. J. Med. 2022, 386, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Libster, R.; Perez Marc, G.; Wappner, D.; Coviello, S.; Bianchi, A.; Braem, V.; Esteban, I.; Caballero, M.T.; Wood, C.; Berrueta, M.; et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N. Engl. J. Med. 2021, 384, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Gharbharan, A.; Jordans, C.C.E.; GeurtsvanKessel, C.; den Hollander, J.G.; Karim, F.; Mollema, F.P.N.; Stalenhoef-Schukken, J.E.; Dofferhoff, A.; Ludwig, I.; Koster, A.; et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat. Commun. 2021, 12, 3189. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Mishevich, J.L.; Mesecar, A.; Mitsuya, H. Recent Drug Development and Medicinal Chemistry Approaches for the Treatment of SARS-CoV-2 and COVID-19. ChemMedChem 2022, 17, e202200440. [Google Scholar] [CrossRef]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Agarwal, A.; Rochwerg, B.; Lamontagne, F.; Siemieniuk, R.A.; Agoritsas, T.; Askie, L.; Lytvyn, L.; Leo, Y.S.; Macdonald, H.; Zeng, L.; et al. A living WHO guideline on drugs for covid-19. BMJ 2020, 370, m3379. [Google Scholar] [CrossRef]
- Kaku, Y.; Kuwata, T.; Zahid, H.M.; Hashiguchi, T.; Noda, T.; Kuramoto, N.; Biswas, S.; Matsumoto, K.; Shimizu, M.; Kawanami, Y.; et al. Resistance of SARS-CoV-2 variants to neutralization by antibodies induced in convalescent patients with COVID-19. Cell Rep. 2021, 36, 109385. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, I.; Pravica, V.; Miljanovic, D.; Cupic, M. Immune Evasion of SARS-CoV-2 Emerging Variants: What Have We Learnt So Far? Viruses 2021, 13, 1192. [Google Scholar] [CrossRef] [PubMed]
- Paniskaki, K.; Anft, M.; Meister, T.L.; Marheinecke, C.; Pfaender, S.; Skrzypczyk, S.; Seibert, F.S.; Thieme, C.J.; Konik, M.J.; Dolff, S.; et al. Immune Response in Moderate to Critical Breakthrough COVID-19 Infection After mRNA Vaccination. Front. Immunol. 2022, 13, 816220. [Google Scholar] [CrossRef] [PubMed]
- Anjan, S.; Khatri, A.; Viotti, J.B.; Cheung, T.; Garcia, L.A.C.; Simkins, J.; Loebe, M.; Phancao, A.; O'Brien, C.B.; Sinha, N.; et al. Is the Omicron variant truly less virulent in solid organ transplant recipients? Transpl. Infect. Dis. 2022, 24, e13923. [Google Scholar] [CrossRef]
- Malahe, S.R.K.; Hoek, R.A.S.; Dalm, V.; Broers, A.E.C.; den Hoed, C.M.; Manintveld, O.C.; Baan, C.C.; van Deuzen, C.M.; Papageorgiou, G.; Bax, H.I.; et al. Clinical characteristics and outcome of immunocompromised patients with COVID-19 caused by the Omicron variant: A prospective observational study. Clin. Infect. Dis. 2022, ciac571, Online ahead of print. [Google Scholar] [CrossRef]
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 Infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/human-regulatroy/overview/public-health-threats/coronavi-rus-disease-covid-19/treatments-vaccines/treatments-covid-19/covid-19-treatments-authorised (accessed on 5 December 2022).
- Available online: https://health.ec.europa.eu/system/files/2021-03/guidance_plasma_covid19_en_0.pdf (accessed on 5 December 2022).
- Schwarzkopf, S.; Krawczyk, A.; Knop, D.; Klump, H.; Heinold, A.; Heinemann, F.M.; Thümmler, L.; Temme, C.; Breyer, M.; Witzke, O.; et al. Cellular Immunity in COVID-19 Convalescents with PCR-Confirmed Infection but with Undetectable SARS-CoV-2-Specific IgG. Emerg Infect. Dis 2021, 27, 122–129. [Google Scholar] [CrossRef]
- Lindemann, M.; Lenz, V.; Knop, D.; Klump, H.; Alt, M.; Aufderhorst, U.W.; Schipper, L.; Schwarzkopf, S.; Meller, L.; Steckel, N.; et al. Convalescent plasma treatment of critically ill intensive care COVID-19 patients. Transfusion 2021, 61, 1394–1403. [Google Scholar] [CrossRef]
- Sarrell, B.A.; Bloch, K.; El Chediak, A.; Kumm, K.; Tracy, K.; Forbes, R.C.; Langone, A.; Thomas, L.; Schlendorf, K.; Trindade, A.J.; et al. Monoclonal antibody treatment for COVID-19 in solid organ transplant recipients. Transplant. Infect. Dis. Off. J. Transplant. Soc. 2022, 24, e13759. [Google Scholar] [CrossRef]
- Bachmann, F.; Budde, K.; Suttorp, N.; Lingscheid, T.; Stegemann, M.S.; Osmanodja, B.; Schrezenmeier, E.; Duettmann, W.; Weber, U.; Naik, M.; et al. Initial Experience With SARS-CoV-2-Neutralizing Monoclonal Antibodies in Kidney or Combined Kidney-Pancreas Transplant Recipients. Transpl. Int. 2022, 35, 10109. [Google Scholar] [CrossRef]
- Wang, A.X.; Busque, S.; Kuo, J.; Singh, U.; Röeltgen, K.; Pinsky, B.A.; Chertow, G.M.; Scandling, J.D.; Lenihan, C.R. SARS-CoV-2 Neutralizing Monoclonal Antibodies for the Treatment of COVID-19 in Kidney Transplant Recipients. Kidney360 2022, 3, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Iketani, S.; Liu, L.; Guo, Y.; Liu, L.; Chan, J.F.; Huang, Y.; Wang, M.; Luo, Y.; Yu, J.; Chu, H.; et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature 2022, 604, 553–556. [Google Scholar] [CrossRef]
- Takashita, E.; Kinoshita, N.; Yamayoshi, S.; Sakai-Tagawa, Y.; Fujisaki, S.; Ito, M.; Iwatsuki-Horimoto, K.; Chiba, S.; Halfmann, P.; Nagai, H.; et al. Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant. N. Engl. J. Med. 2022, 386, 995–998. [Google Scholar] [CrossRef]
- Abolghasemi, H.; Eshghi, P.; Cheraghali, A.M.; Imani Fooladi, A.A.; Bolouki Moghaddam, F.; Imanizadeh, S.; Moeini Maleki, M.; Ranjkesh, M.; Rezapour, M.; Bahramifar, A.; et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfus. Apher. Sci. 2020, 59, 102875. [Google Scholar] [CrossRef]
- Wood, E.M.; Estcourt, L.J.; McQuilten, Z.K. How should we use convalescent plasma therapies for the management of COVID-19? Blood 2021, 137, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.G.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2021, 21, 167–179. [Google Scholar] [CrossRef]
- Beraud, M.; Goodhue Meyer, E.; Lozano, M.; Bah, A.; Vassallo, R.; Brown, B.L. Lessons learned from the use of convalescent plasma for the treatment of COVID-19 and specific considerations for immunocompromised patients. Transfus. Apher. Sci. 2022, 61, 103355. [Google Scholar] [CrossRef] [PubMed]
- Nashaat, H.H.; Anani, M.; Attia, F.M. Convalescent plasma in COVID-19: Renewed focus on the timing and effectiveness of an old therapy. Blood Res. 2022, 57, 6–12. [Google Scholar] [CrossRef]
- Yang, P.; Wang, J.; Zheng, R.; Tan, R.; Li, X.; Liu, X.; Li, Y.; Yuan, Z.; Wang, Y.; Chen, Q.; et al. Convalescent plasma may not be an effective treatment for severe and critically ill covid-19 patients: A Systematic Review & Meta-Analysis of Randomized Controlled Trials. Heart Lung 2022, 53, 51–60. [Google Scholar] [CrossRef]
- Weisser, M.; Khanna, N.; Hedstueck, A.; Sutter, S.T.; Roesch, S.; Stehle, G.; Sava, M.; Deigendesch, N.; Battegay, M.; Infanti, L.; et al. Characterization of pathogen-inactivated COVID-19 convalescent plasma and responses in transfused patients. Transfusion 2022, 62, 1997–2011. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, K.F.A.; Tavernier, S.; Van Roy, N.; De Leeuw, E.; Declercq, J.; Bosteels, C.; Maes, B.; De Bruyne, M.; Bogaert, D.; Bosteels, V.; et al. Case Report: Convalescent Plasma, a Targeted Therapy for Patients with CVID and Severe COVID-19. Front. Immunol. 2020, 11, 596761. [Google Scholar] [CrossRef] [PubMed]
- Garzi, G.; Cinetto, F.; Firinu, D.; Di Napoli, G.; Lagnese, G.; Punziano, A.; Bez, P.; Cinicola, B.L.; Costanzo, G.; Scarpa, R.; et al. Real-life data on monoclonal antibodies and antiviral drugs in Italian inborn errors of immunity patients during COVID-19 pandemic. Front. Immunol. 2022, 13, 947174. [Google Scholar] [CrossRef] [PubMed]

| Group | Treated with mAB (1) | Treated with Plasma (2) | Supportive Care (3) |
|---|---|---|---|
| Total number | 26 | 11 | 18 |
| Sex (w/m) | 17/9 | 5/6 | 8/10 |
| Median age [years] | 58 (17–78) | 63 (44–84) | 75 (35–93) |
| Mean Charlson Comorbidity Index Score, ±SD | 3.23 ± 2.0 | 6.54 ± 2.7 | 5.0 ± 2.12 |
| Severely immunocompromised Patients, n (%) | 17 (65%) | 7 (63%) | 12 (66%) |
| History of pulmonary disease, n (%) | 19 (73%) | 3 (27%) | 5 (27%) |
| Predominant circulating variant? | Alpha B.1.1.7 | Alpha B.1.1.7 | D614G and Alpha B.1.1.7 |
| Range of admission to hospital | 02.2021 to 04.2021 | 07.2020 to 11.2020 | 03.2020 to 02.2021 |
| Number of Patient with moderate disease | 5 (19%) | 3 (27%) | 3 (16%) |
| Progression to severe COVID-19 defined by WHO score ≥ 6, n (%) | 1 (4%) | 6 (54%) | 12 (66%) |
| Parameter | Standardized Coefficients ß | 95% CI | p Value |
|---|---|---|---|
| Sex | 0.371 | −0.8074 to 1.550 | 0.53 |
| Age | −0.0027 | −0.07792 to 0.02377 | 0.29 |
| CCI | 0.139 | −0.1187 to 0.3968 | 0.28 |
| WHO COVID-19 severity score at presentation | 0.81 | 0.01247 to 1.605 | 0.04 * |
| G1 mAb vs. G2 Plasma | 2.002 | 0.3113 to 3.693 | 0.021 * |
| G1 mAb vs. G3 SC | 3.318 | 1.754 to 4.881 | <0.0001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thümmler, L.; Lindemann, M.; Horn, P.A.; Lenz, V.; Konik, M.; Gäckler, A.; Boss, K.; Theodoropoulos, F.; Besa, V.; Taube, C.; et al. Early Treatment with Monoclonal Antibodies or Convalescent Plasma Reduces Mortality in Non-Vaccinated COVID-19 High-Risk Patients. Viruses 2023, 15, 119. https://doi.org/10.3390/v15010119
Thümmler L, Lindemann M, Horn PA, Lenz V, Konik M, Gäckler A, Boss K, Theodoropoulos F, Besa V, Taube C, et al. Early Treatment with Monoclonal Antibodies or Convalescent Plasma Reduces Mortality in Non-Vaccinated COVID-19 High-Risk Patients. Viruses. 2023; 15(1):119. https://doi.org/10.3390/v15010119
Chicago/Turabian StyleThümmler, Laura, Monika Lindemann, Peter A. Horn, Veronika Lenz, Margarethe Konik, Anja Gäckler, Kristina Boss, Fotis Theodoropoulos, Vasiliki Besa, Christian Taube, and et al. 2023. "Early Treatment with Monoclonal Antibodies or Convalescent Plasma Reduces Mortality in Non-Vaccinated COVID-19 High-Risk Patients" Viruses 15, no. 1: 119. https://doi.org/10.3390/v15010119
APA StyleThümmler, L., Lindemann, M., Horn, P. A., Lenz, V., Konik, M., Gäckler, A., Boss, K., Theodoropoulos, F., Besa, V., Taube, C., Brenner, T., Witzke, O., Krawczyk, A., & Rohn, H. (2023). Early Treatment with Monoclonal Antibodies or Convalescent Plasma Reduces Mortality in Non-Vaccinated COVID-19 High-Risk Patients. Viruses, 15(1), 119. https://doi.org/10.3390/v15010119









