Transmission Patterns of Seasonal Influenza in China between 2010 and 2018
Abstract
1. Introduction
2. Methods
2.1. Influenza Surveillance Dataset
2.2. Sequence Data
2.3. Climate and Demographic Data
2.4. Time Series Analysis
2.5. Evolutionary Analysis
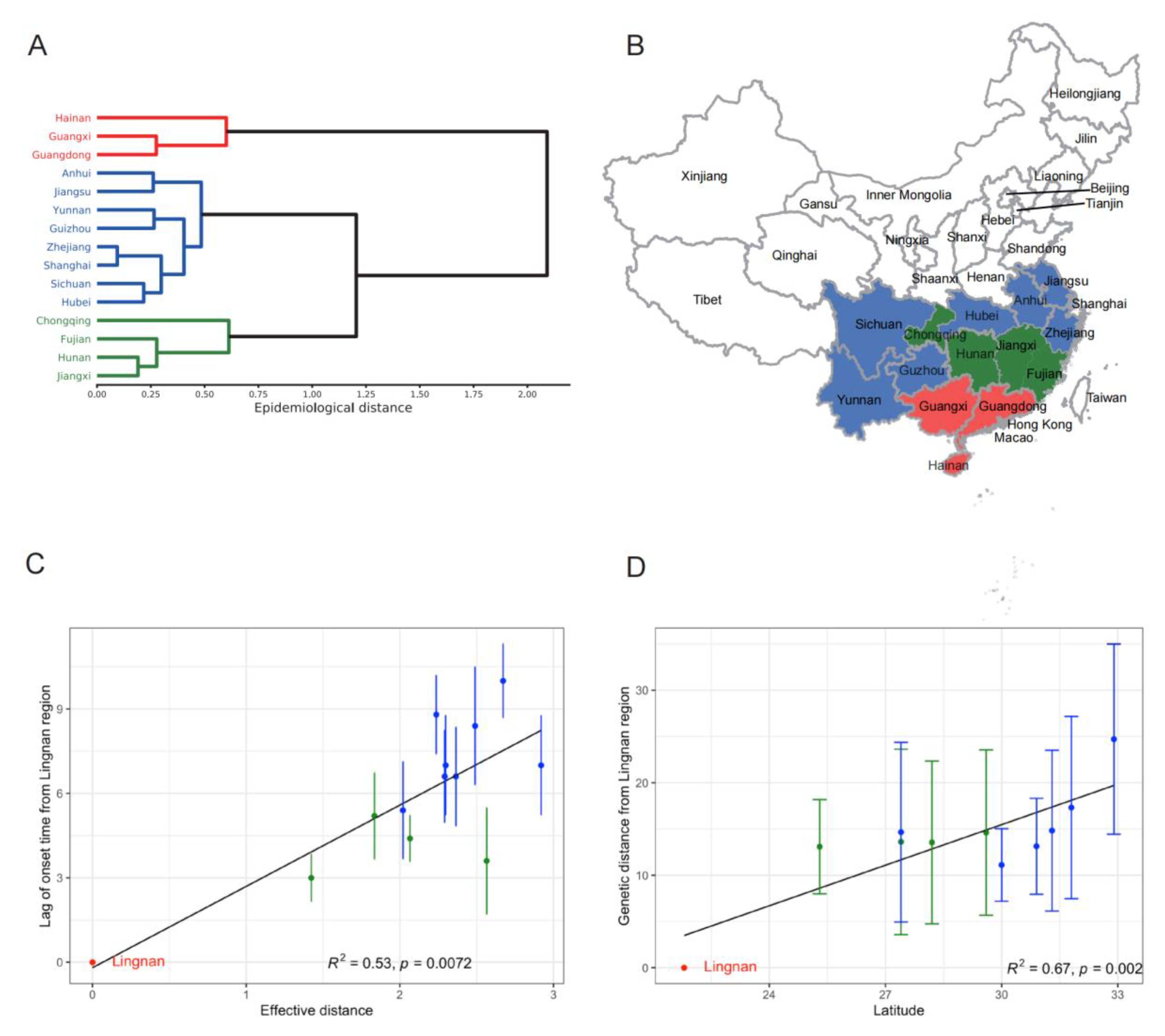
2.6. Clustering Analysis
2.7. Effective Distance
2.8. Statistical and Regression Analysis
3. Results
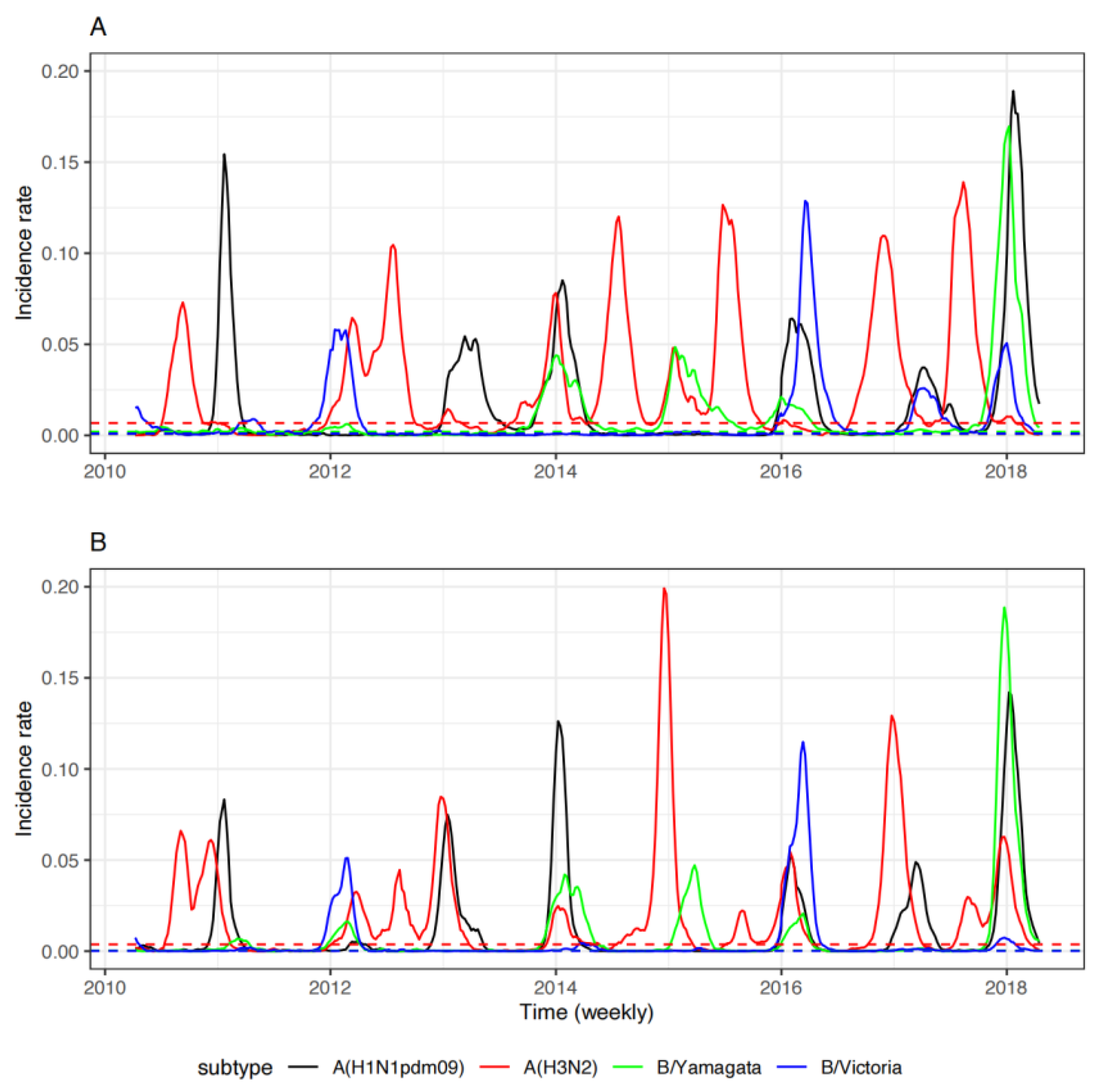
3.1. Surveillance of Seasonal Influenza in China between 2010 and 2018
3.2. Epidemiology of Seasonal Influenza in China
3.3. Spatial Transmission of Seasonal Influenza in China
3.4. Initiating Area and Drivers for Transmission of Influenza A(H3N2) in the Summer
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Liu, Y.; Wu, P.; Peng, Z.; Wang, X.; Chen, T.; Wong, J.Y.T.; Yang, J.; Bond, H.S.; Wang, L.; et al. Influenza-Associated Excess Respiratory Mortality in China, 2010-15: A Population-Based Study. Lancet Public Health 2019, 4, e473–e481. [Google Scholar] [CrossRef]
- Russell, C.A.; Jones, T.C.; Barr, I.G.; Cox, N.J.; Garten, R.J.; Gregory, V.; Gust, I.D.; Hampson, A.W.; Hay, A.J.; Hurt, A.C.; et al. The Global Circulation of Seasonal Influenza A (H3N2) Viruses. Science 2008, 320, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Pybus, O.G.; Nelson, M.I.; Viboud, C.; Taubenberger, J.K.; Holmes, E.C. The Genomic and Epidemiological Dynamics of Human Influenza A Virus. Nature 2008, 453, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Dong, L.; Lan, Y.; Peng, Y.; Wu, A.; Zhang, Y.; Huang, W.; Wang, D.; Wang, M.; Guo, Y.; et al. Mapping of H3N2 Influenza Antigenic Evolution in China Reveals a Strategy for Vaccine Strain Recommendation. Nat. Commun. 2012, 3, 709. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.-L.; Fang, L.-Q.; de Vlas, S.J.; Gao, Y.; Richardus, J.H.; Cao, W.-C. Dual Seasonal Patterns for Influenza, China. Emerg. Infect Dis. 2010, 16, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Alonso, W.J.; Feng, L.; Tan, Y.; Shu, Y.; Yang, W.; Viboud, C. Characterization of Regional Influenza Seasonality Patterns in China and Implications for Vaccination Strategies: Spatio-Temporal Modeling of Surveillance Data. PLoS Med 2013, 10, e1001552. [Google Scholar] [CrossRef] [PubMed]
- Alonso, W.J.; Viboud, C.; Simonsen, L.; Hirano, E.W.; Daufenbach, L.Z.; Miller, M.A. Seasonality of Influenza in Brazil: A Traveling Wave from the Amazon to the Subtropics. Am. J. Epidemiol 2007, 165, 1434–1442. [Google Scholar] [CrossRef]
- Tamerius, J.D.; Shaman, J.; Alonso, W.J.; Alonso, W.J.; Bloom-Feshbach, K.; Uejio, C.K.; Comrie, A.; Viboud, C. Environmental Predictors of Seasonal Influenza Epidemics across Temperate and Tropical Climates. PLoS Pathog. 2013, 9, e1003194. [Google Scholar] [CrossRef]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza Virus Transmission Is Dependent on Relative Humidity and Temperature. PLoS Pathog. 2007, 3, 1470–1476. [Google Scholar] [CrossRef]
- Shaman, J.; Pitzer, V.E.; Viboud, C.; Grenfell, B.T.; Lipsitch, M. Absolute Humidity and the Seasonal Onset of Influenza in the Continental United States. PLoS Biol. 2010, 8, e1000316. [Google Scholar] [CrossRef]
- Gog, J.R.; Ballesteros, S.; Viboud, C.; Simonsen, L.; Bjornstad, O.N.; Shaman, J.; Chao, D.L.; Khan, F.; Grenfell, B.T. Spatial Transmission of 2009 Pandemic Influenza in the US. PLoS Comput. Biol. 2014, 10, e1003635. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, D.; Helbing, D. The Hidden Geometry of Complex, Network-Driven Contagion Phenomena. Science 2013, 342, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Arino, J.; Hu, W.; Raposo, P.; Sears, J.; Calderon, F.; Heidebrecht, C.; Macdonald, M.; Liauw, J.; Chan, A.; et al. Spread of a Novel Influenza A (H1N1) Virus via Global Airline Transportation. N. Engl. J. Med. 2009, 361, 212–214. [Google Scholar] [CrossRef]
- Pei, S.; Kandula, S.; Yang, W.; Shaman, J. Forecasting the Spatial Transmission of Influenza in the United States. Proc. Natl. Acad. Sci. USA 2018, 115, 2752–2757. [Google Scholar] [CrossRef]
- Charu, V.; Zeger, S.; Gog, J.; Bjørnstad, O.N.; Kissler, S.; Simonsen, L.; Grenfell, B.T.; Viboud, C. Human Mobility and the Spatial Transmission of Influenza in the United States. PLoS Comput. Biol. 2017, 13, e1005382. [Google Scholar] [CrossRef]
- Brownstein, J.S.; Wolfe, C.J.; Mandl, K.D. Empirical Evidence for the Effect of Airline Travel on Inter-Regional Influenza Spread in the United States. PLoS Med. 2006, 3, e401. [Google Scholar] [CrossRef] [PubMed]
- Viboud, C.; Bjørnstad, O.N.; Smith, D.L.; Simonsen, L.; Miller, M.A.; Grenfell, B.T. Synchrony, Waves, and Spatial Hierarchies in the Spread of Influenza. Science 2006, 312, 447–451. [Google Scholar] [CrossRef]
- Feng, L.; Shay, D.K.; Jiang, Y.; Zhou, H.; Chen, X.; Zheng, Y.; Jiang, L.; Zhang, Q.; Lin, H.; Wang, S.; et al. Influenza-Associated Mortality in Temperate and Subtropical Chinese Cities, 2003–2008. Bull World Health Organ 2012, 90, 279–288B. [Google Scholar] [CrossRef]
- Liu, X.-X.; Li, Y.; Zhu, Y.; Zhang, J.; Li, X.; Zhang, J.; Zhao, K.; Hu, M.; Qin, G.; Wang, X.-L. Seasonal Pattern of Influenza Activity in a Subtropical City, China, 2010-2015. Sci. Rep. 2017, 7, 17534. [Google Scholar] [CrossRef]
- Shu, Y.; Song, Y.; Wang, D.; Greene, C.M.; Moen, A.; Lee, C.K.; Chen, Y.; Xu, X.; McFarland, J.; Xin, L.; et al. A Ten-Year China-US Laboratory Collaboration: Improving Response to Influenza Threats in China and the World, 2004-2014. BMC Public Health 2019, 19, 520. [Google Scholar] [CrossRef]
- U.S. Influenza Surveillance: Purpose and Methods | CDC. Available online: https://www.cdc.gov/flu/weekly/overview.htm (accessed on 16 September 2020).
- China Statistical Yearbook. 2018. Available online: http://www.stats.gov.cn/tjsj/ndsj/2018/indexeh.htm. (accessed on 16 September 2022).
- China Transport. Statistical Yearbook 2016; Ministry of Transport of the People’s Republic of China; China Communication Press: Beijing, China, 2016.
- Goldstein, E.; Cobey, S.; Takahashi, S.; Miller, J.C.; Lipsitch, M. Predicting the Epidemic Sizes of Influenza A/H1N1, A/H3N2, and B: A Statistical Method. PLoS Med. 2011, 8, e1001051. [Google Scholar] [CrossRef] [PubMed]
- Shaman, J.; Karspeck, A.; Yang, W.; Tamerius, J.; Lipsitch, M. Real-Time Influenza Forecasts during the 2012-2013 Season. Nat. Commun. 2013, 4, 2837. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cowling, B.J.; Lau, E.H.Y.; Shaman, J. Forecasting Influenza Epidemics in Hong Kong. PLoS Comput. Biol. 2015, 11, e1004383. [Google Scholar] [CrossRef]
- Chan, J.; Holmes, A.; Rabadan, R. Network Analysis of Global Influenza Spread. PLoS Comput. Biol. 2010, 6, e1001005. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. Null 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Lu, F.S.; Hattab, M.W.; Clemente, C.L.; Biggerstaff, M.; Santillana, M. Improved State-Level Influenza Nowcasting in the United States Leveraging Internet-Based Data and Network Approaches. Nat. Commun. 2019, 10, 147. [Google Scholar] [CrossRef]
- Szekely, G.J.; Rizzo, M.L. Hierarchical Clustering via Joint Between-Within Distances: Extending Ward’s Minimum Variance Method. J. Classif. 2005, 22, 151–183. [Google Scholar] [CrossRef]
- Smith, D.J.; Lapedes, A.S.; de Jong, J.C.; Bestebroer, T.M.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Mapping the Antigenic and Genetic Evolution of Influenza Virus. Science 2004, 305, 371–376. [Google Scholar] [CrossRef]
- Wen, F.; Bedford, T.; Cobey, S. Explaining the Geographical Origins of Seasonal Influenza A (H3N2). Proc. Biol. Sci. 2016, 283, 1838–20161312. [Google Scholar] [CrossRef]
- Aris-Brosou, S. Inferring Influenza Global Transmission Networks without Complete Phylogenetic Information. Evol. Appl. 2014, 7, 403–412. [Google Scholar] [CrossRef]
- Shaman, J.; Kohn, M. Absolute Humidity Modulates Influenza Survival, Transmission, and Seasonality. Proc. Natl. Acad. Sci. USA 2009, 106, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Cao, Z.; Zou, M.; Tang, K.; Zhang, C.; Tang, J.; Zeng, J.; Wang, Y.; Sun, Q.; Wang, D.; et al. The Effectiveness of Governmental Nonpharmaceutical Interventions against COVID-19 at Controlling Seasonal Influenza Transmission: An Ecological Study. BMC Infect. Dis. 2022, 22, 331. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Xu, M.; Wang, X.; Xie, Y.; Du, X.; Chen, T.; Yang, L.; Wang, D.; Shu, Y. Nonpharmaceutical Interventions Used to Control COVID-19 Reduced Seasonal Influenza Transmission in China. J. Infect Dis. 2020, 222, 1780–1783. [Google Scholar] [CrossRef] [PubMed]

| Province a | Cities (Hospitals) b | Population Size c (M) | Latitude | Longitude | Mean Weekly Temperature in Summer (°C) | Mean Weekly Relative Humidity in Summer (%) | Mean Weekly Temperature in Winter (°C) | Mean Weekly Relative Humidity in Winter (%) | Mean Weekly Specimens Tested | Mean Weekly Influenza Positive |
|---|---|---|---|---|---|---|---|---|---|---|
| Hainan | 5 (6) | 4.89 | 19.6 | 110.1 | 27.8 | 81.7 | 22.0 | 82.6 | 109 | 12 |
| Guangxi | 14 (17) | 48.12 | 22.9 | 108.4 | 26.5 | 79.1 | 16.6 | 76.2 | 318 | 52 |
| Guangdong | 20 (27) | 89.20 | 22.9 | 113.4 | 26.7 | 81.1 | 17.5 | 75.0 | 563 | 90 |
| Yunnan | 14 (17) | 44.57 | 24.8 | 103.0 | 21.1 | 73.7 | 13.5 | 68.5 | 305 | 29 |
| Fujian | 9 (15) | 39.11 | 25.3 | 118.8 | 24.7 | 79.7 | 14.1 | 76.9 | 321 | 56 |
| Guizhou | 8 (13) | 32.27 | 27.4 | 106.8 | 21.7 | 78.5 | 10.3 | 79.6 | 220 | 32 |
| Hunan | 14 (23) | 45.55 | 27.4 | 113.0 | 24.2 | 77.23 | 10.9 | 76.6 | 375 | 46 |
| Jiangxi | 11 (15) | 46.22 | 28.2 | 115.3 | 24.9 | 78.7 | 11.8 | 77.4 | 242 | 40 |
| Chongqing | 1 (7) | 33.90 | 29.6 | 106.6 | 24.1 | 75.7 | 11.7 | 78.9 | 113 | 22 |
| Zhejiang | 12 (16) | 50.37 | 30.0 | 120.4 | 24.2 | 77.8 | 11.3 | 74.1 | 302 | 61 |
| Sichuang | 21 (31) | 83.21 | 30.2 | 104.0 | 18.0 | 70.5 | 7.0 | 62.9 | 347 | 50 |
| Hubei | 13 (18) | 57.17 | 30.9 | 112.6 | 23.7 | 76.9 | 9.7 | 73.7 | 314 | 50 |
| Shanghai | 1 (19) | 24.18 | 31.3 | 121.5 | 24.2 | 72.2 | 10.5 | 69.5 | 317 | 82 |
| Anhui | 17 (25) | 64.16 | 31.8 | 117.5 | 23.4 | 76. 7 | 8.6 | 71.8 | 371 | 61 |
| Jiangsu | 13 (29) | 80.29 | 32.9 | 118.6 | 23.3 | 75.7 | 8.2 | 70.4 | 543 | 73 |
| Shaanxi | 10 (18) | 39.76 | 34.3 | 112.8 | 20.1 | 68.1 | 4.4 | 62.6 | 211 | 32 |
| Henan | 18 (22) | 95.59 | 34.7 | 113.1 | 23.2 | 69.1 | 7.3 | 61.7 | 237 | 39 |
| Gansu | 14 (19) | 23.26 | 35.6 | 104.7 | 17.0 | 53.5 | −0.3 | 52.1 | 204 | 31 |
| Shandong | 17 (27) | 100.05 | 36.3 | 118.4 | 21.6 | 70.3 | 5.1 | 61.2 | 318 | 46 |
| Qinghai | 9 (14) | 5.87 | 36.6 | 101.8 | 10.2 | 55.0 | −4.7 | 42.6 | 126 | 13 |
| Ningxia | 5 (9) | 6.82 | 37.6 | 106.0 | 17.8 | 54.2 | 0.1 | 52.0 | 115 | 14 |
| Shanxi | 11 (17) | 37.02 | 37.8 | 112.8 | 19.2 | 59.9 | 1.2 | 53.5 | 183 | 31 |
| Hebei | 10 (24) | 70.73 | 38.1 | 115.8 | 20.7 | 62.1 | 1.2 | 54.6 | 258 | 37 |
| Tianjin | 1 (10) | 15.57 | 39.2 | 117.2 | 22.6 | 62.4 | 3.5 | 54.4 | 109 | 22 |
| Beijing | 1 (11) | 21.71 | 39.9 | 116.4 | 21.5 | 59.7 | 1.8 | 49.9 | 215 | 37 |
| Liaoning | 14 (21) | 41.97 | 40.7 | 122.6 | 19.3 | 68.4 | −1.4 | 58.5 | 255 | 23 |
| Neimenggu | 12 (19) | 25.28 | 40.8 | 110.8 | 16.8 | 49.1 | −7.2 | 53.1 | 161 | 19 |
| Xinjiang | 13 (16) | 20.57 | 43.8 | 87.6 | 19.4 | 43.1 | −2.2 | 59.1 | 208 | 26 |
| Jilin | 9 (13) | 26.16 | 44.1 | 125.4 | 17.2 | 67.4 | −6.4 | 62.9 | 147 | 18 |
| Heilongjiang | 13 (20) | 35.85 | 46.1 | 126.2 | 16.0 | 68.8 | −10.7 | 66.0 | 225 | 24 |
| R2 (p Value) | ||||
|---|---|---|---|---|
| Summer Season | Winter Season | |||
| Latitude | Longitude | Latitude | Longitude | |
| A(H3N2) | 10−3) | 10−1) | 10−2) | 10−2) |
| A(H1N1)pdm09 | NA | NA | 10−1) | 10−1) |
| B/Yamagata | NA | NA | 10−6) | 10−1) |
| B/Victoria | NA | NA | 10−1) | 10−1) |
| Predictors | Spatial Scale | Temporal Scale | ||
|---|---|---|---|---|
| Coefficients (Standard Error) | p Value | Coefficients (Standard Error) | p Value | |
| Normalized temperature | 20.1 (3.3) | 10−7 | 7.2 (5.4) | 10−1 |
| Normalized relative humidity | 13.6 (2.9) | 10−5 | 10.1 (4.7) | 10−2 |
| Normalized transport volume | −0.4 (1.9) | 10−1 | −7.4 (3.0) | 10−2 |
| Interaction term | −0.5 (1.6) | 10−1 | −14.5 (7.9) | 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, H.; Yang, L.; Wang, G.; Zhang, C.; Xin, Y.; Sun, Q.; Zhang, B.; Chen, T.; Yang, J.; Huang, W.; et al. Transmission Patterns of Seasonal Influenza in China between 2010 and 2018. Viruses 2022, 14, 2063. https://doi.org/10.3390/v14092063
Lei H, Yang L, Wang G, Zhang C, Xin Y, Sun Q, Zhang B, Chen T, Yang J, Huang W, et al. Transmission Patterns of Seasonal Influenza in China between 2010 and 2018. Viruses. 2022; 14(9):2063. https://doi.org/10.3390/v14092063
Chicago/Turabian StyleLei, Hao, Lei Yang, Gang Wang, Chi Zhang, Yuting Xin, Qianru Sun, Bing Zhang, Tao Chen, Jing Yang, Weijuan Huang, and et al. 2022. "Transmission Patterns of Seasonal Influenza in China between 2010 and 2018" Viruses 14, no. 9: 2063. https://doi.org/10.3390/v14092063
APA StyleLei, H., Yang, L., Wang, G., Zhang, C., Xin, Y., Sun, Q., Zhang, B., Chen, T., Yang, J., Huang, W., Xu, M., Xie, Y., Wang, Y., Xu, P., Sun, L., Guo, D., Du, X., Wang, D., & Shu, Y. (2022). Transmission Patterns of Seasonal Influenza in China between 2010 and 2018. Viruses, 14(9), 2063. https://doi.org/10.3390/v14092063






