Management of Chronic Hepatitis B in HIV-Coinfected Patients
Abstract
:1. Introduction
2. Prevalence of HIV/HBV Coinfection
3. Management of HIV/HBV Coinfected Patients
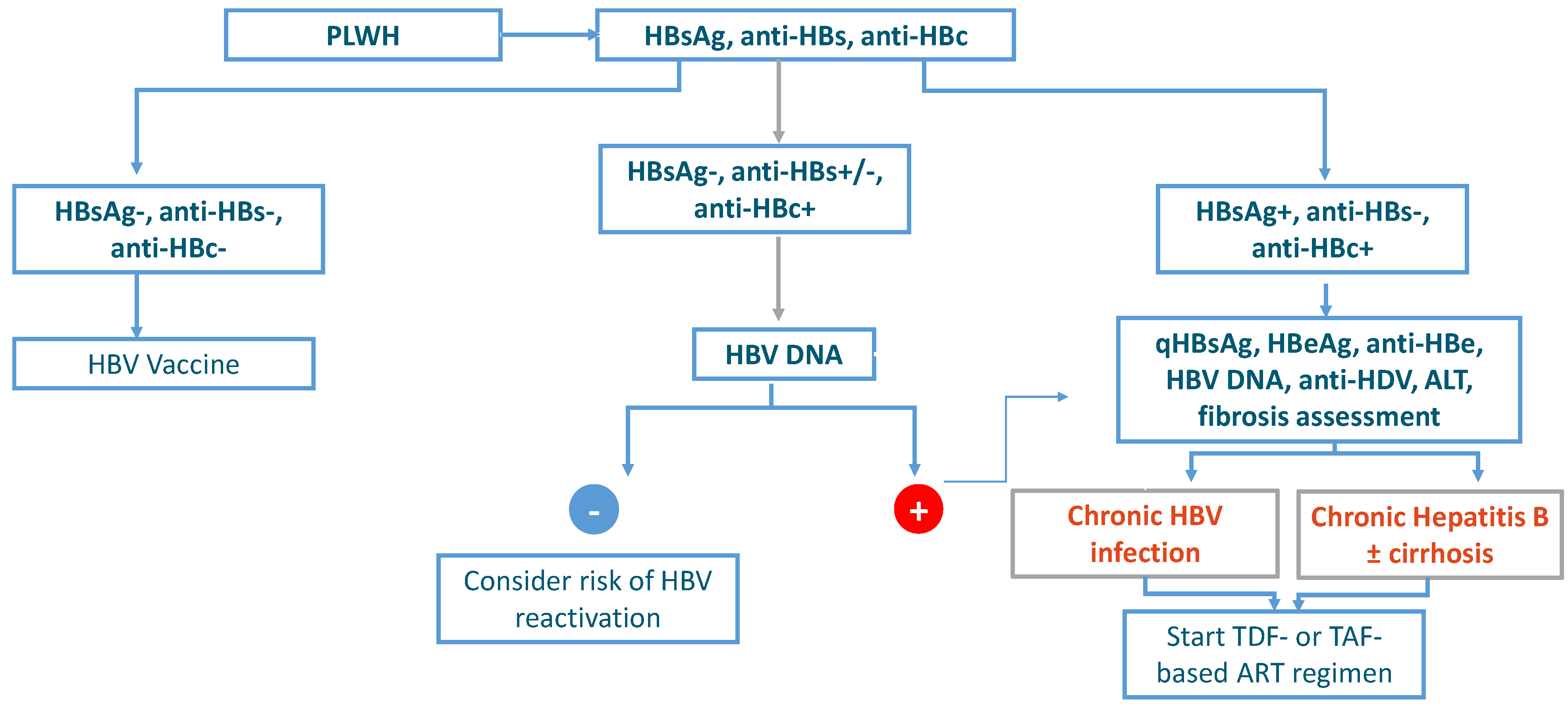
- HBsAg-negative, anti-HBs-negative, and anti-HBc-negative: PLWH without HBV infection.
- HBsAg-negative, anti-HBs-negative/positive, and anti-HBc-positive: PLWH with a past HBV infection.
- HBsAg-positive, anti-HBs-negative, anti-HBc-positive: PLWH with ongoing HBV infection.
4. HBV Seronegative Patients
5. Subjects with a Past HBV Infection
6. HBsAg Positive PLWH
7. Current Treatment Strategies
8. Monitoring of the Response to Therapy and Adverse Events
9. Efficacy and Safety Data from Real-Life Clinical Practice
10. Future Treatment Options
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Hepatitis Report, 2017; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. In Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Shahriar, S.; Araf, Y.; Ahmad, R.; Kattel, P.; Sah, G.S.; Rahaman, T.I.; Hossain, M.G. Insights Into the Coinfections of Human Immunodeficiency Virus-Hepatitis B Virus, Human Immunodeficiency Virus-Hepatitis C Virus, and Hepatitis B Virus-Hepatitis C Virus: Prevalence, Risk Factors, Pathogenesis, Diagnosis, and Treatment. Front. Microbiol. 2022, 12, 780887. [Google Scholar] [CrossRef] [PubMed]
- Weldemhret, L. Epidemiology and challenges of HBV/HIV coinfection among HIV-infected patients in endemic areas: Review. HIV/AIDS Res. Palliat. Care 2021, 13, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Colin, J.F.; Cazals-Hatem, D.; Loriot, M.A.; Martinot-Peignoux, M.; Pham, B.N.; Auperin, A.; Marcellin, P. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology 1999, 29, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Thio, C.L.; Seaberg, E.C.; Skolasky, R., Jr.; Phair, J.; Visscher, B.; Muñoz, A.; Thomas, D.L. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002, 360, 1921–1926. [Google Scholar] [CrossRef]
- Puoti, M.; Torti, C.; Bruno, R.; Filice, G.; Carosi, G. Natural history of chronic hepatitis B in co-infected patients. J. Hepatol. 2006, 44 (Suppl. 1), S65–S70. [Google Scholar] [CrossRef]
- Hoffmann, C.J.; Seaberg, E.C.; Young, S.; Witt, M.D.; D’Acunto, K.; Phair, J.; Thio, C.L. Hepatitis B and long-term HIV outcomes in coinfected HAART recipients. AIDS 2009, 23, 1881–1889. [Google Scholar] [CrossRef]
- Kim, H.N. Chronic Hepatitis B and HIV Coinfection: A Continuing Challenge in the Era of Antiretroviral Therapy. Curr. Hepatol. Rep. 2020, 19, 345–353. [Google Scholar] [CrossRef]
- Platt, L.; French, C.E.; McGowan, C.R.; Sabin, K.; Gower, E.; Trickey, A.; McDonald, B.; Ong, J.; Stone, J.; Easterbrook, P.; et al. Prevalence and burden of HBV co-infection among people living with HIV: A global systematic review and meta-analysis. J. Viral Hepat. 2019, 27, 294–315. [Google Scholar] [CrossRef]
- Report Icona Foundation Cohort–Update December 2021. Available online: www.fondazioneicona.org (accessed on 31 October 2021).
- EACS Guidelines Version 11 October 2021. Available online: www.eacsociety.org (accessed on 31 October 2021).
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf (accessed on 16 August 2021).
- EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol 2017, 67, 370–398. [CrossRef]
- Terrault, N.A.; Lok, A.S.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Wong, J.B. Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Tayal, S.C.; Sankar, K.N. Impaired response to recombinant hepatitis B vaccine in asymptomatic HIV-infected individuals. AIDS 1994, 8, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Nafziger, A.N.; Harro, C.D.; Keyserling, H.L.; Ramsey, K.M.; Drusano, G.L.; Bertino, J.S., Jr. Revaccination of healthy nonresponders with hepatitis B vaccine and prediction of seroprotection response. Vaccine 2003, 21, 1174–1179. [Google Scholar] [CrossRef]
- Launay, O.; van der Vliet, D.; Rosenberg, A.R.; Michel, M.L.; Piroth, L.; Rey, D.; Carrat, F. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs. standard hepatitis B vaccine regimen in adults with HIV-1: A randomized controlled trial. JAMA 2011, 305, 1432–1440. [Google Scholar] [CrossRef]
- Mena, G.; García-Basteiro, A.; Bayas, J. Hepatitis B and A vaccination in HIV-infected adults: A review. Hum. Vaccines Immunother. 2015, 11, 2582–2598. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, G.; Locarnini, S.; Pollicino, T.; Levrero, M.; Zoulim, F.; Lok, A.S.; The Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J. Hepatol. 2019, 71, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Rouphaela, N.G.; Talatia, N.J.; Rimland, D. Hepatitis B reverse seroconversion in HIV-positive patients: Case series and review of the literature. AIDS 2007, 21, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Salpini, R.; D’Anna, S.; Alkhatib, M.; Piermatteo, L.; Tavelli, A.; Quiros, E.; Cingolani, A.; Papalini, C.; Carrara, S.; Malagnino, V.; et al. Reactivation of hepatitis B virus is a frequent event in anti-HBc positive/HBsAg-negative HIV-infected patients switching to Tenofovir sparing therapy as revealed by highly sensitive HBV assays. In Proceedings of the 14th Congress National—ICAR, Montreal, QC, Canada, 30 May–3 June 2022. [Google Scholar]
- Perrillo, R.P.; Gish, R.; Falck-Ytter, Y.T. American Gastroenterological Association Institute Technical Review on prevention and treatment of hepatitis B reactivation during immunosuppressive drug therapy. Gastroenterology 2015, 148, 221. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.S.; Miller, R.H.; Marion, P.L. Hepadnaviruses and retroviruses share genome homology and features of replication. Hepatology 1987, 7, 64S–73S. [Google Scholar] [CrossRef]
- Papatheodoridis, G.V.; Sypsa, V.; Dalekos, G.; Yurdaydin, C.; van Boemmel, F.; Buti, M.; Goulis, J.; Calleja, J.L.; Chi, H.; Manolakopoulos, S.; et al. Eight-year survival in chronic hepatitis B patients under long-term entecavir or tenofovir therapy is similar to the general population. J. Hepatol. 2018, 68, 1129–1136. [Google Scholar] [CrossRef]
- Lampertico, P.; Invernizzi, F.; Viganò, M.; Loglio, A.; Mangia, G.; Facchetti, F.; Colombo, M. The long-term benefits of nucleos(t)ide analogs on esophageal varices in compensated HBV cirrhotics with no or small esophageal varices: A 12-year prospective cohort study. J. Hepatol. 2015, 63, 1118–1125. [Google Scholar] [CrossRef]
- Papatheodoridis, G.V.; Lampertico, P.; Manolakopoulos, S.; Lok, A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: A systematic review. J. Hepatol. 2010, 53, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Arends, P.; Sonneveld, M.J.; Zoutendijk, R.; Carey, I.; Brown, A.; Fasano, M.; Janssen, H.L. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: Limited role for risk scores in Caucasians. Gut 2014, 64, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Idilman, R.; Dalekos, G.N.; Buti, M.; Chi, H.; Van Boemmel, F.; Calleja-Panero, J.L.; Sypsa, V.; Goulis, J.; Manolakopoulos, S.; et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology 2017, 66, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Bottero, J.; Miailhes, P.; Lascoux-Combe, C.; Rougier, H.; Girard, P.M.; Lacombe, K. Liver fibrosis regression and progression during controlled hepatitis B virus infection among HIV-HBV patients treated with tenofovir disoproxil fumarate in France: A prospective cohort study. J. Int. AIDS Soc. 2017, 20, 21426. [Google Scholar] [CrossRef]
- Re, V.L., III; Newcomb, C.W.; Carbonari, D.M.; Roy, J.A.; Althoff, K.N.; Kitahata, M.M.; Kim, H.N. Determinants of Liver Complications Among HIV/Hepatitis B Virus-Coinfected Patients. J. Acquir. Immune Defic. Syndr. 2019, 82, 71–80. [Google Scholar]
- Mallet, V.; Hamed, K.; Schwarzinger, M. Prognosis of patients with chronic hepatitis B in France (2008–2013): A nationwide, observational and hospital-based study. J. Hepatol. 2017, 66, 514–520. [Google Scholar] [CrossRef]
- Calcagno, A.; Alessandro, C.; Saracino, A.; De Luca, A.; Colella, E.; Cingolani, A.; Monforte, A.A. Long-term Follow-up of HIV-HBV Coinfected Patients According to the Use of Anti-HBV Active Drugs. In Proceedings of the HIV/Drug Therapy, Glasgow, UK, 28–31 October 2018. [Google Scholar]
- Yip, T.C.F.; Wong, G.L.H.; Wong, V.W.S.; Tse, Y.K.; Lui, G.C.Y.; Lam, K.L.Y.; Chan, H.L.Y. Durability of hepatitis B surface antigen seroclearance in untreated and nucleos(t)ide analogue-treated patients. J. Hepatol. 2018, 68, 63–72. [Google Scholar] [CrossRef]
- Chihota, B.V.; Wandeler, G.; Chilengi, R.; Mulenga, L.; Chung, R.T.; Bhattacharya, D.; Vinikoor, M.J. High rates of hepatitis B virus (HBV) functional cure among human immunodeficiency virus-HBV coinfected patients on antiretroviral therapy in Zambia. J. Infect Dis. 2020, 221, 218–222. [Google Scholar] [CrossRef]
- Zoutendijk, R.; Zaaijer, H.L.; De Vries-Sluijs, T.E.M.S.; Reijnders, J.G.P.; Mulder, J.W.; Kroon, F.P.; Richter, C.; Van Der Eijk, A.A.; Sonneveld, M.J.; Hansen, B.; et al. Hepatitis B Surface Antigen Declines and Clearance During Long-Term Tenofovir Therapy in Patients Coinfected With HBV and HIV. J. Infect. Dis. 2012, 206, 974–980. [Google Scholar] [CrossRef]
- Kim, G.A.; Lim, Y.S.; An, J.; Lee, D.; Shim, J.H.; Kim, K.M.; Suh, D.J. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepa-titis B: Clinical outcomes and durability. Gut 2014, 63, 1325–1332. [Google Scholar] [CrossRef]
- Fanning, G.C.; Zoulim, F.; Hou, J.; Bertoletti, A. Therapeutic strategies for hepatitis B virus infection: Towards a cure. Nat. Rev. Drug Discov. 2019, 18, 827–844. [Google Scholar] [CrossRef] [PubMed]
| Regimen | Drugs | Duration | Route of Administration | |
|---|---|---|---|---|
| HBV monoinfection | Monotherapy |
| Long-term until HBsAg loss | Oral |
| Monotherapy |
| 48 weeks | Subcutaneous injection | |
| HIV/HBV coinfection | Combination regimen |
| Indefinitely | Oral |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasano, M.; Poliseno, M.C.; Fiore, J.R.; Lo Caputo, S.; D’Arminio Monforte, A.; Santantonio, T.A. Management of Chronic Hepatitis B in HIV-Coinfected Patients. Viruses 2022, 14, 2022. https://doi.org/10.3390/v14092022
Fasano M, Poliseno MC, Fiore JR, Lo Caputo S, D’Arminio Monforte A, Santantonio TA. Management of Chronic Hepatitis B in HIV-Coinfected Patients. Viruses. 2022; 14(9):2022. https://doi.org/10.3390/v14092022
Chicago/Turabian StyleFasano, Massimo, Maria Cristina Poliseno, Josè Ramon Fiore, Sergio Lo Caputo, Antonella D’Arminio Monforte, and Teresa Antonia Santantonio. 2022. "Management of Chronic Hepatitis B in HIV-Coinfected Patients" Viruses 14, no. 9: 2022. https://doi.org/10.3390/v14092022
APA StyleFasano, M., Poliseno, M. C., Fiore, J. R., Lo Caputo, S., D’Arminio Monforte, A., & Santantonio, T. A. (2022). Management of Chronic Hepatitis B in HIV-Coinfected Patients. Viruses, 14(9), 2022. https://doi.org/10.3390/v14092022







