The ORF45 Protein of Kaposi’s Sarcoma-Associated Herpesvirus and Its Critical Role in the Viral Life Cycle
Abstract
1. Introduction
2. Expression of ORF45 as an Immediate-Early Gene
3. ORF45 Structure and Localization
4. ORF45 as a Component of the Tegument of KSHV
5. ORF45 Interactions with Viral Proteins
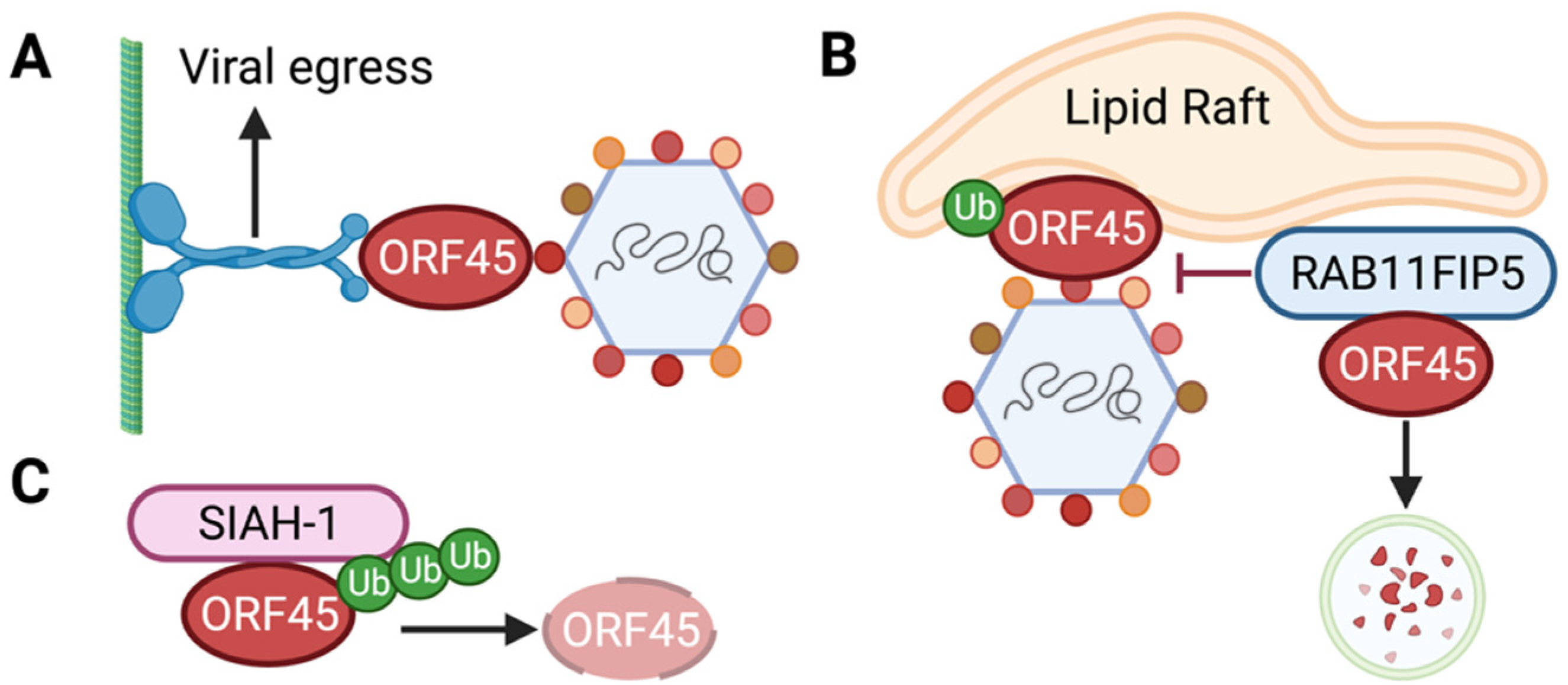
6. Role of ORF45 in the Viral Life Cycle
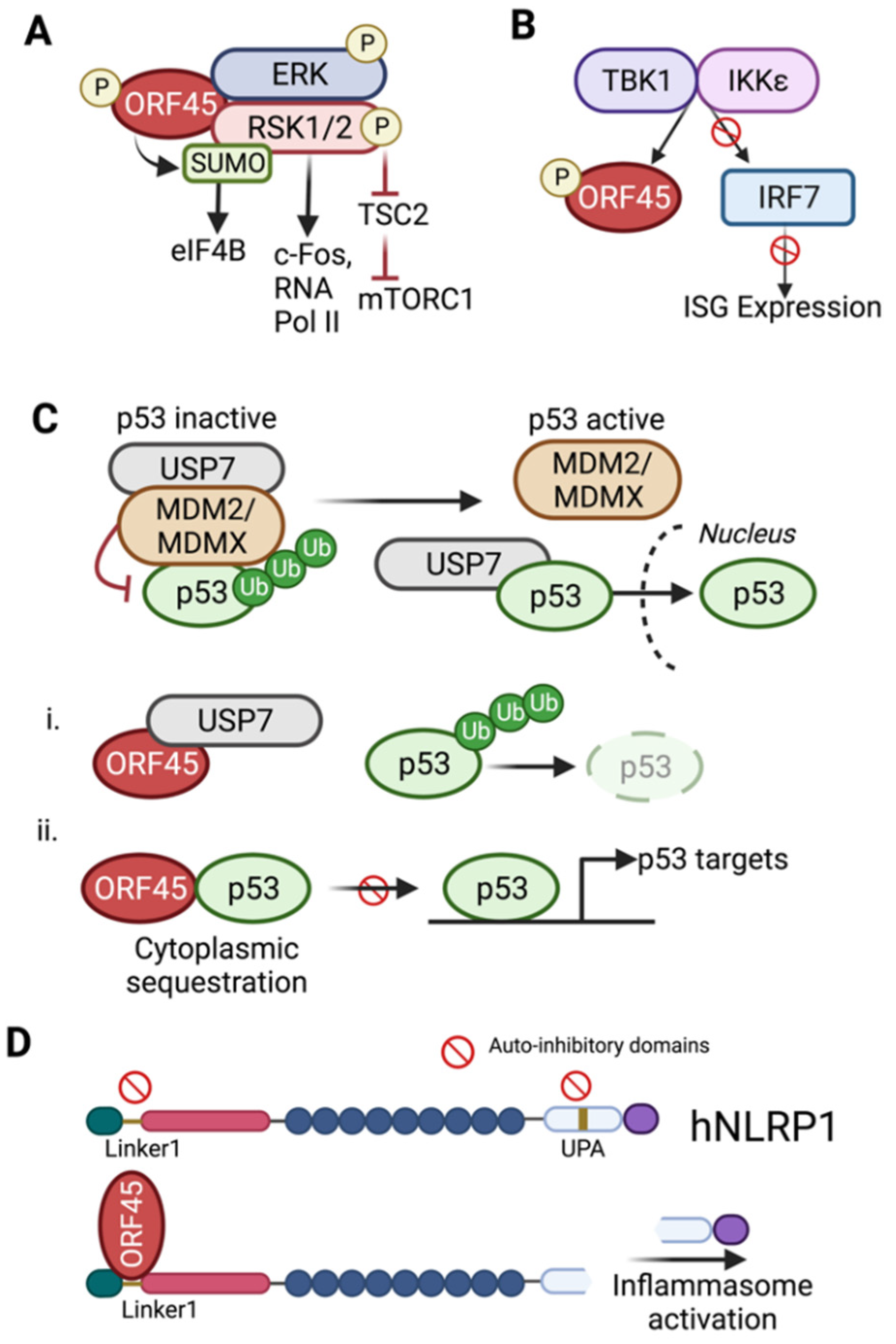
7. ORF45-Mediated Sustained Activation of RSK
8. Regulation of Cellular p53 Signaling
9. Evasion of Host Defenses
10. ORF45 Homologs
10.1. MHV68 ORF45
10.2. RRV ORF45
10.3. EBV BKRF4
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef]
- Cesarman, E.; Chang, Y.; Moore, P.S.; Said, J.W.; Knowles, D.M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 1995, 332, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Soulier, J.; Grollet, L.; Oksenhendler, E.; Cacoub, P.; Cazals-Hatem, D.; Babinet, P.; d’Agay, M.F.; Clauvel, J.P.; Raphael, M.; Degos, L. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 1995, 86, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Bechtel, J.T.; Liang, Y.; Hvidding, J.; Ganem, D. Host range of Kaposi’s sarcoma-associated herpesvirus in cultured cells. J. Virol. 2003, 77, 6474–6481. [Google Scholar] [CrossRef] [PubMed]
- Myoung, J.; Ganem, D. Infection of lymphoblastoid cell lines by Kaposi’s sarcoma-associated herpesvirus: Critical role of cell-associated virus. J. Virol. 2011, 85, 9767–9777. [Google Scholar] [CrossRef] [PubMed]
- Myoung, J.; Ganem, D. Active lytic infection of human primary tonsillar B cells by KSHV and its noncytolytic control by activated CD4+ T cells. J. Clin. Investig. 2011, 121, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Duus, K.M.; Lentchitsky, V.; Wagenaar, T.; Grose, C.; Webster-Cyriaque, J. Wild-type Kaposi’s sarcoma-associated herpesvirus isolated from the oropharynx of immune-competent individuals has tropism for cultured oral epithelial cells. J. Virol. 2004, 78, 4074–4084. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Ganem, D. A Unique Herpesviral Transcriptional Program in KSHV-Infected Lymphatic Endothelial Cells Leads to mTORC1 Activation and Rapamycin Sensitivity. Cell Host Microbe 2013, 13, 429–440. [Google Scholar] [CrossRef]
- Golas, G.; Alonso, J.D.; Toth, Z. Characterization of de novo lytic infection of dermal lymphatic microvascular endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Virology 2019, 536, 27–31. [Google Scholar] [CrossRef]
- Grundhoff, A.; Ganem, D. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Investig. 2004, 113, 124–136. [Google Scholar] [CrossRef]
- Lee, H.R.; Lee, S.; Chaudhary, P.M.; Gill, P.; Jung, J.U. Immune evasion by Kaposi’s sarcoma-associated herpesvirus. Future Microbiol. 2010, 5, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Coscoy, L. Immune evasion by Kaposi’s sarcoma-associated herpesvirus. Nat. Rev. Immunol. 2007, 7, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.X.; King, S.M.; Smith, E.J.; Levy, D.E.; Yuan, Y. A Kaposi’s sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. USA 2002, 99, 5573–5578. [Google Scholar] [CrossRef]
- Preston, C.M.; Rinaldi, A.; Nicholl, M.J. Herpes simplex virus type 1 immediate early gene expression is stimulated by inhibition of protein synthesis. J. Gen. Virol. 1998, 79, 117–124. [Google Scholar] [CrossRef]
- Lacoste, V.; de la Fuente, C.; Kashanchi, F.; Pumfery, A. Kaposi’s sarcoma-associated herpesvirus immediate early gene activity. Front. Biosci-Landmark 2004, 9, 2245–2272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, F.X.; Cusano, T.; Yuan, Y. Identification of the immediate-early transcripts of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 1999, 73, 5556–5567. [Google Scholar] [CrossRef]
- Arias, C.; Weisburd, B.; Stern-Ginossar, N.; Mercier, A.; Madrid, A.S.; Bellare, P.; Holdorf, M.; Weissman, J.S.; Ganem, D. KSHV 2.0: A comprehensive annotation of the Kaposi’s sarcoma-associated herpesvirus genome using next-generation sequencing reveals novel genomic and functional features. PLoS Pathog. 2014, 10, e1003847. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, P.; Thakker, S.; Verma, S.C. Transcriptome Analysis of Kaposi’s Sarcoma-Associated Herpesvirus during De Novo Primary Infection of Human B and Endothelial Cells. J. Virol. 2015, 89, 3093–3111. [Google Scholar] [CrossRef]
- Chang, P.J.; Wang, S.S.; Chen, L.Y.; Hung, C.H.; Huang, H.Y.; Shih, Y.J.; Yen, J.B.; Liou, J.Y.; Chen, L.W. ORF50-dependent and ORF50-independent activation of the ORF45 gene of Kaposi’s sarcoma-associated herpesvirus. Virology 2013, 442, 38–50. [Google Scholar] [CrossRef]
- Wang, S.S.; Chang, P.J.; Chen, L.W.; Chen, L.Y.; Hung, C.H.; Liou, J.Y.; Yen, J.B. Positive and negative regulation in the promoter of the ORF46 gene of Kaposi’s sarcoma-associated herpesvirus. Virus Res. 2012, 165, 157–169. [Google Scholar] [CrossRef]
- Russo, J.J.; Bohenzky, R.A.; Chien, M.C.; Chen, J.; Yan, M.; Maddalena, D.; Parry, J.P.; Peruzzi, D.; Edelman, I.S.; Chang, Y.; et al. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 1996, 93, 14862–14867. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.J.; Hung, C.H.; Wang, S.S.; Tsai, P.H.; Shih, Y.J.; Chen, L.Y.; Huang, H.Y.; Wei, L.H.; Yen, J.B.; Lin, C.L.; et al. Identification and Characterization of Two Novel Spliced Genes Located in the orf47-orf46-orf45 Gene Locus of Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2014, 88, 10092–10109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.J.; Zhu, F.X. Identification of the Nuclear Export and Adjacent Nuclear Localization Signals for ORF45 of Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2009, 83, 2531–2539. [Google Scholar] [CrossRef]
- Sander, G.; Konrad, A.; Thurau, M.; Wies, E.; Leubert, R.; Kremmer, E.; Dinkel, H.; Schulz, T.; Neipel, F.; Stürzl, M. Intracellular localization map of human herpesvirus 8 proteins. J. Virol. 2008, 82, 1908–1922. [Google Scholar] [CrossRef]
- Kuang, E.; Tang, Q.Y.; Maul, G.G.; Zhu, F.X. Activation of p90 ribosomal S6 kinase by ORF45 of Kaposi’s sarcoma-associated herpesvirus and its role in viral lytic replication. J. Virol. 2008, 82, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.X.; Yuan, Y. The ORF45 protein of Kaposi’s sarcoma-associated herpesvirus is associated with purified virions. J. Virol. 2003, 77, 4221–4230. [Google Scholar] [CrossRef]
- Zhu, F.X.; Chong, J.M.; Wu, L.J.; Yuan, Y. Virion proteins of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2005, 79, 800–811. [Google Scholar] [CrossRef]
- Guo, H.T.; Shen, S.; Wang, L.L.; Deng, H.Y. Role of tegument proteins in herpesvirus assembly and egress. Protein Cell 2010, 1, 987–998. [Google Scholar] [CrossRef]
- Sathish, N.; Wang, X.; Yuan, Y. Tegument Proteins of Kaposi’s Sarcoma-Associated Herpesvirus and Related Gamma-Herpesviruses. Front. Microbiol. 2012, 3, 98. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Che, Y.C.; Li, Q.H. HSV-1 tegument protein and the development of its genome editing technology. Virol. J. 2016, 13. [Google Scholar] [CrossRef]
- Dai, X.H.; Gong, D.Y.; Wu, T.T.; Sun, R.; Zhou, Z.H. Organization of Capsid-Associated Tegument Components in Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2014, 88, 12694–12702. [Google Scholar] [CrossRef] [PubMed]
- Nabiee, R.; Syed, B.; Castano, J.R.; Lalani, R.; Totonchy, J.E. An Update of the Virion Proteome of Kaposi Sarcoma-Associated Herpesvirus. Viruses 2020, 12, 1382. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Dai, X.; Xiao, Y.; Du, Y.; Chapa, T.J.; Johnson, J.R.; Li, X.; Krogan, N.J.; Deng, H.; Wu, T.T.; et al. Virus-Like Vesicles of Kaposi’s Sarcoma-Associated Herpesvirus Activate Lytic Replication by Triggering Differentiation Signaling. J. Virol. 2017, 91, e00362-17. [Google Scholar] [CrossRef] [PubMed]
- Rozen, R.; Sathish, N.; Li, Y.; Yuan, Y. Virion-wide protein interactions of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2008, 82, 4742–4750. [Google Scholar] [CrossRef]
- Gillen, J.; Li, W.W.; Liang, Q.M.; Avey, D.; Wu, J.J.; Wu, F.Y.; Myoung, J.; Zhu, F.X. A Survey of the Interactome of Kaposi’s Sarcoma-Associated Herpesvirus ORF45 Revealed Its Binding to Viral ORF33 and Cellular USP7, Resulting in Stabilization of ORF33 That Is Required for Production of Progeny Viruses. J. Virol. 2015, 89, 4918–4931. [Google Scholar] [CrossRef]
- Wu, J.J.; Avey, D.; Li, W.W.; Gillen, J.; Fu, B.S.; Miley, W.; Whitby, D.; Zhu, F.X. ORF33 and ORF38 of Kaposi’s Sarcoma-Associated Herpesvirus Interact and Are Required for Optimal Production of Infectious Progeny Viruses. J. Virol. 2016, 90, 1741–1756. [Google Scholar] [CrossRef]
- Guo, H.T.; Wang, L.L.; Peng, L.; Zhou, Z.H.; Deng, H.Y. Open Reading Frame 33 of a Gammaherpesvirus Encodes a Tegument Protein Essential for Virion Morphogenesis and Egress. J. Virol. 2009, 83, 10582–10595. [Google Scholar] [CrossRef]
- Gillen, J.; Zhu, F.X. Disruption of the Interaction between ORF33 and the Conserved Carboxyl-Terminus of ORF45 Abolishes Progeny Virion Production of Kaposi Sarcoma-Associated Herpesvirus. Viruses 2021, 13, 1828. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.S.; Reyes, R.A.; Izumiya, Y.; Wisdom, R.; Kung, H.J.; Luciw, P.A. ORF36 protein kinase of Kaposi’s sarcoma herpesvirus activates the c-Jun N-terminal kinase signaling pathway. J. Biol. Chem. 2004, 279, 38325–38330. [Google Scholar] [CrossRef]
- Avey, D.; Tepper, S.; Pifer, B.; Bahga, A.; Williams, H.; Gillen, J.; Li, W.W.; Ogden, S.; Zhu, F.X. Discovery of a Coregulatory Interaction between Kaposi’s Sarcoma-Associated Herpesvirus ORF45 and the Viral Protein Kinase ORF36. J. Virol. 2016, 90, 5953–5964. [Google Scholar] [CrossRef]
- Liang, Q.M.; Fu, B.S.; Wu, F.Y.; Li, X.J.; Yuan, Y.; Zhu, F.X. ORF45 of Kaposi’s Sarcoma-Associated Herpesvirus Inhibits Phosphorylation of Interferon Regulatory Factor 7 by IKK epsilon and TBK1 as an Alternative Substrate. J. Virol. 2012, 86, 10162–10172. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.S.; Kuang, E.; Li, W.W.; Avey, D.; Li, X.J.; Turpin, Z.; Valdes, A.; Brulois, K.; Myoung, J.J.; Zhu, F.X. Activation of p90 Ribosomal S6 Kinases by ORF45 of Kaposi’s Sarcoma-Associated Herpesvirus Is Critical for Optimal Production of Infectious Viruses. J. Virol. 2015, 89, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Alzhanova, D.; Meyo, J.O.; Juarez, A.; Dittmer, D.P. The ORF45 Protein of Kaposi Sarcoma-Associated Herpesvirus Is an Inhibitor of p53 Signaling during Viral Reactivation. J. Virol. 2021, 95, e01459-21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, X.; Liu, C.; Deng, H.; Li, W.; Xu, X.; Xiao, M.Z.X.; Wang, C.; Zhang, Y.; Fu, J.; et al. The SUMO E3 ligase activity of ORF45 determines KSHV lytic replication. PLoS Pathog. 2022, 18, e1010504. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, N.N.; Li, W.W.; Zhu, F.X.; Wang, Y.; Yuan, Y. Mono-ubiquitylated ORF45 Mediates Association of KSHV Particles with Internal Lipid Rafts for Viral Assembly and Egress. PLoS Pathog. 2015, 11, e1005332. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, J.F.; Liu, C.R.; Qu, Y.F.; Wang, W.L.; Xiao, M.Z.X.; Zhu, F.X.; Liu, Z.S.; Liang, Q.M. KSHV-encoded ORF45 activates human NLRP1 inflammasome. Nat. Immunol. 2022, 23, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.C.; Zhang, Y.J.; Deng, J.H.; Wang, X.P.; Pan, H.Y.; Hettler, E.; Gao, S.J. Efficient infection by a recombinant Kaposi’s sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: Application for genetic analysis. J. Virol. 2002, 76, 6185–6196. [Google Scholar] [CrossRef] [PubMed]
- Brulois, K.F.; Chang, H.; Lee, A.S.Y.; Ensser, A.; Wong, L.Y.; Toth, Z.; Lee, S.H.; Lee, H.R.; Myoung, J.; Ganem, D.; et al. Construction and Manipulation of a New Kaposi’s Sarcoma-Associated Herpesvirus Bacterial Artificial Chromosome Clone. J. Virol. 2012, 86, 9708–9720. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.X.; Li, X.J.; Zhou, F.C.; Gao, S.H.; Yuan, Y. Functional characterization of Kaposi’s sarcoma-associated herpesvirus ORF45 by bacterial artificial chromosome-based mutagenesis. J. Virol. 2006, 80, 12187–12196. [Google Scholar] [CrossRef] [PubMed]
- Sathish, N.; Zhu, F.X.; Yuan, Y. Kaposi’s Sarcoma-Associated Herpesvirus ORF45 Interacts with Kinesin-2 Transporting Viral Capsid-Tegument Complexes along Microtubules. PLoS Pathog. 2009, 5, e1000332. [Google Scholar] [CrossRef]
- Wei, X.Q.; Dong, J.Z.; Cheng, C.C.; Ji, M.J.; Yu, L.; Luo, S.Q.; Wu, S.W.; Bai, L.; Lan, K. Host RAB11FIP5 protein inhibits the release of Kaposi’s sarcoma-associated herpesvirus particles by promoting lysosomal degradation of ORF45. PLoS Pathog. 2020, 16, e1009099. [Google Scholar] [CrossRef] [PubMed]
- Abada, R.; Dreyfuss-Grossman, T.; Herman-Bachinsky, Y.; Geva, H.; Masa, S.R.; Sarid, R. SIAH-1 interacts with the Kaposi’s sarcoma-associated herpesvirus-encoded ORF45 protein and promotes its ubiquitylation and proteasomal degradation. J. Virol. 2008, 82, 2230–2240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, G.; Chung, Y.L.; Glover, T.; Valentine, V.; Look, A.T.; Fearon, E.R. Characterization of human homologs of the Drosophila seven in absentia (sina) gene. Genomics 1997, 46, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhang, S.; Vidal, M.; LaBaer, J.; Xu, T.; Fearon, E.R. Mammalian homologs of seven in absentia regulate DCC via the ubiquitin-proteasome pathway. Genes Dev. 1997, 11, 2701–2714. [Google Scholar] [CrossRef]
- DuShane, J.K.; Maginnis, M.S. Human DNA Virus Exploitation of the MAPK-ERK Cascade. Int. J. Mol. Sci. 2019, 20, 3427. [Google Scholar] [CrossRef] [PubMed]
- Sharma-Walia, N.; Krishnan, H.H.; Naranatt, P.P.; Zeng, L.; Smith, M.S.; Chandran, B. ERK1/2 and MEK1/2 induced by Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J. Virol. 2005, 79, 10308–10329. [Google Scholar] [CrossRef] [PubMed]
- Anjum, R.; Blenis, J. The RSK family of kinases: Emerging roles in cellular signalling. Nat. Rev. Mol. Cell Biol. 2008, 9, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Romeo, Y.; Zhang, X.C.; Roux, P.P. Regulation and function of the RSK family of protein kinases. Biochem. J. 2012, 441, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Davis, Z.H.; Verschueren, E.; Jang, G.M.; Kleffman, K.; Johnson, J.R.; Park, J.; Von Dollen, J.; Maher, M.C.; Johnson, T.; Newton, W.; et al. Global Mapping of Herpesvirus-Host Protein Complexes Reveals a Transcription Strategy for Late Genes. Mol. Cell 2015, 57, 349–360. [Google Scholar] [CrossRef]
- Sorgeloos, F.; Peeters, M.; Hayashi, Y.; Borghese, F.; Capelli, N.; Drappier, M.; Cesaro, T.; Colau, D.; Stroobant, V.; Vertommen, D.; et al. A case of convergent evolution: Several viral and bacterial pathogens hijack RSK kinases through a common linear motif. Proc. Natl. Acad. Sci. USA 2022, 119, e2114647119. [Google Scholar] [CrossRef]
- Alexa, A.; Sok, P.; Gross, F.; Albert, K.; Kobori, E.; Poti, A.L.; Gogl, G.; Bento, I.; Kuang, E.S.; Taylor, S.S.; et al. A non-catalytic herpesviral protein reconfigures ERK-RSK signaling by targeting kinase docking systems in the host. Nat. Commun. 2022, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Kuang, E.; Wu, F.Y.; Zhu, F.X. Mechanism of Sustained Activation of Ribosomal S6 Kinase (RSK) and ERK by Kaposi Sarcoma-associated Herpesvirus ORF45 multiprotein complexes retain active phosphorylated ERK and RSK and protect them from dephosphorylation. J. Biol. Chem. 2009, 284, 13958–13968. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Huang, L.; Xiao, Y.J.; Yao, X.Y.; Long, X.B.; Zhu, F.X.; Kuang, E.S. Development of an ORF45-Derived Peptide To Inhibit the Sustained RSK Activation and Lytic Replication of Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2019, 93, e02154-18. [Google Scholar] [CrossRef] [PubMed]
- Shahbazian, D.; Roux, P.P.; Mieulet, V.; Cohen, M.S.; Raught, B.; Taunton, J.; Hershey, J.W.B.; Blenis, J.; Pende, M.; Sonenberg, N. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. Embo. J. 2006, 25, 2781–2791. [Google Scholar] [CrossRef]
- Kuang, E.S.; Fu, B.S.; Liang, Q.M.; Myoung, J.; Zhu, F.X. Phosphorylation of Eukaryotic Translation Initiation Factor 4B (EIF4B) by Open Reading Frame 45/p90 Ribosomal S6 Kinase (ORF45/RSK) Signaling Axis Facilitates Protein Translation during Kaposi Sarcoma-associated Herpesvirus (KSHV) Lytic Replication. J. Biol. Chem. 2011, 286, 41171–41182. [Google Scholar] [CrossRef]
- Liu, Z.S.; Liu, C.R.; Wang, X.; Li, W.W.; Zhou, J.F.; Dong, P.X.; Xiao, M.Z.X.; Wang, C.X.; Zhang, Y.C.; Fu, J.Y.; et al. RSK1 SUMOylation is required for KSHV lytic replication. PLoS Pathog. 2021, 17, e1010123. [Google Scholar] [CrossRef]
- Huang, L.M.; Chao, M.F.; Chen, M.Y.; Shih, H.M.; Chiang, Y.P.; Chuang, C.Y.; Lee, C.Y. Reciprocal regulatory interaction between human herpesvirus 8 and human immunodeficiency virus type 1. J. Biol. Chem. 2001, 276, 13427–13432. [Google Scholar] [CrossRef] [PubMed]
- Karijolich, J.; Zhao, Y.; Peterson, B.; Zhou, Q.; Glaunsinger, B. Kaposi’s Sarcoma-Associated Herpesvirus ORF45 Mediates Transcriptional Activation of the HIV-1 Long Terminal Repeat via RSK2. J. Virol. 2014, 88, 7024–7035. [Google Scholar] [CrossRef]
- Li, X.J.; Du, S.M.; Avey, D.; Li, Y.Q.; Zhu, F.X.; Kuang, E.S. ORF45-Mediated Prolonged c-Fos Accumulation Accelerates Viral Transcription during the Late Stage of Lytic Replication of Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2015, 89, 6895–6906. [Google Scholar] [CrossRef]
- Li, X.J.; Kuang, E.S. RSK-c-Fos in KSHV lytic progression. Oncotarget 2015, 6, 24588–24589. [Google Scholar] [CrossRef]
- Stallone, G.; Schena, A.; Infante, B.; Di Paolo, S.; Loverre, A.; Maggio, G.; Ranieri, E.; Gesualdo, L.; Schena, F.P.; Grandaliano, G. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N. Engl. J. Med. 2005, 352, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ley, B.; Grillo, E.; Rios-Buceta, L.; Paoli, J.; Moreno, C.; Vano-Galvan, S.; Jaen-Olasolo, P. Classic Kaposi’s sarcoma treated with topical rapamycin. Dermatol. Ther. 2015, 28, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Alzhanova, D.; Corcoran, K.; Bailey, A.G.; Long, K.; Taft-Benz, S.; Graham, R.L.; Broussard, G.S.; Heise, M.; Neumann, G.; Halfmann, P.; et al. Novel modulators of p53-signaling encoded by unknown genes of emerging viruses. PLoS Pathog. 2021, 17, e1009033. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.G.; Hilton, I.B.; Staudt, M.R.; Burd, C.E.; Dittmer, D.P. Distinct p53, p53: LANA, and LANA Complexes in Kaposi’s Sarcoma-Associated Herpesvirus Lymphomas. J. Virol. 2010, 84, 3898–3908. [Google Scholar] [CrossRef] [PubMed]
- Laura, M.V.; de la Cruz-Herrera, C.F.; Ferreiros, A.; Baz-Martinez, M.; Lang, V.; Vidal, A.; Munoz-Fontela, C.; Rodriguez, M.S.; Collado, M.; Rivas, C. KSHV latent protein LANA2 inhibits sumo2 modification of p53. Cell Cycle 2015, 14, 277–282. [Google Scholar] [CrossRef]
- Nakamura, H.; Li, M.; Zarycki, J.; Jung, J.U. Inhibition of p53 tumor suppressor by viral interferon regulatory factor. J. Virol. 2001, 75, 7572–7582. [Google Scholar] [CrossRef]
- Shin, Y.C.; Nakamura, H.; Liang, X.Z.; Feng, P.H.; Chang, H.S.; Kowalik, T.F.; Jung, J.U. Inhibition of the ATM/p53 signal transduction pathway by Kaposi’s sarcoma-associated herpesvirus interferon regulatory factor 1. J. Virol. 2006, 80, 2257–2266. [Google Scholar] [CrossRef]
- Qi, S.M.; Cheng, G.; Cheng, X.D.; Xu, Z.; Xu, B.; Zhang, W.D.; Qin, J.J. Targeting USP7-Mediated Deubiquitination of MDM2/MDMX-p53 Pathway for Cancer Therapy: Are We There Yet? Front. Cell Dev. Biol. 2020, 8, 233. [Google Scholar] [CrossRef]
- Au, W.C.; Moore, P.A.; LaFleur, D.W.; Tombal, B.; Pitha, P.M. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J. Biol. Chem. 1998, 273, 29210–29217. [Google Scholar] [CrossRef]
- Sato, M.; Hata, N.; Asagiri, M.; Nakaya, T.; Taniguchi, T.; Tanaka, N. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 1998, 441, 106–110. [Google Scholar] [CrossRef]
- Zhu, F.X.; Sathish, N.; Yuan, Y. Antagonism of Host Antiviral Responses by Kaposi’s Sarcoma-Associated Herpesvirus Tegument Protein ORF45. PLoS ONE 2010, 5, e10573. [Google Scholar] [CrossRef] [PubMed]
- Sathish, N.; Zhu, F.X.; Golub, E.E.; Liang, Q.M.; Yuan, Y. Mechanisms of Autoinhibition of IRF-7 and a Probable Model for Inactivation of IRF-7 by Kaposi’s Sarcoma-associated Herpesvirus Protein ORF45. J. Biol. Chem. 2011, 286, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Virgin, H.W.; Latreille, P.; Wamsley, P.; Hallsworth, K.; Weck, K.E.; Dal Canto, A.J.; Speck, S.H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 1997, 71, 5894–5904. [Google Scholar] [CrossRef]
- Jia, X.; Shen, S.; Lv, Y.; Zhang, Z.W.; Guo, H.T.; Deng, H.Y. Tegument Protein ORF45 Plays an Essential Role in Virion Morphogenesis of Murine Gammaherpesvirus 68. J. Virol. 2016, 90, 7587–7592. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.M.; Sun, R. Inhibition of gammaherpesvirus replication by RNA interference. J. Virol. 2003, 77, 3301–3306. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Dutia, B.M.; Roberts, K.L.; Garcia-Ramirez, J.J.; Dickinson, P.; Stewart, J.P.; Ghazal, P.; Roy, D.J.; Nash, A.A. Transcriptome profile of murine gammaherpesvirus-68 lytic infection. J. Gen. Virol. 2003, 84, 99–109. [Google Scholar] [CrossRef]
- Bortz, E.; Whitelegge, J.P.; Jia, Q.M.; Zhou, Z.H.; Stewart, J.P.; Wu, T.T.; Sun, R. Identification of proteins associated with murine gammaherpesvirus 68 virions. J. Virol. 2003, 77, 13425–13432. [Google Scholar] [CrossRef]
- Vidick, S.; Leroy, B.; Palmeira, L.; Machiels, B.; Mast, J.; Francois, S.; Wattiez, R.; Vanderplasschen, A.; Gillet, L. Proteomic Characterization of Murid Herpesvirus 4 Extracellular Virions. PLoS ONE 2013, 8, e83842. [Google Scholar] [CrossRef]
- Bortz, E.; Wang, L.L.; Jia, Q.M.; Wu, T.T.; Whitelegge, J.P.; Deng, H.Y.; Zhou, Z.H.; Sun, R. Murine gammaherpesvirus 68 ORF52 encodes a tegument protein required for virion morphogenesis in the cytoplasm. J. Virol. 2007, 81, 10137–10150. [Google Scholar] [CrossRef]
- Jia, Q.M.; Chernishof, V.; Bortz, E.; McHardy, I.; Wu, T.T.; Liao, H.I.; Sun, R. Murine gammaherpesvirus 68 open reading frame 45 plays an essential role during the immediate-early phase of viral replication. J. Virol. 2005, 79, 5129–5141. [Google Scholar] [CrossRef]
- Desrosiers, R.C.; Sasseville, V.G.; Czajak, S.C.; Zhang, X.M.; Mansfield, K.G.; Kaur, A.; Johnson, R.P.; Lackner, A.A.; Jung, J.U. A herpesvirus of rhesus monkeys related to the human Kaposi’s sarcoma-associated herpesvirus. J. Virol. 1997, 71, 9764–9769. [Google Scholar] [CrossRef] [PubMed]
- Searles, R.P.; Bergquam, E.P.; Axthelm, M.K.; Wong, S.W. Sequence and genomic analysis of a rhesus macaque rhadinovirus with similarity to Kaposi’s sarcoma-associated herpesvirus human herpesvirus 8. J. Virol. 1999, 73, 3040–3053. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Damania, B.; Kedes, D.H. De novo infection with rhesus monkey Rhadinovirus leads to the accumulation of multiple intranuclear capsid species during lytic replication but favors the release of genome-containing virions. J. Virol. 2003, 77, 13439–13447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Connor, C.M.; Kedes, D.H. Mass spectrometric analyses of purified rhesus monkey rhadinovirus reveal 33 virion-associated proteins. J. Virol. 2006, 80, 1574–1583. [Google Scholar] [CrossRef]
- Anderson, M.S.; Loftus, M.S.; Kedes, D.H. Maturation and Vesicle-Mediated Egress of Primate Gammaherpesvirus Rhesus Monkey Rhadinovirus Require Inner Tegument Protein ORF52. J. Virol. 2014, 88, 9111–9128. [Google Scholar] [CrossRef]
- Woodson, E.N.; Kedes, D.H. Distinct Roles for Extracellular Signal-Regulated Kinase 1 (ERK1) and ERK2 in the Structure and Production of a Primate Gammaherpesvirus. J. Virol. 2012, 86, 9721–9736. [Google Scholar] [CrossRef]
- Woodson, E.N.; Anderson, M.S.; Loftus, M.S.; Kedes, D.H. Progressive Accumulation of Activated ERK2 within Highly Stable ORF45-Containing Nuclear Complexes Promotes Lytic Gammaherpesvirus Infection. PLoS Pathog. 2014, 10, e1004147. [Google Scholar] [CrossRef]
- Young, L.S.; Murray, P.G. Epstein-Barr virus and oncogenesis: From latent genes to tumours. Oncogene 2003, 22, 5108–5121. [Google Scholar] [CrossRef]
- Ko, Y.H. EBV and human cancer. Exp. Mol. Med. 2015, 47, e130. [Google Scholar] [CrossRef]
- Al Masud, H.M.A.; Watanabe, T.; Yoshida, M.; Sato, Y.; Goshima, F.; Kimura, H.; Murata, T. Epstein-Barr Virus BKRF4 Gene Product Is Required for Efficient Progeny Production. J. Virol. 2017, 91, e00975-17. [Google Scholar] [CrossRef]
- Gan, Y.Y.; Fonestan, A.; Chan, S.H.; Tsao, S.Y.; Li, B.; Tan, W.H. Molecular cloning and expression of Epstein-Barr virus antigens in the lambda-GT11 expression vector-antibodies towards proteins from the BORF2 and BKRF4 reading frames in nasopharyngeal carcinoma patients. Intervirology 1994, 37, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Lau, R.; Middeldorp, J.; Farrell, P.J. Epstein-Barr virus gene expression in oral hairy leukoplakia. Virology 1993, 195, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.H.; Sitz, J.; Shen, Q.T.; Leblanc-Lacroix, A.; Campos, E.I.; Borozan, I.; Marcon, E.; Greenblatt, J.; Fradet-Turcotte, A.; Jin, D.Y.; et al. A Screen for Epstein-Barr Virus Proteins That Inhibit the DNA Damage Response Reveals a Novel Histone Binding Protein. J. Virol. 2018, 92, e00262-18. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, E.; Luftig, M.; Chase, M.R.; Weicksel, S.; Cahir-McFarland, E.; Illanes, D.; Sarracino, D.; Kieff, E. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 2004, 101, 16286–16291. [Google Scholar] [CrossRef]
- Salsman, J.; Zimmerman, N.; Chen, T.; Domagala, M.; Frappier, L. Genome-wide screen of three herpesviruses for protein subcellular localization and alteration of PML nuclear bodies. PLoS Pathog. 2008, 4, e1000100. [Google Scholar] [CrossRef]
- Al Masud, H.M.A.; Watanabe, T.; Sato, Y.; Goshima, F.; Kimura, H.; Murata, T. The BOLF1 gene is necessary for effective Epstein-Barr viral infectivity. Virology 2019, 531, 114–125. [Google Scholar] [CrossRef]
- Konishi, N.; Narita, Y.; Hijioka, F.; Al Masud, H.M.A.; Sato, Y.; Kimura, H.; Murata, T. BGLF2 Increases Infectivity of Epstein-Barr Virus by Activating AP-1 upon De Novo Infection. Msphere 2018, 3, e00138-18. [Google Scholar] [CrossRef]
- Liu, X.Q.; Cohen, J.I. Epstein-Barr Virus (EBV) Tegument Protein BGLF2 Promotes EBV Reactivation through Activation of the p38 Mitogen-Activated Protein Kinase. J. Virol. 2016, 90, 1129–1138. [Google Scholar] [CrossRef]

| ORF45 Mutant/Motif | Function | Reference |
|---|---|---|
| S41A/S162A | IKKε and TBK1 phosphorylation sites | [41] |
| F66A | ERK/RSK binding and activation | [42] |
| A144G/V146G | SIAH-1 binding site | [34] |
| E223A(G224E)/S226A | USP7 binding site | [35,43] |
| 237IVDL240/328VIII331 mutant | SIM1 and SIM2 binding | [44] |
| V284A/L285A; I289A/L291A | ORF45 restricted to the nucleus (NES mutant) | [23] |
| K297R | ORF45 restricted to the cytoplasm (NLS mutant) | [23] |
| K297R, K99R, (297–300)4A | Targeting capsid to lipid rafts | [45] |
| Δ300-332 | hNLRP1 binding site | [46] |
| W403A/W405A | ORF33 binding site | [38] |
| Protein | Length (aa) | Expression Kinetics | Conserved Motif | Known Functions |
|---|---|---|---|---|
| KSHV ORF45 | 407 | Tegument, immediate-early | N terminus C terminus | ERK/RSK activation, ORF33 binding, production of viral progeny, IRF7 inhibition, inflammasome activation, SUMO E3 ligase |
| MHV 68 ORF45 | 217 | Tegument, early/late | N terminus C terminus | ORF33 binding, production of viral progeny |
| RRV ORF45 | 353 | Tegument, early | N terminus C terminus | ERK/RSK activation, ORF33 binding, production of viral progeny, SUMO E3 ligase |
| EBV BKRF4 | 206 | Tegument, early/late | N terminus C terminus | BGLF2 (ORF33) binding, production of viral progeny, inhibition of host DNA damage response |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atyeo, N.; Papp, B. The ORF45 Protein of Kaposi’s Sarcoma-Associated Herpesvirus and Its Critical Role in the Viral Life Cycle. Viruses 2022, 14, 2010. https://doi.org/10.3390/v14092010
Atyeo N, Papp B. The ORF45 Protein of Kaposi’s Sarcoma-Associated Herpesvirus and Its Critical Role in the Viral Life Cycle. Viruses. 2022; 14(9):2010. https://doi.org/10.3390/v14092010
Chicago/Turabian StyleAtyeo, Natalie, and Bernadett Papp. 2022. "The ORF45 Protein of Kaposi’s Sarcoma-Associated Herpesvirus and Its Critical Role in the Viral Life Cycle" Viruses 14, no. 9: 2010. https://doi.org/10.3390/v14092010
APA StyleAtyeo, N., & Papp, B. (2022). The ORF45 Protein of Kaposi’s Sarcoma-Associated Herpesvirus and Its Critical Role in the Viral Life Cycle. Viruses, 14(9), 2010. https://doi.org/10.3390/v14092010






